Performance of Dry-Seeded Rice Genotypes under Varied Soil Moisture Regimes and Foliar-Applied Hormones
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Crop Management, Plant Samplings, and Data-Recording
2.4. Plant Samplings for Biochemical Analysis
2.4.1. Estimation of Activity of Superoxide Dismutase (SOD), Peroxidase (POD), and Malondialdehyde (MDA) Lipid Peroxide Content
2.4.2. Proline, Free Amino Acids, and Total Soluble Proteins in Leaves
2.4.3. Total Chlorophyll Content
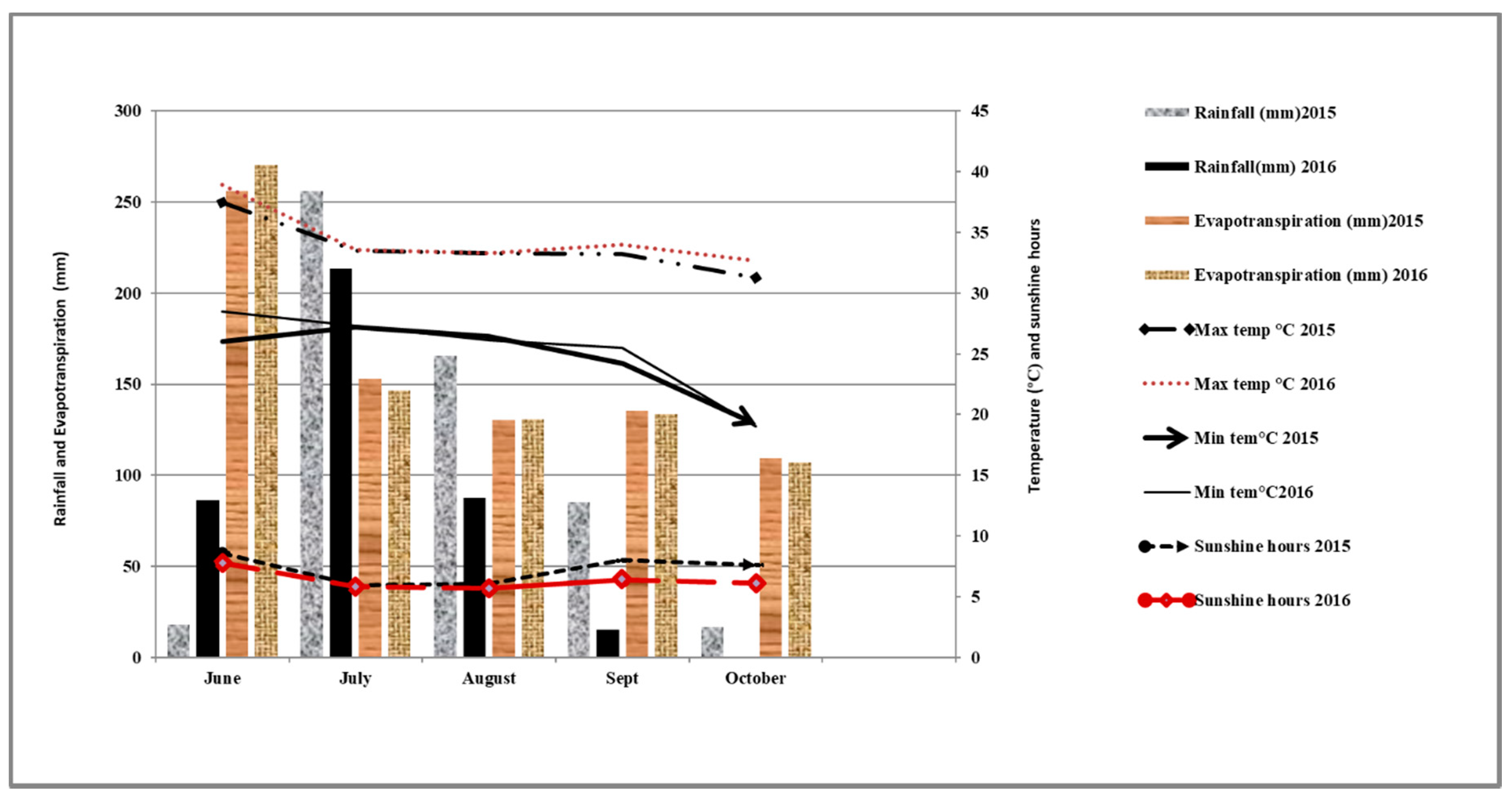
2.5. Weather Data
2.6. Statistical Analysis
3. Results and Discussion
3.1. Plant Height, Leaf Area Index, and Dry Matter Translocation
3.2. Yield, Yield-Contributing Characters, and Harvest Index
3.3. Antioxidants Activity and Osmoregulation
3.4. Correlation Studies
4. Conclusions and Future Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohanty, S. Trends in global rice consumption. Rice Today 2013, 12, 44–45. [Google Scholar]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Current status, challenges and opportunities in rice production. In Rice Production Worldwide; Chauhan, B.S., Khawar, J., Mahajan, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–32. [Google Scholar]
- Chauhan, B.S.; Mahajan, G.; Sardana, V.; Timsina, J.; Jat, M.L. Productivity and sustainability of rice-wheat cropping system in Indo-Gangetic plains of the Indian subcontinent: Problems, opportunities, and strategies. Adv. Agron. 2012, 117, 315–369. [Google Scholar]
- Rodell, M.; Velicigna, I.; Famiglietti, J.S. Satellite-based estimates of ground water-depletion in India. Nature 2009, 60, 999–1002. [Google Scholar] [CrossRef]
- Hira, G.S. Water management in northern states and the food security of India. J. Crop Improv. 2009, 23, 136–157. [Google Scholar] [CrossRef]
- Bhushan, L.; Ladha, J.K.; Gupta, R.K.; Singh, S.; Tirol-Padre, A.; Saharawi, Y.S.; Guthega, M.; Pathak, H. Saving of water and labour in a rice-wheat system with no-tillage and direct seeding technologies. Agron. J. 2007, 99, 1288–1296. [Google Scholar] [CrossRef]
- Gopal, R.; Jat, R.K.; Malik, R.K.; Kumar, V.; Alam, M.M.; Jat, M.L.; Mazid, M.A.; Saharawat, Y.S.; McDonald, A.; Gupta, R. Direct Dry-Seeded Rice Production Technology and Weed Management in Rice Based Systems; Tec. Bullet; International Maize and Wheat Improvement Centre: New Delhi, India, 2010; p. 28. [Google Scholar]
- Liu, H.; Hussain, S.; Zheng, M.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Dry direct-seeded rice as an alternative to transplanted-flooded rice in central China. Agron. Sust. Devl. 2015, 35, 285–294. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B.S.; Gill, M.S. Dry seeded rice culture in Punjab state of India; lesson learned from farmers. Field Crop. Res. 2013, 144, 89–99. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B.S.; Johnson, D.E. Weed management in aerobic rice in northwestern Indo-Gangetic Plains. J. Crop Improv. 2009, 23, 366–382. [Google Scholar] [CrossRef]
- Pal, R.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Impact of sowing date on yield, dry matter and nitrogen accumulation and nitrogen translocation in dry-seeded rice in North-West India. Field Crop Res. 2017, 206, 138–148. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defence mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 26. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Asthir, B. Transformation of sucrose to starches and protein in rice leaves and grains under two establishment methods. Rice Sci. 2016, 23, 255–265. [Google Scholar] [CrossRef]
- Kumari, M.; Asthir, B. Characterisation of rice cultivars on the basis of antioxidant defence response. Indian J. Plant Physiol. 2016, 21, 107–113. [Google Scholar] [CrossRef]
- Leshem, Y.Y.; Wurzburger, J.; Grossman, S. Cytokinin interaction with free radical metabolism and senescence effects on endogenous lipoxygenase and purine oxidation. Physiol. Plant. 1981, 53, 9–12. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, B.; Xu, V.Q. Effects of abscisic acid on drought responses of kentucky bluegrass. J. Amer. Soc. Hort. Sci. 2003, 128, 36–41. [Google Scholar] [CrossRef]
- Li, C.; Yin, C.; Liu, S. Different responses of two contrasting Populus davidiana populations to exogenous abscisic acid application. Environ. Exp. Bot. 2004, 51, 237–246. [Google Scholar] [CrossRef]
- Rajasekaran, L.R.; Blake, T.J. Early growth invigoration of jack pine seedlings by natural plant growth regulators. Trees 1998, 12, 420–423. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L.; Smith, M.; Beckett, R.P. Abscisic acid modifies the changes in lipid brought about by water stress in the moss (Atrichum androgynum). New Phytol. 2002, 156, 255–264. [Google Scholar]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef]
- Yoshida, K.; Igarashi, E.; Mukai, M.; Hirata, K.; Miyamoto, K. Induction of tolerance to oxidative stress in the green alga, Chlamydomonas reinhardtii, by abscisic acid. Plant Cell Environ. 2003, 26, 451–457. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Cherkozyanova, A.; Dodd, I.C. Effect of partial root zone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J. Exp. Bot. 2007, 58, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vanova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.K. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Zavaleta-Mancera, H.A.; López-Delgado, H.; Loza-Tavera, H.; Mora, H.M.; Trevilla-García, C.; Vargas-Suárez, M.; Ougham, H. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J. Plant Physiol. 2007, 164, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Baque, M.A.; Hahn, E.J.; Paek, K.Y. Growth, secondary metabolite production and antioxidant enzyme response of Morinda citrifolia adventitious root as affected by auxin and cytokinin. Plant Biotech. Rep. 2010, 4, 109–116. [Google Scholar] [CrossRef]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photo-respiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Gianfagna, T.; Huang, B. Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J. Exp.Bot. 2011, 62, 5311–5333. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Frebort, I.; Pospısilova, H.; Martı´nez-Andujar, C.; Acosta, M.; Sanchez-Bravo, J.; Lutts, S.; Dodd, I.C.; et al. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef]
- Synkova, H.; Semoradova, S.; Schnablova, R.; Witters, E.; Husak, M.; Valcke, R. Cytokinin-induced activity of antioxidant enzymes in transgenic Pssu-ipt tobacco during plant ontogeny. Biol. Plant. 2006, 50, 31–41. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Cox, M.C.; Qualset, C.O.; Rains, D.W. Genetic variation for nitrogen assimilation and translocation in wheat. III. Nitrogen translocation in relation to grain yield and protein. Crop Sci. 1986, 26, 737–740. [Google Scholar] [CrossRef]
- Papakosta, D.K.; Gagianas, A.A. Nitrogen and dry matter accumulation, remobilization and losses for mediterranean wheat during grain filling. Agron. J. 1991, 83, 864–870. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 16, 559–566. [Google Scholar] [CrossRef]
- Goyal, M.; Asthir, B. Polyamine catabolism influences antioxidant defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul. 2010, 60, 13–25. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photo-per-oxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid per-oxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lee, Y.P.; Takahashi, T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Canad. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenol oxidases in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Mahajan, G.; Sharma, N.; Sardana, V. Enhancing grain yield and nitrogen-use efficiency in rice through foliarly applied gibberellic acid in dry-direct-seeded rice. J. Crop Impro. 2015, 29, 65–81. [Google Scholar] [CrossRef]
- Watanabe, H.; Hase, S.; Saigusa, M. Effect of combine application of ethephon and gibberellins on growth and nutrient uptake of rice seedling growing. Plant Prod. Sci. 2007, 10, 468–472. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Pandey, P.; Giri, S.P.; Dwivedi, J.L. Effect of GA3 and other plant growth regulators on hybrid rice seed production. Asian. J. Plant. Sci. 2011, 10, 1–7. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Liu, K.; Wang, P. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 2006, 57, 149–160. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Yuan, L.; Wang, Z.; Yang, J.; Zhang, J. Post-anthesis alternate wetting and moderate soil drying enhance activities of key enzymes in sucrose-to-starch conversion in inferior spikelets of rice. J. Exp. Bot. 2012, 63, 215–227. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Wang, J.; Wang, Z.; Yang, J.; Zhang, J. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J. Exp. Bot. 2013, 64, 2523–2538. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Liu, L.J. Water deficit induced senescence and its relationships to remobilization of pre-stored carbon in wheat during grain filling. Agron. J. 2001, 93, 196–206. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, T.T.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Involvement of cytokinins in the grain filling of rice under alternate wetting and drying irrigation. J. Exp. Bot. 2010, 61, 3719–3733. [Google Scholar] [CrossRef]
- Roitsch, T.; Ehne, R. Regulation of source/sink relations. Plant Growth Regul. 2000, 32, 359–367. [Google Scholar] [CrossRef]
- Tang, W.-B.; Zhang, G.L.; Xiao, Y.-H.; Deng, H.-B.; Guo-hua, K.F.; Liu, L.C. Physiological and biochemical characteristics in flag leaves of the C Lingyou series of rice hybrid combinations at late growth stages. Rice Science. 17 (English version). J. Rice Sci. 2010, 24, 169–174. [Google Scholar]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Lui, L.J. Absicsic acid and cytokinins in the root exudates and leaves and their relationships to senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Planta 2002, 215, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Tuna, A.L.; Alfredo, A.C.A. Gibberellic acid improves water deficit tolerance in maize plants. Acta Physiol.Plant. 2006, 4, 331–337. [Google Scholar] [CrossRef]
- Arteca, R.N. Plant Growth Substances: Principles and Applications; CBS Publishers: New Delhi, India, 1997. [Google Scholar]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Davis, P.J. Introduction. In Plant Hormones, Biosynthesis, Signal Transduction, Action; Davis, P.J., Ed.; Kluwer, Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1–35. [Google Scholar]
- Van, J.S.; Davey, J.E. The synthesis, transport, and metabolism of endogenous cytokinnins. Plant Cell Environ. 1979, 2, 93–106. [Google Scholar]
- Arguest, C.T.; Ferreria, F.J.; Kieber, J.J. Environmental perceptions avenues: The interaction of cytokinin and environmental response pathway. Plant Cell Environ. 2009, 32, 1147–1160. [Google Scholar] [CrossRef]
- Arshad, M.; Frankenberger, J. Microbial production of plant growth regulators. In Soil Microbial Ecology; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Gurmani, A.R.; Bano, A.N.; Zhang, J.; Khan, S.U.; Flower, T.J. Exogenous applied silicates and abscisic acid ameliorates the growth of salinity stressed wheat (Triticum aestivum) seedling through Na+ exclusion. Aust. J. Crop Sci. 2013, 7, 1123–1130. [Google Scholar]
- Yang, J.C.; Zhang, J.H.; Weng, Z.Q.; Zhu, Q.S.; Liu, L.J. Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserve in wheat subjected to water stress during grain filling. Plant Cell Environ. 2003, 26, 1621–1631. [Google Scholar] [CrossRef]
- Cao, S.Q.; Zhang, R.X.; Lu, W.; Dong, Z.R.; Zeng, Q.M. The involvement of cytokinin and abscisic levels in roots in the regulation of photosynthetic function in flag leaves during grain filling in super-high-yielding rice (Oryza stiva). J. Agron. Crop Sci. 2004, 190, 73–80. [Google Scholar]
- Tietz, A.; Ludwig, M.; Dingkuhn, M.; Dorffling, K. Effect of abscisic acid on the transport of assimilates in barley. Planta 1981, 152, 557–561. [Google Scholar] [CrossRef]
- Clifford, P.E.; Offler, C.E.; Patrick, J.W. Growth regulators have rapid effects on photosynthate unloading from seed coats of Phaseolus vulgaris L. Plant Physiol. 1986, 80, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.L.; Cheikh, N. The role of hormones in photosynthate partitioning and seed filling. In Plant Hormones, Physiology, Biochemistry and Molecular Biology; Davis, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 649–670. [Google Scholar]
- Yang, J.; Wang, Z.; Zhu, Q.; Su, B. Regulation of ABA and GA to the grain filling of rice. Acta Agron. Sini. 1999, 25, 341–348. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, L.; Zhang, S.; Xiao, Y.; Zhizhou, H.; Dongyang, L. Effect of high temperature stress on protective enzyme activities and membrane permeability of flag leaf in rice. Acta Agron. Sin. 2006, 32, 1306–1310. [Google Scholar]
- Ghorbanli, M.; Gafarabad, M.; Amirkian, T.; Allahverdi, B.M. Investigation of proline, total protein, chlorophyll, ascorbate and dehydro ascorbate changes under drought stress in Akria and Mobil tomato cultivars. Iran J. Plant Physiol. 2013, 2, 651–658. [Google Scholar]
- Khan, S.U.; Gurmani, A.R.; Din, J.U.; Qayyum, A.; Kashif, A.S.; Muhammad, L.; Zahoor, A. Exogenously applied gibberellic acid, indole acetic acid and kinetin as potential regulators of source-sink relationship of physiological and yield attributes in rice (Oryza sativa L.) genotypes under water deficit conditions. Int. J. Agric. Bio. 2016, 18, 139–145. [Google Scholar] [CrossRef]
- Stevens, J.T.; Senaratna, S.K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilization. Plant Growth Regul. 2006, l49, 77–83. [Google Scholar]
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Plant Bio. 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Kumar, N.A.R.; Vijayalakshmi, C.; Vijayalakshmi, D. Osmolytes accumulation, membrane stability and ABA profiles in rice genotypes exposed to heat and drought stress. Int. J. Bior. Stress Mgt. 2015, 6, 117–122. [Google Scholar] [CrossRef]
- Danai-Tambhale, S.; Kumar, V.; Shriram, V. Differential response of two scented indica rice (Oryza sativa) cultivars under salt stress. J. Stress Physiol. Biochem. 2011, 7, 387–397. [Google Scholar]
- Tuna, A.L.; Kaya, A.C.; Dikilitas, M.; Higgs, D. The combined effect of gibberellic acid and salinity on some antioxidant enzymes activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Meena, R.K.; Verulkar, S.B.; Chandel, G. Nutrient characters analysis in rice genotypes under different environmental conditions. Bult. Environ. Pharmaco. Life Sci. 2012, 1, 61–64. [Google Scholar]
- Tan, C.L.; Zhang, H.X.; Dai, Z.Y.; Zhao, B.H.; Xu, M.L.; Liu, X.B.; Zhao, G.X. Characteristics of sink, source and flow in good quality indica rice Yangdao 6. Sci. Agric. Sin. 2003, 36, 26–30, (In Chinese with English Abstract). [Google Scholar]
- Pan, S.; Rasul, F.; Li, W.; Tian, H.; Mo, Z.; Duan, M.; Tang, X. Role of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Bano, A.; Ullah, F.; Nosheen, A. Role of abscisic acid and drought stress on activities of antioxidant enzymes in wheat. Plant Soil Environ. 2012, 42, 181–185. [Google Scholar] [CrossRef]


| Plant Hormones | Plant Height (cm) | |
|---|---|---|
| Genotypes | ||
| PR-111 | PR-123 | |
| GA3 (40 mg kg−1) | 81.3 | 90.6 |
| ABA (20 mg kg−1) | 77.0 | 80.0 |
| CK (40 mg kg−1) | 76.0 | 82.7 |
| Water spray (control) | 73.3 | 78.7 |
| LSD0.05 | 3.7 | |
| Treatments | Plant Height at Maturity (cm) | Leaf Area Index at Flowering | Leaf Area Index at Maturity | Dry Matter Translocation (Mg per ha) | Total Chlorophyll Leaf (mg per g fw) | |
|---|---|---|---|---|---|---|
| at Flowering before Spray | at 15 Days after Spray | |||||
| Soil moisture tension regimes | ||||||
| 10 kPa | 82.0 | 4.30 | 3.90 | 2.85 | 2.87 | 2.58 |
| 20 kPa | 78.0 | 3.70 | 3.18 | 2.30 | 3.02 | 2.61 |
| LSD0.05 | 3.0 | 0.11 | 0.16 | 0.48 | 0.09 | NS |
| Genotypes | ||||||
| PR-111 | 76.9 | 4.10 | 3.48 | 2.66 | 2.70 | 2.35 |
| PR-123 | 83.0 | 3.95 | 3.60 | 2.47 | 3.19 | 2.85 |
| LSD0.05 | 3.0 | 0.11 | NS | NS | 0.03 | 0.06 |
| Plant hormones | ||||||
| GA3 (40 mg kg−1) | 86.0 | 4.0 | 3.63 | 2.78 | 2.95 | 2.58 |
| ABA (20 mg kg−1) | 78.5 | 4.0 | 3.73 | 3.11 | 2.93 | 2.63 |
| CK (40 mg kg−1) | 78.0 | 4.0 | 3.73 | 2.46 | 2.95 | 2.66 |
| Water spray (control) | 77.4 | 4.0 | 3.10 | 1.91 | 2.94 | 2.52 |
| LSD0.05 | 2.6 | NS | 0.23 | 0.50 | NS | 0.08 |
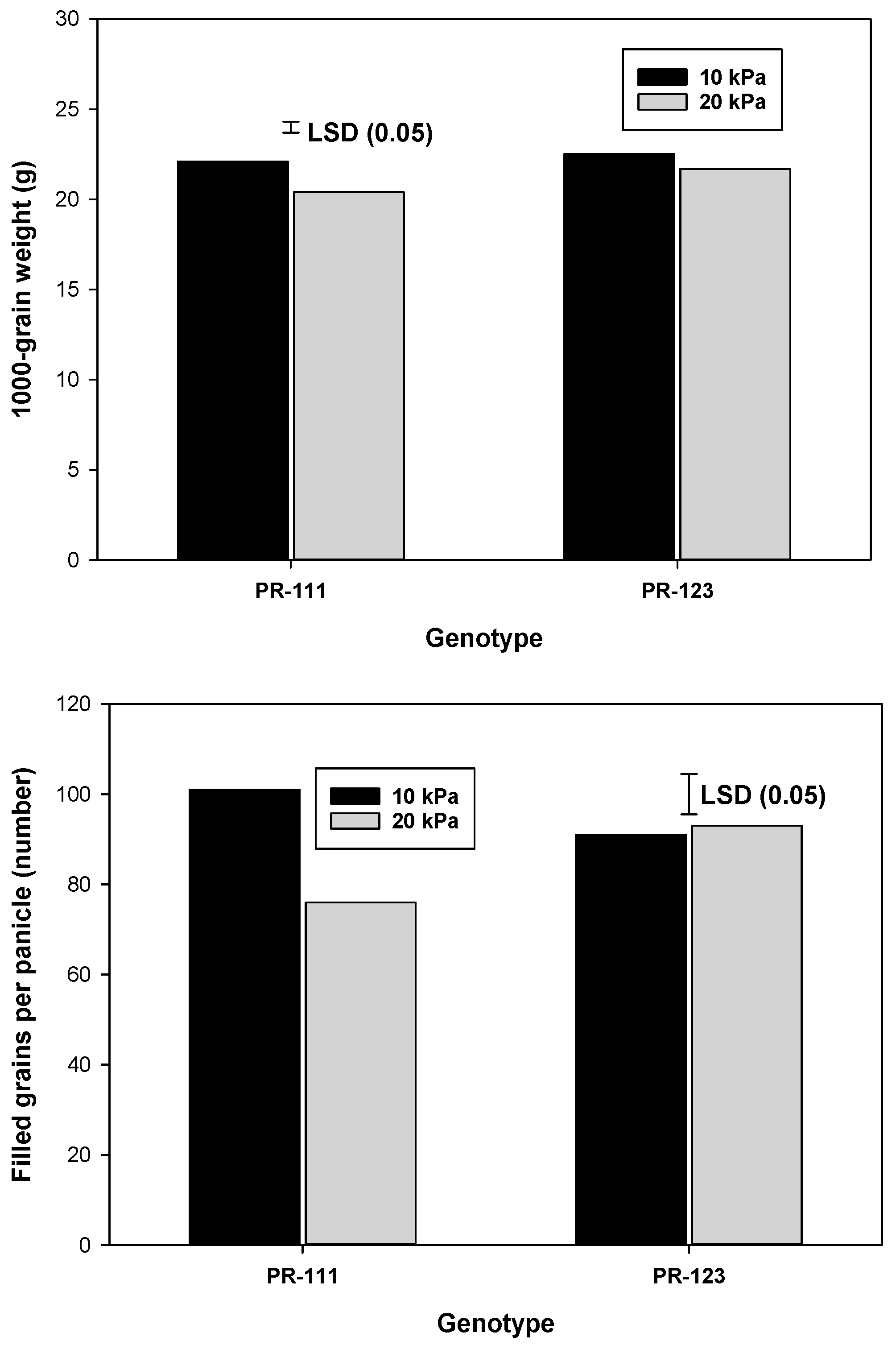
| Treatments | Grain Yield (t ha−1) | Panicles m−2 (Numbers) | Filled Grain per Panicle (Numbers) | 1000-Grain Weight (g) | Harvest Index |
|---|---|---|---|---|---|
| Soil moisture tension regimes | |||||
| 10 kPa | 5.8 | 383 | 96 | 22.3 | 0.35 |
| 20 kPa | 4.7 | 372 | 84 | 21.0 | 0.30 |
| LSD0.05 | 0.3 | NS | 6 | 0.4 | 0.02 |
| Genotypes | |||||
| PR-111 | 5.3 | 386 | 88 | 21.3 | 0.35 |
| PR-123 | 5.2 | 368 | 92 | 22.1 | 0.31 |
| LSD0.05 | NS | 12 | NS | 0.4 | 0.02 |
| Plant hormones | |||||
| GA (40 mg kg−1) | 5.5 | 388 | 93 | 21.5 | 0.34 |
| ABA (20 mg kg−1) | 5.4 | 376 | 94 | 22.0 | 0.35 |
| CK (40 mg kg−1) | 5.4 | 380 | 90 | 21.8 | 0.32 |
| Water spray (control) | 4.6 | 366 | 83 | 21.3 | 0.30 |
| LSD0.05 | 0.4 | NS | 6 | 0.4 | 0.02 |
| Plant Hormones | Soil Moisture Tension Regimes | |||
|---|---|---|---|---|
| 10 kPa | 20 kPa | |||
| Genotypes | ||||
| PR-111 | PR-123 | PR-111 | PR-123 | |
| Proline contents ( µg per g of fresh leaves) | ||||
| GA3 (40 mg kg−1) | 170.6 | 129.5 | 154.5 | 180.6 |
| ABA (20 mg kg−1) | 147.8 | 147.5 | 214.1 | 198.8 |
| CK (40 mg kg−1) | 133.7 | 144.7 | 217.4 | 154.3 |
| Water spray (control) | 143.1 | 138.7 | 170.6 | 106.6 |
| LSD0.05 | 16.2 | |||
| Soluble protein (%) | ||||
| GA3 (40 mg kg−1) | 8.6 | 9.2 | 7.8 | 7.3 |
| ABA (20 mg kg−1) | 7.7 | 7.5 | 7.3 | 7.1 |
| CK (40 mg kg−1) | 7.0 | 8.4 | 6.9 | 6.8 |
| Water spray (control) | 7.3 | 5.9 | 7.0 | 6.6 |
| LSD0.05 | 0.8 | |||
| Amino acids (%) | ||||
| GA3 (40 mg kg−1) | 0.59 | 0.34 | 0.57 | 0.61 |
| ABA (20 mg kg−1) | 0.60 | 0.46 | 0.43 | 0.65 |
| CK (40 mg kg−1) | 0.59 | 0.65 | 0.68 | 0.62 |
| Water spray (control) | 0.40 | 0.46 | 0.45 | 0.52 |
| LSD0.05 | 0.04 | |||
| Plant Hormones | Soil Moisture Tension Regimes | |||
|---|---|---|---|---|
| 10 kPa | 20 kPa | |||
| Genotypes | ||||
| PR-111 | PR-123 | PR-111 | PR-123 | |
| Activity of SOD (U per mg of protein) | ||||
| GA3 (40 mg kg−1) | 3.71 | 4.33 | 5.52 | 4.75 |
| ABA (20 mg kg−1) | 4.87 | 6.16 | 5.68 | 5.71 |
| CK (40 mg kg−1) | 3.75 | 4.31 | 3.54 | 4.21 |
| Water spray (control) | 3.52 | 4.20 | 2.63 | 2.40 |
| LSD0.05 | 0.62 | |||
| Activity of POD (µ moles per minute per gram of fw) | ||||
| GA3 (40 mg kg−1) | 5.62 | 6.50 | 6.03 | 5.99 |
| ABA (20 mg kg−1) | 5.10 | 5.12 | 7.73 | 6.81 |
| CK (40 mg kg−1) | 5.03 | 6.79 | 7.08 | 5.35 |
| Water spray (control) | 3.92 | 4.45 | 4.98 | 5.01 |
| LSD0.05 | 0.36 | |||
| MDA production in flag leaf (mg per g of fw) | ||||
| GA3 (40 mg kg−1) | 7.60 | 9.70 | 7.20 | 6.90 |
| ABA (20 mg kg−1) | 5.70 | 8.40 | 5.80 | 6.00 |
| CK (40 mg kg−1) | 9.90 | 12.1 | 11.80 | 6.00 |
| Water spray (control) | 15.2 | 14.5 | 15.4 | 10.9 |
| LSD0.05 | 1.05 | |||
| GY | P m−2 | G P−1 | 1000-GW | HI | P. Ht. (F) | LAI (F) | LAI (M) | DMT | |
|---|---|---|---|---|---|---|---|---|---|
| GY | |||||||||
| P per m2 | 0.56 | ||||||||
| G per P | 0.96 * | 0.52 | |||||||
| 1000-GW | 0.98 * | 0.51 | 0.93 * | ||||||
| HI | 0.98 * | 0.61 | 0.98 * | 0.95 * | |||||
| Pl. Ht (F) | −0.04 | −0.20 | 0.07 | 0.13 | −0.12 | ||||
| LAI (F) | 0.91 * | 0.54 | 0.95 * | 0.86 * | 0.81 * | 0.95 * | |||
| LAI (M) | 0.72 * | 0.50 | 0.68 | 0.77 * | 0.69 | 0.71 * | 0.23 | ||
| DMT | 0.34 | 0.43 | 0.30 | 0.37 | 0.30 | 0.39 | 0.26 | 0.49 |
| GY | P m−2 | G P−1 | 1000-GW | HI | P. Ht. (F) | LAI (F) | LAI (M) | DMT | |
|---|---|---|---|---|---|---|---|---|---|
| GY | |||||||||
| P per m2 | 0.12 | ||||||||
| G per P | 0.86 * | −0.32 | |||||||
| 1000 GW | 0.34 | −0.58 | 0.40 | ||||||
| HI | 0.85 * | 0.03 | 0.76 * | 0.42 | |||||
| Pl. Ht. (F) | −0.15 | −0.07 | −0.25 | 0.28 | −0.12 | ||||
| LAI (F) | −0.22 | −0.15 | −0.22 | 0.41 | 0.27 | −0.38 | |||
| LAI (M) | 0.21 | 0.00 | 0.01 | 0.55 | −0.09 | 0.21 | −0.25 | ||
| DMT | 0.72 * | 0.21 | 0.49 | 0.47 | 0.28 | 0.52 | −0.29 | 0.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, R.; Mahajan, G.; Sardana, V.; Asthir, B.; Chauhan, B.S. Performance of Dry-Seeded Rice Genotypes under Varied Soil Moisture Regimes and Foliar-Applied Hormones. Plants 2020, 9, 539. https://doi.org/10.3390/plants9040539
Pal R, Mahajan G, Sardana V, Asthir B, Chauhan BS. Performance of Dry-Seeded Rice Genotypes under Varied Soil Moisture Regimes and Foliar-Applied Hormones. Plants. 2020; 9(4):539. https://doi.org/10.3390/plants9040539
Chicago/Turabian StylePal, Rajinder, Gulshan Mahajan, Virender Sardana, Bavita Asthir, and Bhagirath Singh Chauhan. 2020. "Performance of Dry-Seeded Rice Genotypes under Varied Soil Moisture Regimes and Foliar-Applied Hormones" Plants 9, no. 4: 539. https://doi.org/10.3390/plants9040539
APA StylePal, R., Mahajan, G., Sardana, V., Asthir, B., & Chauhan, B. S. (2020). Performance of Dry-Seeded Rice Genotypes under Varied Soil Moisture Regimes and Foliar-Applied Hormones. Plants, 9(4), 539. https://doi.org/10.3390/plants9040539






