Preliminary Investigation of Effect of Neem-Derived Pesticides on Plasmopara halstedii Pathotype 704 in Sunflower under In Vitro and In Vivo Conditions
Abstract
1. Introduction
2. Results
2.1. HPLC Analysis of Neem Leaf Extract (NLE)
2.2. Pathotype Characterization of the Tested P. halstedii Isolate
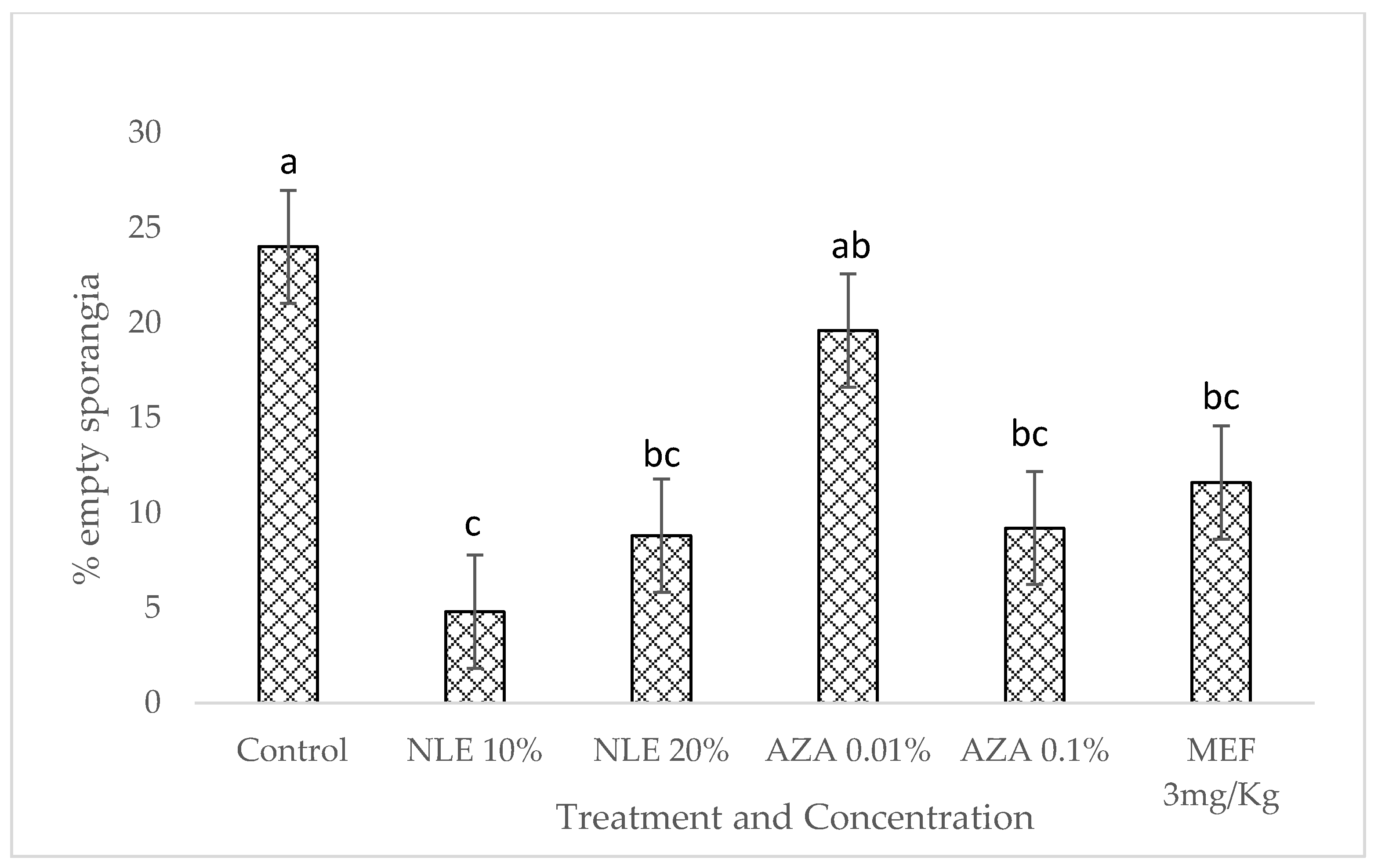
2.3. In Vitro Experiment: Microscopic Examination of the Effect of Neem-Derived Pesticides on P. halstedii Inoculum
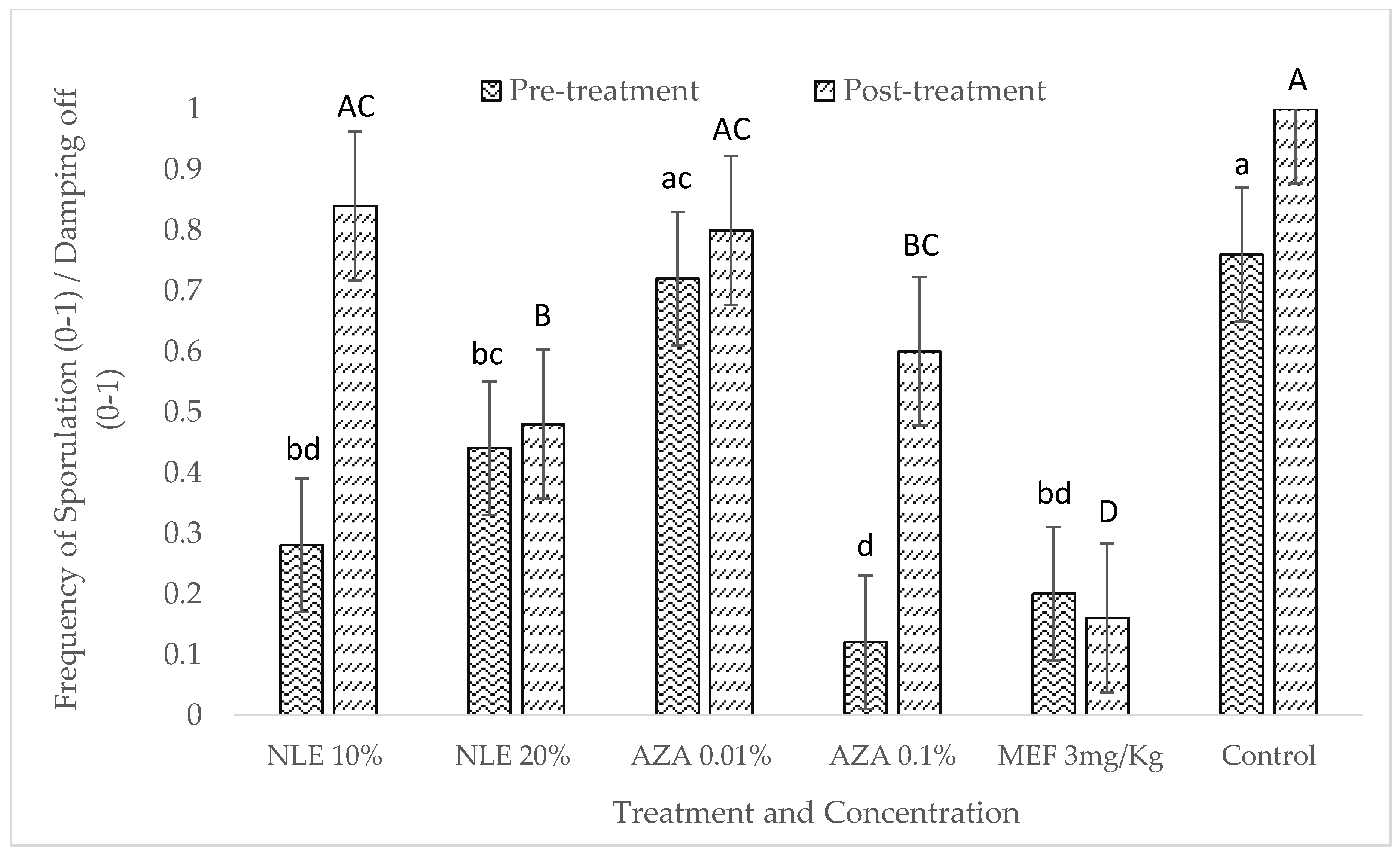
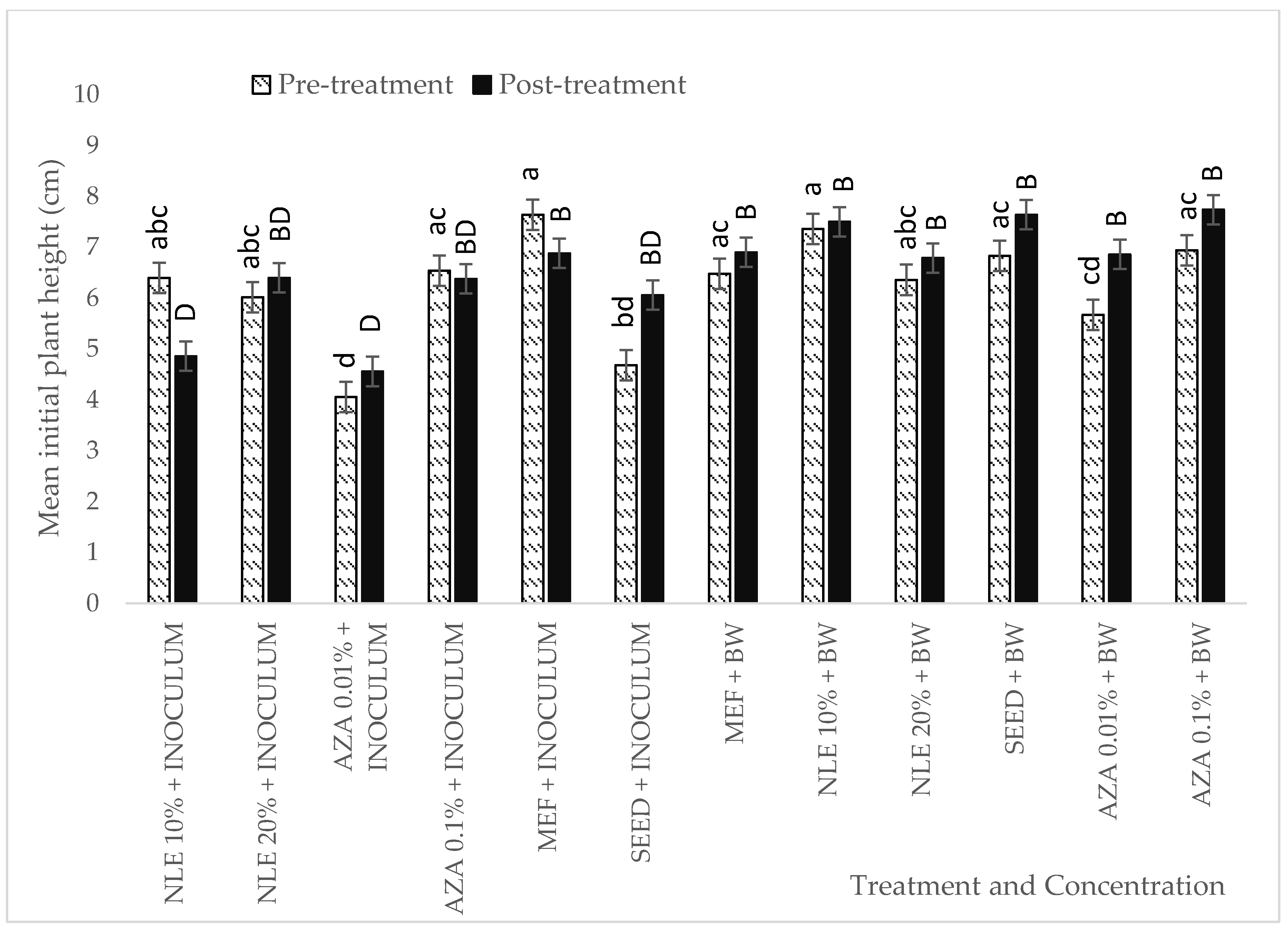
2.4. In Vivo Experiment: Pre- and Post-Treatment Effect of Neem-Derived Pesticides Against P. halstedii in Sunflower
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of Neem Leaf Extract (NLE)
5.2. Preparation of Azadirachtin (NeemAzal T/S) (AZA)
5.3. Preparation of Mefenoxam (MEF)
5.4. HPLC Analysis of Neem Leaf Extract
5.5. Pathotype Characterization of the Tested P. halstedii Isolate
5.6. P. halstedii Sporangia Collection and Preparation of Inoculum
5.7. In Vitro Experiment: Examination of the Effect of Neem-Derived Pesticides on P. halstedii Sporangial Germination
5.8. In Vivo Experiment: Pre- and Post-Treatment Effect of Neem-Derived Pesticides on P. halstedii in Sunflower
5.8.1. Pre-Treatment Effect of Neem-Derived Pesticides Against P. halstedii in Sunflower
- Seedlings inoculated with Plasmopara halstedii sporangial suspension.
- Seedlings treated with bidistilled water (BW).
- Treated seeds (Mefenoxam) inoculated with Plasmopara halstedii sporangial solution.
- Treated seeds (Mefenoxam) inoculated with bidistilled water (BW).
- Seedlings pre-treated with AZA 0.1% inoculated with Plasmopara halstedii sporangial solution.
- Seedlings pre-treated with AZA 0.1% inoculated with bidistilled water (BW).
- Seedlings pre-treated with AZA 0.01% inoculated with Plasmopara halstedii sporangial solution.
- Seedlings pre-treated with AZA 0.01% inoculated with bidistilled water (BW).
- Seedlings pre-treated with NLE 10% inoculated with Plasmopara halstedii sporangial solution.
- Seedlings pre-treated with NLE 10% inoculated with bidistilled water (BW).
- Seeds pre-treated with NLE 20% inoculated with Plasmopara halstedii sporangial solution.
- Seedlings pre-treated with NLE 20% inoculated with double distilled water (BW).
5.8.2. Post-treatment Effect of Neem-Derived Pesticides Against P. halstedii in Sunflower
- Seeds inoculated with Plasmopara halstedii sporangial suspension.
- Seeds inoculated with bidistilled water (BW).
- Pre-treated seeds (Mefenoxam) inoculated with Plasmopara halstedii sporangial solution.
- Pre-treated seeds (Mefenoxam) inoculated with bidistilled water (BW).
- Seeds inoculated with Plasmopara halstedii sporangial solution followed by AZA 0.1% solution.
- Seeds inoculated with bidistilled water followed by AZA 0.1% solution (BW).
- Seeds inoculated with Plasmopara halstedii sporangial solution followed by AZA 0.01% solution.
- Seeds inoculated with bidistilled water followed by AZA 0.01% solution (BW).
- Seeds inoculated with Plasmopara halstedii sporangial solution, followed by NLE 10% solution.
- Seeds inoculated with bidistilled water followed by NLE 10% solution (BW).
- Seeds inoculated with Plasmopara halstedii sporangial solution followed by NLE 20% solution.
- Seeds inoculated with bidistilled water followed by NLE 20% solution (BW).
5.9. Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Göre, M.E. Epidemic outbreaks of downy mildew caused by Plasmopara halstedii on sunflower in Thrace, part of the Marmara region of Turkey. Plant Pathol. 2009, 58, 209–218. [Google Scholar] [CrossRef]
- Radwan, O.; Bouzidi, M.F.; Mouzeyar, S. Molecular characterization of two types of resistance in sunflower to Plasmopara halstedii, the causal agent of downy mildew. Phytopathology 2011, 101, 970–979. [Google Scholar] [CrossRef]
- Ioos, R.; Laugustin, L.; Rose, S.; Tourvieille, J.; de Labrouhe, D.T. Development of a PCR test to detect the downy mildew causal agent Plasmopara halstedii in sunflower seeds. Plant Pathol. 2007, 56, 209–218. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Trojanová, Z.D.; Sedlářová, M.; Pospíchalová, R.; Lebeda, A. Pathogenic variability of Plasmopara halstedii infecting sunflower in the Czech Republic. Plant Pathol. 2018, 66, 136–144. [Google Scholar] [CrossRef]
- Meliala, C.; Vear, F.; de Labrouhe, D.T. Relation between date of infection of sunflower downy mildew (Plasmopara halstedii) and symptoms development. Helia 2000, 23, 35–44. [Google Scholar]
- Viranyi, F.; Spring, O. Advances in sunflower downy mildew research. Eur. J. Plant Pathol. 2011, 129, 207–220. [Google Scholar] [CrossRef]
- Cohen, T.; Sackston, W.E. Factors affecting infection of sunflowers by Plasmopara halstedii. Can. J. Bot. 1973, 51, 15–22. [Google Scholar] [CrossRef]
- Sackston, W.E. Downy mildew of sunflower. In The Downy Mildews; Spencer, D.M., Ed.; Academic Press: London, UK; New York, NY, USA, 1981; pp. 545–575. [Google Scholar]
- Gascuel, Q.; Martinez, Y.; Boniface, M.C.; Vear, F.; Pichon, M.; Godiard, L. The sunflower downy mildew pathogen Plasmopara halstedii. Mol. Plant Pathol. 2015, 16, 109–122. [Google Scholar] [CrossRef]
- Sedlářová, M.; Pospíchalová, R.; Drábková Trojanová, Z.; Bartůšek, T.; Slobodianová, L.; Lebeda, A. First report of Plasmopara halstedii new races 705 and 715 on sunflower from the Czech Republic—Short communication. Plant Protect. Sci. 2016, 52, 182–187. [Google Scholar]
- Bán, R.; Kovács, A.; Körösi, K.; PerczeL, M.; Turóczi, G.; Pálinkás, Z.; Égei, M. First Report on the Occurrence of a Globally New Pathotype, 724, of Plasmopara halstedii Downy Mildew in Hungary. Plant Dis. 2018, 102, 1861. [Google Scholar] [CrossRef] [PubMed]
- Albourie, J.M.; Tourville, J.; De Labrouhe, D.T. Resistance to metalaxyl in isolates of the sunflower pathogen Plasmopara halstedii. Eur. J. Plant Pathol. 1998, 194, 235–242. [Google Scholar] [CrossRef]
- Rashid, A.; Ahmad, I.; Iram, S.; Mirza, J.I.; Rauf, C.A. Efficacy of different Neem (Azadirachta indica A. Juss) products against various life stages of Phytophthora infestans (Mont) de bary. Pak. J. Bot. 2004, 36, 881–886. [Google Scholar]
- Schmutterer, H. Potential of azadirachtin-containing pesticides for integrated pest control in developing and industrialized countries. J. Insect Physiol. 1988, 34, 713–719. [Google Scholar] [CrossRef]
- Girish, K.; Bhat, S.S. Neem—A Green Treasure. Electron. J. Biol. 2008, 4, 102–111. [Google Scholar]
- Achimu, P.; Schlösser, E. Effect of neem extracts (Azadirchta indica A. Juss) against downy mildew (Plasmopora viticola) of grapevein. International symposium on crop protection 5 May. Gent (Belgium) 1992, 44, 423–431. [Google Scholar]
- Ghimeray, A.K.; Jin, C.W.; Ghimire, B.K.; Cho, D.H. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta indica A. Juss grown in foothills of Nepal. Afr. J. Biotech. 2009, 8, 3084–3091. [Google Scholar]
- Kumar, C.S.; Srinivas, M.; Yakkundi, S. Limonoids from the seeds of Azadirachta indica. Phytochemistry 1996, 43, 451–455. [Google Scholar] [CrossRef]
- Mirza, J.I.; Hameed, S.; Ahmad, I.; Ayub, N.; Strang, R.H.C. In Vitro Antifungal Activity of Neem Products against Phytophthora Infestans. Pak. J. Biol. Sci. 2000, 3, 824–828. [Google Scholar]
- Mboussi, S.B.; Ambang, Z.; Ndogho, A.; Ngoh Dooh, J.P.; Manga Essouma, F. In vitro Antifungal Potential of Aqueous Seeds Extracts of Azadirachta indica and Thevetia peruviana against Phytophthora megakarya in Cameroon. J. Appl. Life Sci. Int. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Ngadze, E. In vitro and greenhouse evaluation of botanical extracts for antifungal activity against Phythopthora infestans. J. Biopest 2014, 7, 199–204. [Google Scholar]
- Rovesti, L.; Di Marco, S.; Pancaldi, D. Effect of neem kernel extract on some phytopathogenic fungi under greenhouse conditions/Wirkung von Nimsamenextrakt auf einige phytopathogene Pilze unter Gewächshausbedingungen. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 1992, 99, 293–296. [Google Scholar]
- Krzyzaniak, Y.; Trouvelot, S.; Negrel, J.; Cluzet, S.; Valls, J.; Richard, T.; Bougaud, A.; Jacquens, L.; Klinguer, A.; Chiltz, A.; et al. A plant extract acts both as a resistance inducer and an oomycide against grapevine downy mildew. Front. Plant Sci. 2018, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Shakywar, R.C.; Pathak, S.P.; Kumar, S.; Singh, A.K. Evaluation of fungicides and plant extracts (botanicals) against Phytophthora colocasiae raciborski causing leaf blight of Taro. J. Plant Dis. Sci. 2012, 7, 197–200. [Google Scholar]
- Fernandez, A.M.; Gomez, M.V.; Velasco, A.; Camacho, A.M.; Fernandez, C.; Altarejos, J. In Vivo Antifungal Activity of the Essential Oil of Bupleurum gibraltarium against Plasmopara halstedii in Sunflower. J. Agric. Food Chem. 2004, 52, 6414. [Google Scholar] [CrossRef]
- Naumann, K.; Rankin, L.J.; Isman, M.B. Systemic action of neem seed extract on mountain pine beetle (Coleoptera: Scolytidae) in lodgepole pine. J. Econ. Entomol. 1994, 87, 1580–1585. [Google Scholar] [CrossRef]
- Osman, M.Z.; Port, G.R. Systemic action of neem seed substances against Piens brassicae. Entomol. Exp. Appl. 1990, 54, 297–300. [Google Scholar] [CrossRef]
- Marion, D.F.; Larew, H.G.; Knodel, J.J.; Natoli, W. Systemic activity of neem extract against the leaf birch miner. J. Arboric. 1990, 16, 12–16. [Google Scholar]
- Goel, N.; Sahi, A.N.; Paul, P.K. Age as a factor in induction of systemic acquired resistance in tomato against bacterial speck by aqueous fruit extracts of Azadirachta indica. Arch. Phytopathol. Plant Prot. 2013, 46, 1696–1706. [Google Scholar] [CrossRef]
- Bhuvaneswari, V.; Srivastava, K.; Paul, P.K. Azadirachta indica induce systemic acquired resistance in barley against Drechslera graminea. Arch. Phytopathol. Plant Prot. 2012, 45, 898–908. [Google Scholar] [CrossRef]
- Lehmann, W. Fungizide Inhaltsstoffe aus den Kernen des Neembaumes (Azadirachta indica A. Juss)—Ihre Gewinnung und Wirkung auf Wurzelbranderreger der Zuckerrübe. Ph.D. Thesis, Mitt. a. d. Biol, Bundesanst. f.Land- und Forstwirtschaft, Göttingen, Germany, 1991. [Google Scholar]
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of neem (Azadiracta indica). Curr. Sci. 2002, 82, 1336–1345. [Google Scholar]
- Doshi, P.; Mészárosné Póss, A.; Tóth, F.; Szalai, M.; Turóczi, G. Effect of neem-derived plant protection products on the isopod species Porcellionides pruinosus (Brandt, 1833). ZooKeys 2018, 801, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Petrikovszki, R.; Doshi, P.; Turóczi, G.; Tóth, F.; Nagy, P. Investigating the Side-Effects of Neem-Derived Pesticides on Commercial Entomopathogenic and Slug-Parasitic Nematode Products under Laboratory Conditions. Plants 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Trojanová, Z.; Sedlářová, M.; Gulya, T.J.; Lebeda, A. Methodology of virulence screening and race characterization of Plasmopara halstedii, and resistance evaluation in sunflower—A review. Plant Pathol. 2017, 66, 171–185. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 26 September 2019).
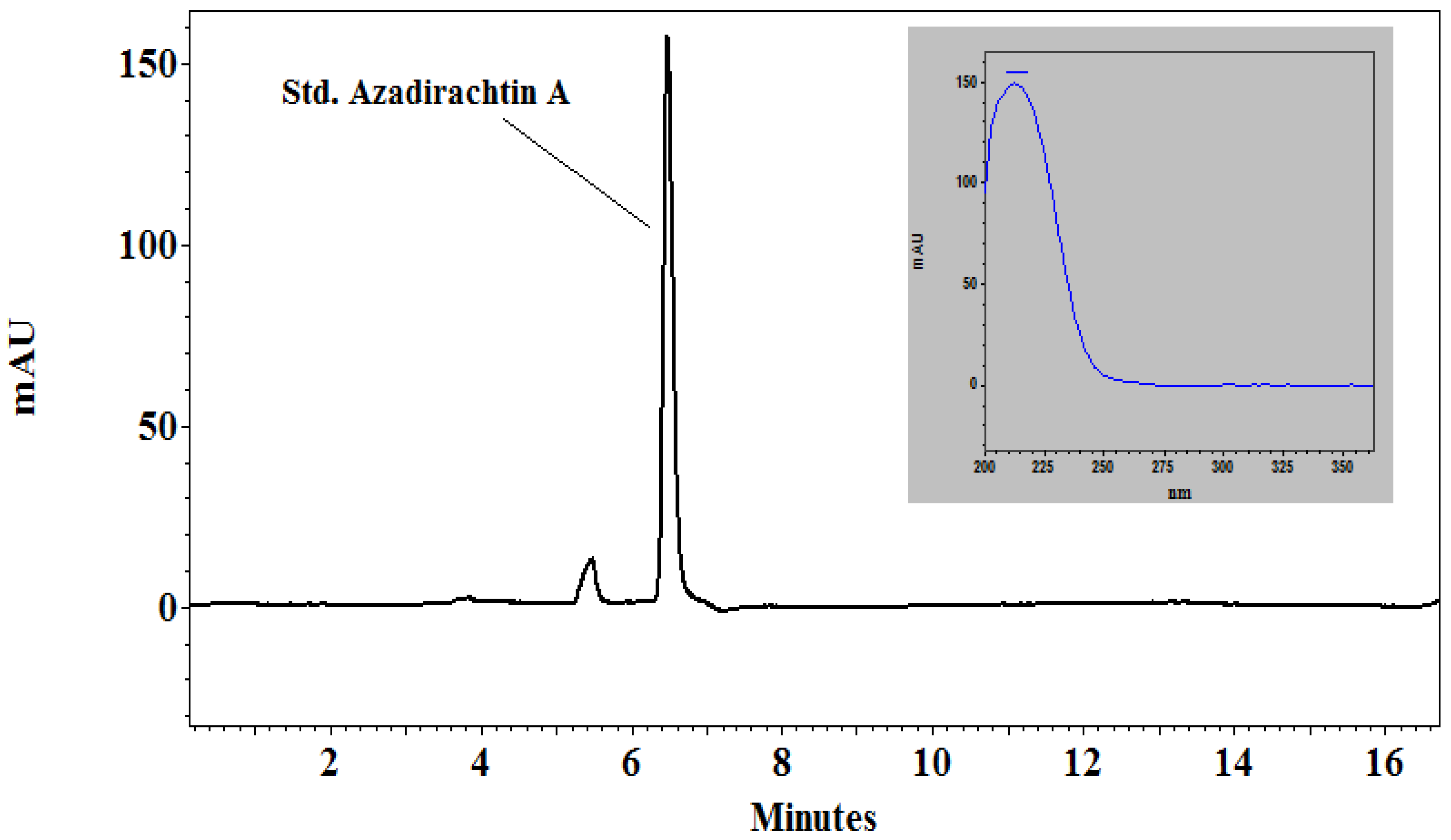
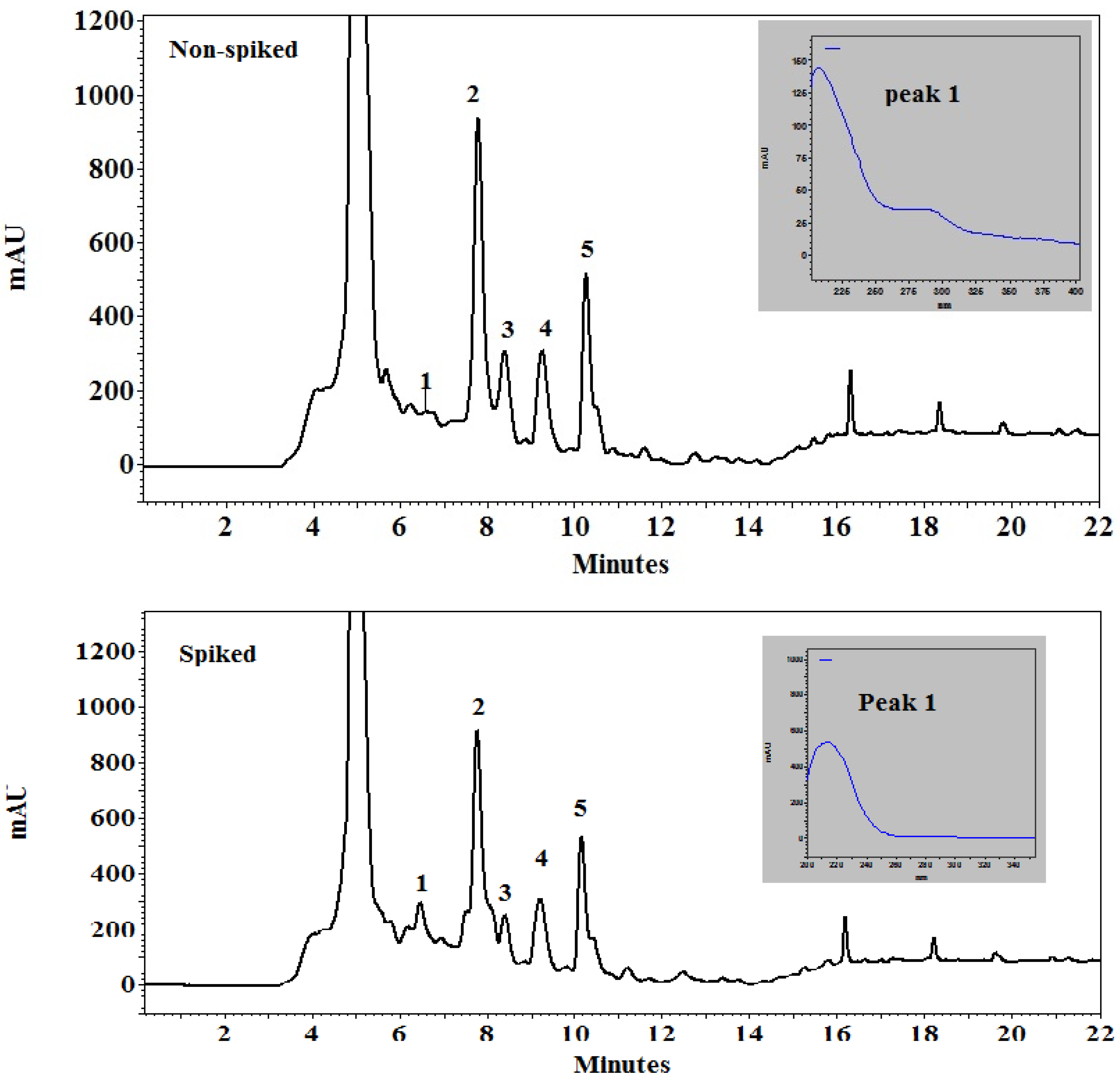
| Sample | Peak No | Area (From Chromatogram) | Area × 909 (Factor) | Azadirachtin (µg/g) | Azadirachtin (mg/5 g) |
|---|---|---|---|---|---|
| Non-Spiked | 1 | 0.23 | 209 | 1 ** | |
| 2 | 47.2 | 42,909 | 214.5 | ||
| 3 | 15.5 | 14,089 | 70 | ||
| 4 | 18.06 | 16,425 | 80 | ||
| 5 | 18.6 | 16,907 | 84.5 | ||
| Spiked | 1 | 5.5 | 4999 | 24.5 ** | |
| 2 | 37.7 | 34,269 | 171 | ||
| 3 | 6.5 | 5908 | 29.5 | ||
| 4 | 17.5 | 15,907 | 79.5 | ||
| 5 | 18.4 | 16,725 | 83.5 |
| Differential Lines | First Evaluation (%) | Second Evaluation (%) | Reaction of Plants | Score | Pathotype Code |
|---|---|---|---|---|---|
| Iregi szürke csíkos | 96.7± 3.9 | 100 | S | 1 | |
| RHA-265 | 91.7 ± 6.4 | 100 | S | 2 | 7 |
| RHA-274 | 100 | 100 | S | 4 | |
| PMI-3 | 0 | 0 | R | 0 | |
| PM-17 | 0 | 0 | R | 0 | 0 |
| 803-1 | 0 | 0 | R | 0 | |
| HAR-4 | 0 | 0 | R | 0 | |
| QHP-2 | 0 | 0 | R | 0 | 4 |
| HA-335 | 93.3 ± 5.4 | 100 | S | 4 |
| Treatment | Df | Sum Sq | Mean Sq | F Value | p Value |
|---|---|---|---|---|---|
| Pre-treatment | 11 | 22.33 | 2.03 | 21.31 | <0.05 |
| Post-treatment | 11 | 42.68 | 3.88 | 48.66 | <0.05 |
| Sunflower Differential Line | Iregi Szürke Csíkos | RHA-265 | RHA-274 | PMI-3 | PM-17 | 803-1 | HAR-4 | QHP-2 | HA-335 |
|---|---|---|---|---|---|---|---|---|---|
| Resistance gene to P. halstedii | No Pl gene | Pl1 | Pl2/Pl21 | PlPMI3 | Pl5 | Pl5+ | Pl15 | Pl1/Pl15 | Pl6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doshi, P.; Nisha, N.; Yousif, A.I.A.; Körösi, K.; Bán, R.; Turóczi, G. Preliminary Investigation of Effect of Neem-Derived Pesticides on Plasmopara halstedii Pathotype 704 in Sunflower under In Vitro and In Vivo Conditions. Plants 2020, 9, 535. https://doi.org/10.3390/plants9040535
Doshi P, Nisha N, Yousif AIA, Körösi K, Bán R, Turóczi G. Preliminary Investigation of Effect of Neem-Derived Pesticides on Plasmopara halstedii Pathotype 704 in Sunflower under In Vitro and In Vivo Conditions. Plants. 2020; 9(4):535. https://doi.org/10.3390/plants9040535
Chicago/Turabian StyleDoshi, Pratik, Nisha Nisha, Ahmed Ibrahim Alrashid Yousif, Katalin Körösi, Rita Bán, and György Turóczi. 2020. "Preliminary Investigation of Effect of Neem-Derived Pesticides on Plasmopara halstedii Pathotype 704 in Sunflower under In Vitro and In Vivo Conditions" Plants 9, no. 4: 535. https://doi.org/10.3390/plants9040535
APA StyleDoshi, P., Nisha, N., Yousif, A. I. A., Körösi, K., Bán, R., & Turóczi, G. (2020). Preliminary Investigation of Effect of Neem-Derived Pesticides on Plasmopara halstedii Pathotype 704 in Sunflower under In Vitro and In Vivo Conditions. Plants, 9(4), 535. https://doi.org/10.3390/plants9040535






