Severe Plastid Genome Size Reduction in a Mycoheterotrophic Orchid, Danxiaorchis singchiana, Reveals Heavy Gene Loss and Gene Relocations
Abstract
1. Introduction
2. Results
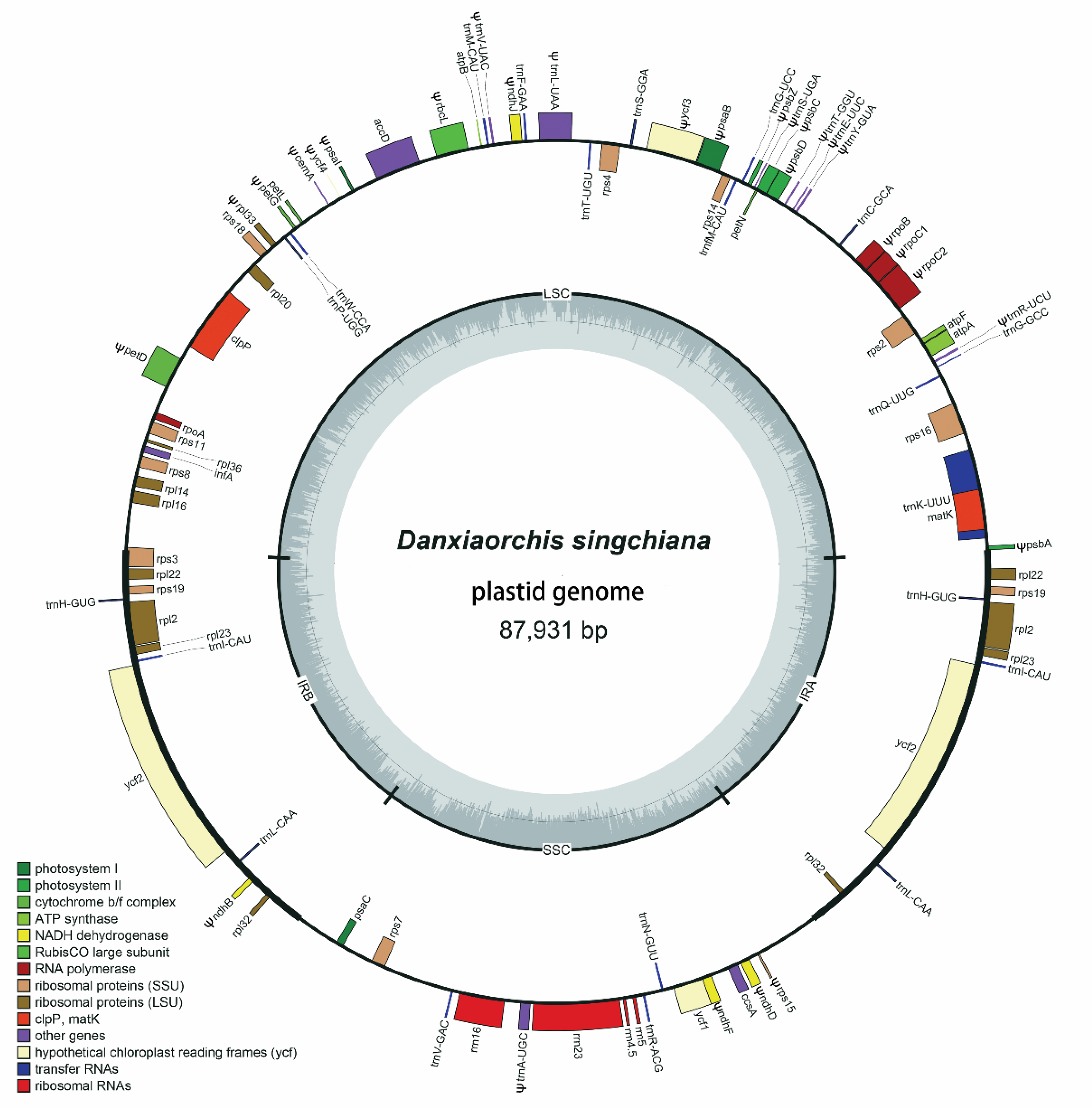
2.1. Plastome Size and Structure
2.2. Gene Content and Order
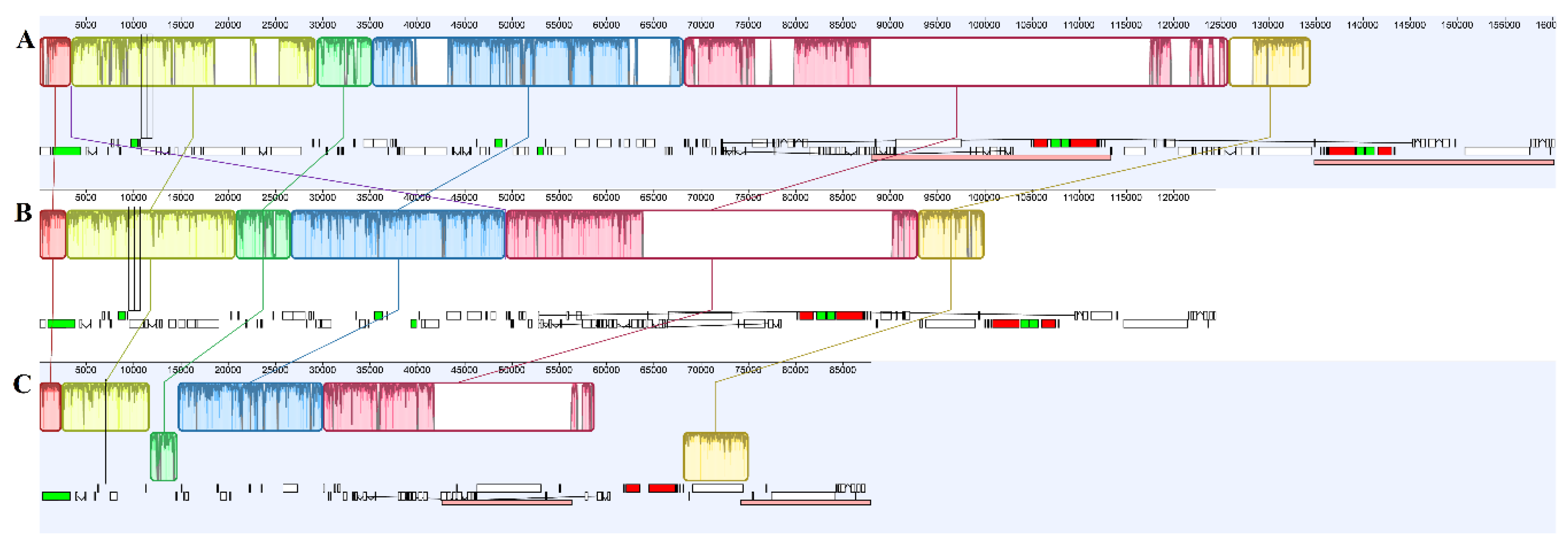
2.3. Genome Comparison and Selection Pressure Analyses
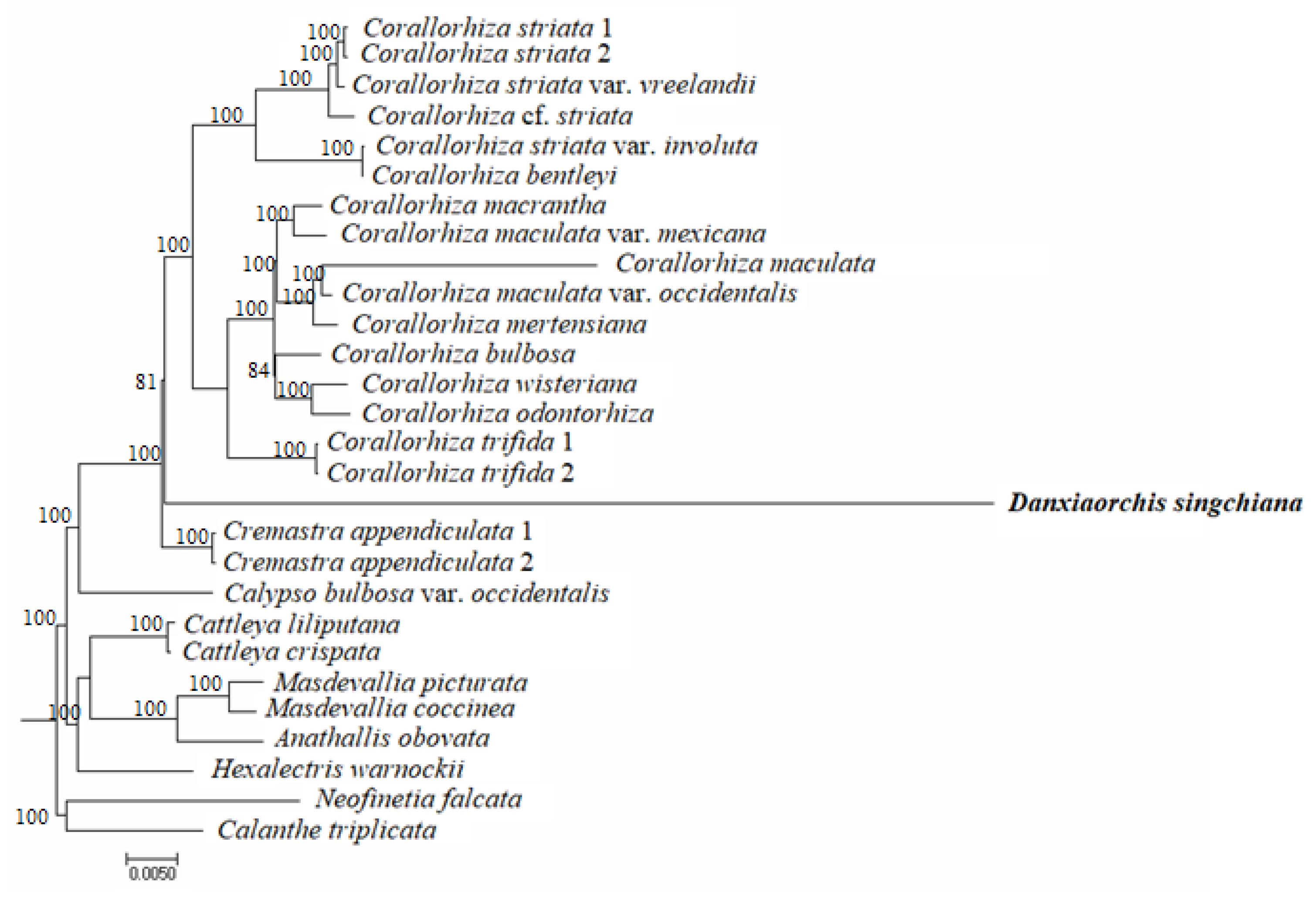
2.4. Phylogenomic Analysis
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Govaerts, R.; Bernet, P.; Kratochvil, K.; Gerlach, G.; Carr, G.; Alrich, P.; Pridgeon, A.M.; Pfahl, J.; Campacci, M.A.; Baptista, D.H.; et al. World checklist of Orchidaceae. Available online: http://apps.kew.org/wcsp/ (accessed on 20 December 2019).
- Merckx, V.S.F.T.; Mennes, C.B.; Peay, K.G.; Geml, J. Evolution and diversification. In Mycoheterotrophy; Merckx, V.S.F.T., Ed.; Springer: New York, NY, USA, 2013; pp. 215–244. [Google Scholar]
- Wolfe, K.H.; Katz-Downie, D.S.; Morden, C.W.; Palmer, J.D. Evolution of the plastid ribosomal RNA operon in a nongreen parasitic plant: Accelerated sequence evolution, altered promoter structure, and tRNA pseudogenes. Plant Mol. Biol. 1992, 18, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.F.; Freudenstein, J.V.; Li, J.; Mayfield-Jones, D.R.; Perez, L.; Pires, J.C.; Santos, C. Investigating the path of plastid genome degradation in an early-transitional clade of heterotrophic orchids, and implications for heterotrophic angiosperms. Mol. Biol. Evol. 2014, 31, 3095–3112. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.F.; Wicke, S.; Sass, C. Dense infraspecific sampling reveals rapid and independent trajectories of plastome degradation in a heterotrophic orchid complex. New Phytol. 2018, 218, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, E.; Fujii, S.; Colas des Francs-Small, C.; Brundrett, M.; Small, I. Rampant gene loss in the underground orchid Rhizanthella gardneri highlights evolutionary constraints on plastid genomes. Mol. Biol. Evol. 2011, 28, 2077–2086. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jo, S.; Cheon, S.H.; Joo, M.J.; Hong, J.R.; Kwak, M.H.; Kim, K.J. Extensive losses of photosynthesis genes in the plastome of a mycoheterotrophic orchid, Cyrtosia septentrionalis (Vanilloideae: Orchidaceae). Genome Biol. Evol. 2019, 11, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Logacheva, M.D.; Schelkunov, M.I.; Penin, A.A. Sequencing and analysis of plastid genome in mycoheterotrophic orchid Neottia nidus-avis. Genome Biol. Evol. 2011, 3, 1296–1303. [Google Scholar] [CrossRef]
- Barrett, C.F.; Davis, J.I. The plastid genome of the mycoheterotrophic Corallorhiza striata (Orchidaceae) is in the relatively early stages of degradation. Am. J. Bot. 2012, 99, 1513–1523. [Google Scholar] [CrossRef]
- Schelkunov, M.I.; Shtratnikova, V.Y.; Nuraliev, M.S.; Selosse, M.A.; Penin, A.A.; Logacheva, M.D. Exploring the limits for reduction of plastid genomes: A case study of the mycoheterotrophic orchids Epipogium aphyllum and Epipogium roseum. Genome Biol. Evol. 2015, 7, 1179–1191. [Google Scholar] [CrossRef]
- Feng, Y.L.; Wicke, S.; Li, J.W.; Han, Y.; Lin, C.S.; Li, D.Z.; Zhou, T.T.; Huang, W.C.; Huang, L.Q.; Jin, X.H. Lineage-specific reductions of plastid genomes in an orchid tribe with partially and fully mycoheterotrophic species. Genome Biol. Evol. 2016, 8, 2164–2175. [Google Scholar] [CrossRef]
- Barrett, C.F.; Kennedy, A.H. Plastid genome degradation in the endangered, mycoheterotrophic, North American orchid Hexalectris warnockii. Genome Biol. Evol. 2018, 10, 1657–1662. [Google Scholar] [CrossRef]
- Huo, X.; Zhao, Y.; Qian, Z.; Liu, M. Characterization of the complete chloroplast genome of Eulophia zollingeri, an endangered orchid in China. Conserv. Genet. Resour. 2018, 10, 817–819. [Google Scholar] [CrossRef]
- Kim, H.T.; Shin, C.H.; Sun, H.; Kim, J.H. Sequencing of the plastome in the leafless green mycoheterotroph Cymbidium macrorhizon helps us to understand an early stage of fully mycoheterotrophic plastome structure. Plant Syst. Evol. 2018, 304, 245–258. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, X.; Liu, J.; Zhao, X.; Zhou, J.; Wang, X.; Wang, D.; Lai, C.; Xu, W.; Huang, J.; et al. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018, 9, 1615. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.F.; Sinn, B.T.; Kennedy, A.H. Unprecedented parallel photosynthetic losses in a heterotrophic orchid genus. Mol. Biol. Evol. 2019, 36, 1884–1901. [Google Scholar] [CrossRef]
- Shevtsov, S.; Murik, O.; Zer, H.; Weinstein, O.; Keren, N.; Fragman-Sapir, O.; Ostersetzer-Biran, O. The complete plastid genome sequence and the photosynthetic activity of the putative mycoheterotrophic orchid Limodorum abortivum. Isr. J. Plant Sci. 2019, 66, 69–88. [Google Scholar] [CrossRef]
- Zhai, J.W.; Zhang, G.Q.; Chen, L.J.; Xiao, X.J.; Liu, K.W.; Tsai, W.C.; Hsiao, Y.Y.; Tian, H.Z.; Zhu, J.Q.; Wang, M.N.; et al. A new orchid genus, Danxiaorchis, and phylogenetic analysis of the tribe Calypsoeae. PLoS ONE 2013, 8, e60371. [Google Scholar] [CrossRef][Green Version]
- Freudenstein, J.V.; Yukawa, T.; Luo, Y.B. A reanalysis of relationships among Calypsoinae (Orchidaceae: Epidendroideae): Floral and vegetative evolution and the placement of Yoania. Syst. Bot. 2017, 42, 17–26. [Google Scholar] [CrossRef]
- Chase, M.W.; Cameron, K.M.; Barrett, R.L.; Freudenstein, J.V. DNA data and Orchidaceae systematics: A new phylogenetic classification. In Orchid Conservation; Dixon, K.W., Kell, S.P., Barrett, R.L., Cribb, P.J., Eds.; Natural History Publications: Sabah, Malaysia, 2003; pp. 69–89. [Google Scholar]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef]
- Palmer, J.D. Contrasting modes and tempos of genome evolution in land plant organelles. Trends Genet. 1990, 6, 115–120. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, 6–11. [Google Scholar] [CrossRef]
- Lin, C.S.; Chen, J.J.; Chiu, C.C.; Hsiao, H.C.; Yang, C.J.; Jin, X.H.; Leebens-Mack, J.; de Pamphilis, C.W.; Huang, Y.T.; Yang, L.H.; et al. Concomitant loss of NDH complex-related genes within chloroplast and nuclear genomes in some orchids. Plant J. 2017, 90, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Chen, J.J.; Huang, Y.T.; Chan, M.T.; Daniell, H.; Chang, W.J.; Hsu, C.T.; Liao, D.C.; Wu, F.H.; Lin, S.Y.; et al. The location and translocation of ndh genes of chloroplast origin in the Orchidaceae family. Sci. Rep. 2015, 5, 9040. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Müller, K.F.; de Pamphilis, C.W.; Quandt, D.; Wickett, N.J.; Zhang, Y.; Renner, S.S.; Schneeweiss, G.M. Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. Plant Cell 2013, 25, 3711–3725. [Google Scholar] [CrossRef] [PubMed]
- Logacheva, M.D.; Schelkunov, M.I.; Nuraliev, M.S.; Samigullin, T.H.; Penin, A.A. The plastid genome of mycoheterotrophic monocot Petrosavia stellaris exhibits both gene losses and multiple rearrangements. Genome Biol. Evol. 2014, 6, 238–246. [Google Scholar] [CrossRef]
- Lim, G.S.; Barrett, C.F.; Pang, C.C.; Davis, J.I. Drastic reduction of plastome size in the mycoheterotrophic Thismia tentaculata relative to that of its autotrophic relative Tacca chantrieri. Am. J. Bot. 2016, 103, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M.; Depamphilis, C.W.; Müller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef]
- Arnon, D.I.; Allen, M.B.; Whatley, F.R. Photosynthesis by isolated chloroplasts. Nature 1954, 174, 394–396. [Google Scholar]
- Arnon, D.I.; Whatley, F.R.; Allen, M.B. Triphosphopyridine nucleotide as a catalyst of photosynthetic phosphorylation. Nature 1957, 180, 182–185. [Google Scholar]
- Hill, R.; Bendall, F. Function of the two cytochrome components in chloroplasts: A working hypothesis. Nature 1960, 186, 136–137. [Google Scholar]
- Kamikawa, R.; Tanifuji, G.; Ishikawa, S.A.; Ishii, K.I.; Matsuno, Y.; Onodera, N.T.; Ishida, K.I.; Hashimoto, T.; Miyashita, H.; Mayama, S.; et al. Proposal of a twin aarginine translocator system-mediated constraint against loss of ATP synthase genes from nonphotosynthetic plastid genomes. Mol. Biol. Evol. 2015, 32, 2598–2604. [Google Scholar] [CrossRef]
- Oshima, K.; Kakizawa, S.; Nishigawa, H.; Jung, H.Y.; Wei, W.; Suzuki, S.; Arashida, R.; Nakata, D.; Miyata, S.; Ugaki, M.; et al. Reductive evolution suggested from the complete genome sequence of a plant-pathogenic phytoplasma. Nat. Genet. 2004, 36, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, T.T.; Scharff, L.B.; Alkatib, S.; Hasdorf, S.; Schöttler, M.A.; Bock, R. Nonessential plastid-encoded ribosomal proteins in tobacco: A developmental role for plastid translation and implications for reductive genome evolution. Plant Cell 2011, 23, 3137–3155. [Google Scholar] [CrossRef] [PubMed]
- Scharff, L.B.; Bock, R. Synthetic biology in plastids. Plant J. 2014, 78, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Alkatib, S.; Scharff, L.B.; Rogalski, M.; Fleischmann, T.T.; Matthes, A.; Seeger, S.; Schöttler, M.A.; Ruf, S.; Bock, R. The contributions of wobbling and superwobbling to the reading of the genetic code. PLoS Genet. 2012, 8, e1003076. [Google Scholar] [CrossRef]
- Kumar, A.M.; Schaub, U.; Söll, D.; Ujwal, M.L. Glutamyl-transfer RNA: At the crossroad between chlorophyll and protein biosynthesis. Trends Plant Sci. 1996, 1, 371–376. [Google Scholar] [CrossRef]
- Barbrook, A.C.; Howe, C.J.; Purton, S. Why are plastid genomes retained in non-photosynthetic organisms? Trends Plant Sci. 2006, 11, 101–108. [Google Scholar] [CrossRef]
- Marechal-Drouard, L.; Weil, J.H.; Dietrich, A. Transfer RNAs and transfer RNA genes in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 13–32. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, S.; Jiang, Y.; Luo, H.; Xiong, D.; Zhai, J.; Li, B. Danxiaorchis yangii sp. nov.(Orchidaceae: Epidendroideae), the second species of Danxiaorchis. Phytotaxa 2017, 306, 287–295. [Google Scholar] [CrossRef][Green Version]
- Luo, J.; Hou, B.W.; Niu, Z.T.; Liu, W.; Xue, Q.Y.; Ding, X.Y. Comparative chloroplast genomes of photosynthetic orchids: Insights into evolution of the Orchidaceae and development of molecular markers for phylogenetic applications. PLoS ONE 2014, 9, e99016. [Google Scholar] [CrossRef]
- Huang, J. On the distribution of Danxia landforms in China. Econ. Geogr. 1999, 19, 31–35. [Google Scholar]
- Xu, J.; Jiang, X.L.; Deng, M.; Westwood, M.; Song, Y.G.; Zheng, S.S. Conservation genetics of rare trees restricted to subtropical montane cloud forests in southern China: A case study from Quercus arbutifolia (Fagaceae). Tree Genet. Genomes 2016, 12, 90. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, Z.H.; Schuiteman, A.; Chase, M.W.; Li, J.W.; Huang, W.C.; Hidayat, A.; Wu, S.S.; Jin, X.H. Phylogenomics of Orchidaceae based on plastid and mitochondrial genomes. Mol. Phylogenet. Evol. 2019, 139, 106540. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Genomic plant DNA preparation from fresh tissue-CTAB method. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2016, 45, e18. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, w59–w64. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2010; pp. 1–8. [Google Scholar]
| Type of Genes | Group of Gene | Gene Name |
|---|---|---|
| Housekeeping | Large subunit of ribosome | rpl2*; rpl14; rpl16; rpl20; rpl22*; rpl23*; rpl32*; rpl33; rpl36 |
| Small subunit of ribosome | rps2; rps3*; rps4; rps7; rps8; rps11; rps12; rps14; rps16; rps18; rps19* | |
| Ribosomal RNA genes | rrn4.5; rrn5; rrn16; rrn23 | |
| Transfer RNA genes | trnC-GCA; trnF-GAA; trnfM-CAU; trnG-GCC; trnG-UCC; trnH-GUG*; trnI-CAU*; trnI-GAU; trnK-UUU; trnL-CAA*; trnM-CAU; trnN-GUU; trnP-UGG; trnQ-UUG; trnR-ACG; trnS-GGA; trnT-UGU; trnV-GAC; trnW-CCA | |
| Translational initiation factor | infA | |
| Maturase | matK | |
| Subunit of acetyl-CoA carboxylase | accD | |
| Subunit of protease Clp | clpP | |
| Component of TIC complex | ycf1; ycf2* | |
| Photosynthesis-related | Subunits of photosystem I | psaC |
| Subunits of cytochrome b6f | petL; petN | |
| Plastid-encoded RNA polymerase | Subunits of the plastid-encoded RNA polymerase | rpoA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Meng, K.; Wang, H.; Zhou, R.; Liao, W.; Chen, F.; Zhang, S.; Fan, Q. Severe Plastid Genome Size Reduction in a Mycoheterotrophic Orchid, Danxiaorchis singchiana, Reveals Heavy Gene Loss and Gene Relocations. Plants 2020, 9, 521. https://doi.org/10.3390/plants9040521
Lee SY, Meng K, Wang H, Zhou R, Liao W, Chen F, Zhang S, Fan Q. Severe Plastid Genome Size Reduction in a Mycoheterotrophic Orchid, Danxiaorchis singchiana, Reveals Heavy Gene Loss and Gene Relocations. Plants. 2020; 9(4):521. https://doi.org/10.3390/plants9040521
Chicago/Turabian StyleLee, Shiou Yih, Kaikai Meng, Haowei Wang, Renchao Zhou, Wenbo Liao, Fang Chen, Shouzhou Zhang, and Qiang Fan. 2020. "Severe Plastid Genome Size Reduction in a Mycoheterotrophic Orchid, Danxiaorchis singchiana, Reveals Heavy Gene Loss and Gene Relocations" Plants 9, no. 4: 521. https://doi.org/10.3390/plants9040521
APA StyleLee, S. Y., Meng, K., Wang, H., Zhou, R., Liao, W., Chen, F., Zhang, S., & Fan, Q. (2020). Severe Plastid Genome Size Reduction in a Mycoheterotrophic Orchid, Danxiaorchis singchiana, Reveals Heavy Gene Loss and Gene Relocations. Plants, 9(4), 521. https://doi.org/10.3390/plants9040521




