DNA Fingerprinting and Species Identification Uncovers the Genetic Diversity of Katsouni Pea in the Greek Islands Amorgos and Schinoussa
Abstract
1. Introduction
2. Results
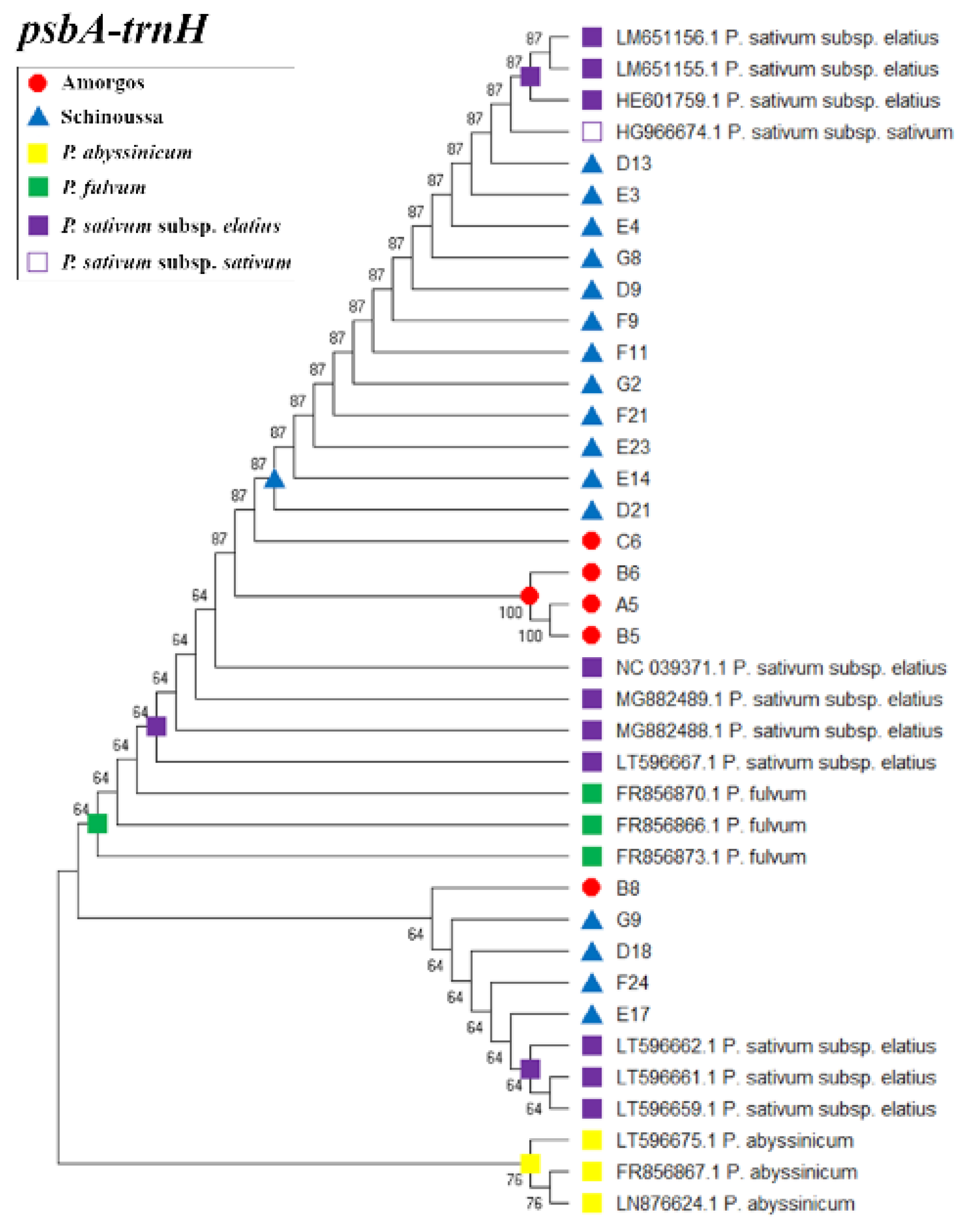
2.1. DNA Barcoding, Sequencing and Tree Analysis
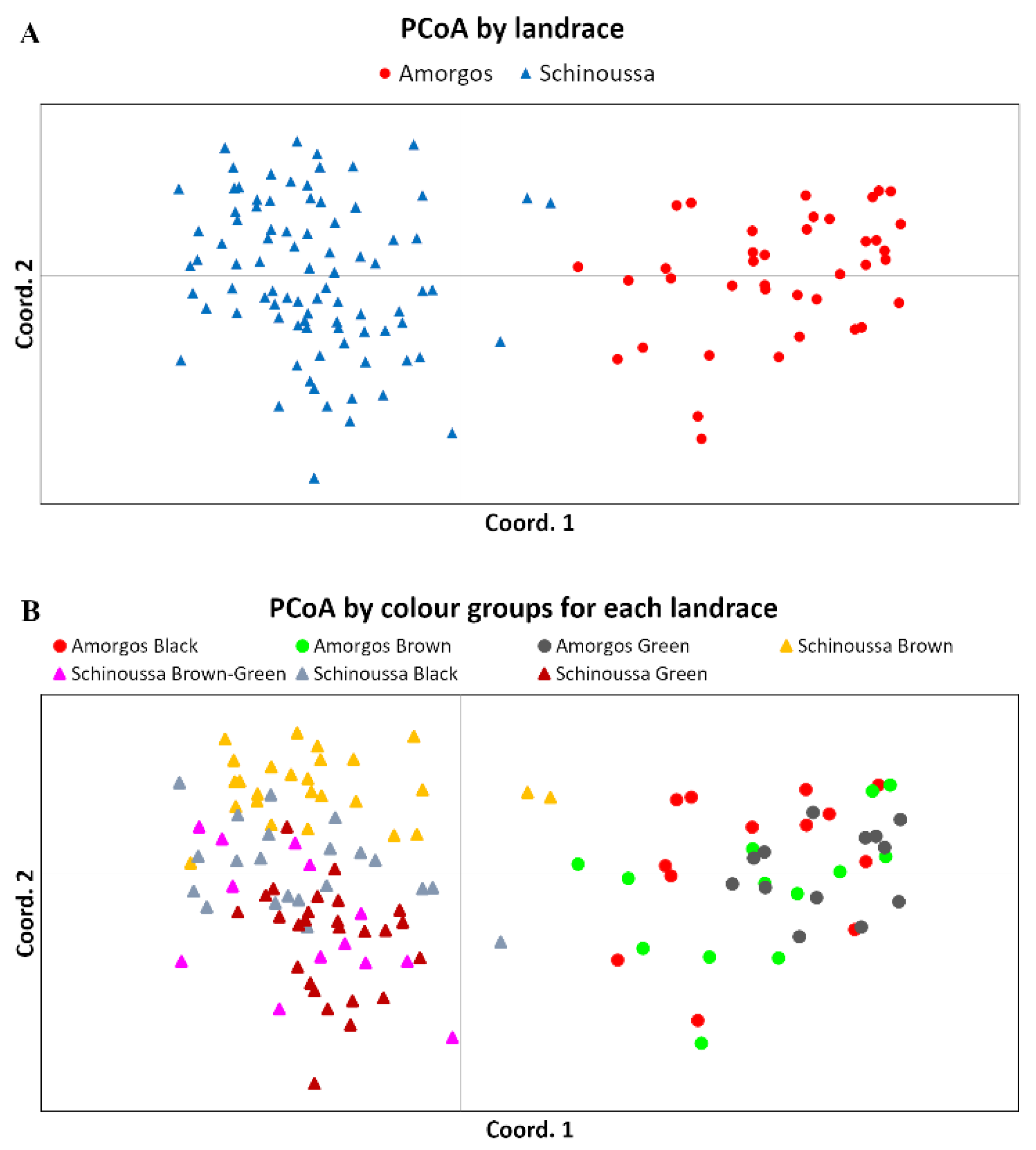
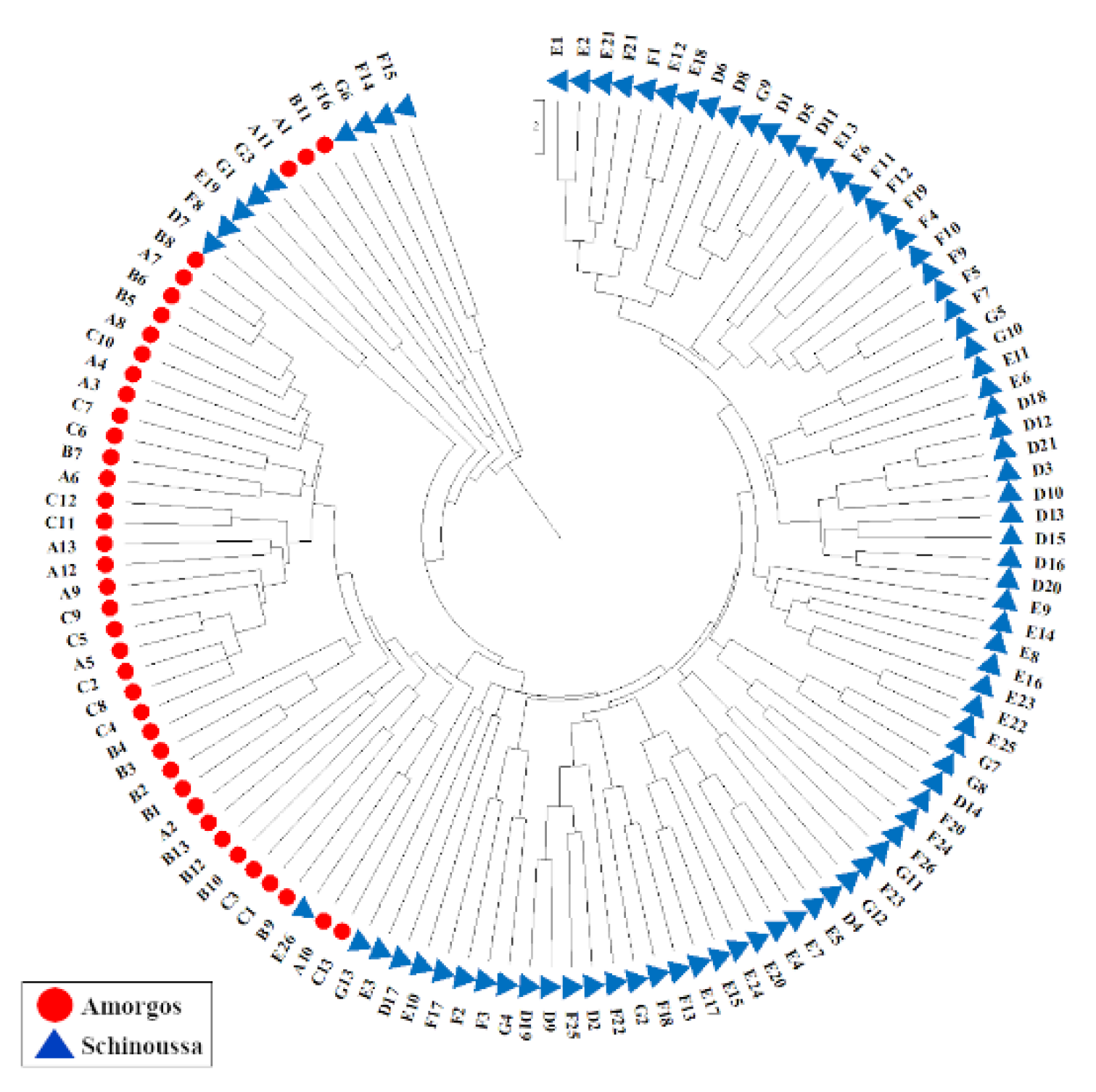
2.2. ISSR Genotyping
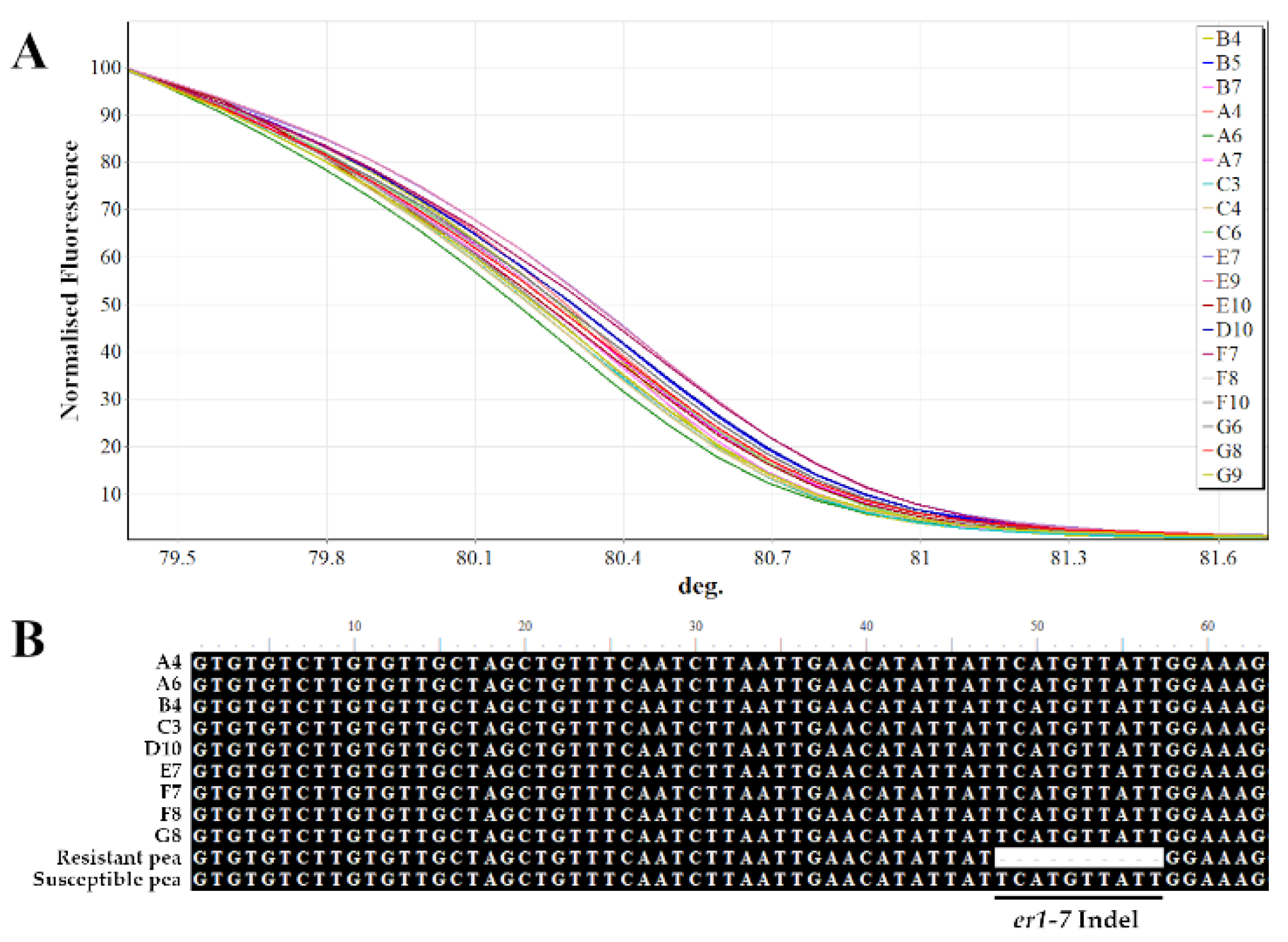
2.3. Molecular Screening for Powdery Mildew Using HRM Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and DNA Isolation
4.2. DNA Barcoding and Sequencing Analysis
4.3. ISSR Genotyping and Data Analysis
4.4. Molecular Screening for Powdery Mildew Resistance Using HRM Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiss, E. Current state of the art. In Domestication of Plants in the Old World—The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press: Oxford, UK, 2012; pp. 1–8. [Google Scholar]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Hillen, C.; Robinson, J.G. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Crews, T.E.; Peoples, M.B. Legume versus fertilizer sources of nitrogen: Ecological tradeoffs and human needs. Agric. Ecosyst. Environ. 2004, 102, 279–297. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 1–13. [Google Scholar]
- Maxted, N.; Ambrose, M. Peas (Pisum L.). In Plant Genetic Resources of Legumes in the Mediterranean; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 181–190. [Google Scholar]
- Smýkal, P.; Hradilová, I.; Trn, O.; Brus, J.; Rathore, A.; Das, R.R.; Bhattacharyya, D.; Richards, C.; Coyne, J.C.; Pirintsos, S. Genomic diversity and macroecology of the crop wild relatives of domesticated pea. Nat. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Kreplak, J.; Madoui, M.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Weeden, N.F. Domestication of pea (Pisum sativum L.): The case of the Abyssinian pea. Front. Plant Sci. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Pagani, L.; Kivisild, T.; Tarekegn, A.; Ekong, R.; Plaster, C.; Gallego Romero, I.; Ayub, Q.; Mehdi, S.Q.; Thomas, M.G.; Luiselli, D.; et al. Ethiopian genetic diversity reveals linguistic stratification and complex influences on the Ethiopian gene pool. Am. J. Hum. Genet. 2012, 91, 83–96. [Google Scholar] [CrossRef]
- Trněný, O.; Brus, J.; Hradilová, I.; Rathore, A.; Das, R.R.; Kopecký, P.; Coyne, C.J.; Reeves, P.; Richards, C.; Smýkal, P. Molecular evidence for two domestication events in the pea crop. Genes 2018, 9, 535–555. [Google Scholar] [CrossRef]
- Jing, R.; Vershinin, A.; Grzebyta, J.; Shaw, P.; Smýkal, P.; Marshall, D.; Ambrose, M.J.; Ellis, T.H.N.; Flavell, A.J. The genetic diversity and evolution of field pea (Pisum) studied by high throughput retrotransposon based insertion polymorphism (RBIP) marker analysis. BMC Evol. Biol. 2010, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Smýkal, P.; Kenicer, G.; Flavell, A.J.; Corander, J.; Kosterin, O.; Redden, R.J.; Ford, R.; Coyne, C.J.; Maxted, N.; Ambrose, M.J.; et al. Phylogeny, phylogeography and genetic diversity of the Pisum genus. Plant Genet. Resour. Charact. Util. 2011, 9, 4–18. [Google Scholar] [CrossRef]
- Kosterin, O.E. Abyssinian pea (Lathyrus schaeferi Kosterin nom. nov. pro Pisum abyssinicum A. Br.) is a problematic taxon. Acta Biol. Sib. 2017, 21, 158–169. [Google Scholar] [CrossRef]
- Ellis, T.H.N. Pisum. In Wild Crop Relatives: Genomic and Breeding Resources Legume Crops and Forages; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–248. ISBN 9783642204463. [Google Scholar]
- Jing, R.; Ambrose, M.A.; Knox, M.R.; Smykal, P.; Hybl, M.; Ramos, Á.; Caminero, C.; Burstin, J.; Duc, G.; van Soest, L.J.M.; et al. Genetic diversity in European Pisum germplasm collections. Theor. Appl. Genet. 2012, 125, 367–380. [Google Scholar] [CrossRef]
- Nisar, M.; Ghafoor, A.; Ahmad, H.; Khan, M.R.; Qureshi, A.S.; Ali, H.; Islam, M. Evaluation of genetic diversity of pea germplasm through phenotypic trait analysis. Pak. J. Bot. 2008, 40, 2081–2086. [Google Scholar]
- Smýkal, P.; Horáček, J.; Dostálová, R.; Hýbl, M. Variety discrimination in pea (Pisum sativum L.) by molecular, biochemical and morphological markers. J. Appl. Genet. 2008, 49, 155–166. [Google Scholar] [CrossRef]
- Ouafi, L.; Alane, F.; Rahal-Bouziane, H.; Abdelguerfi, A. Agro-morphological diversity within field pea (Pisum sativum L.) genotypes. Afr. J. Agric. Res. 2016, 11, 4039–4047. [Google Scholar]
- Singh, S.R.; Ahmed, N.; Singh, D.B.; Srivastva, K.K.; Singh, R.K.; Mir, A. Genetic variability determination in garden pea (Pisum sativum L sub sp. hortense Asch. and Graebn.) by using the multivariate analysis. Legum. Res. Int. J. 2017, 40, 416–422. [Google Scholar] [CrossRef][Green Version]
- Newmaster, S.G.; Ragupathy, S. Testing plant barcoding in a sister species complex of pantropical Acacia (Mimosoideae, Fabaceae ). Mol. Ecol. Resour. 2009, 9, 172–180. [Google Scholar]
- Tar’an, B.; Zhang, C.; Warkentin, T.; Tullu, A.; Vandenberg, A. Genetic diversity among varieties and wild species accessions of pea (Pisum sativum L.) based on molecular markers, and morphological and physiological characters. Genome 2005, 48, 257–272. [Google Scholar] [CrossRef]
- Kapila, R.K.; Naryal, S.; Dhiman, K.C.; Singh, S.K.; Sharma, J.K. Identification of field and Garden Pea (Pisum sativum L.) varieties using RAPD and ISSR markers. Seed Res. 2012, 40, 1–9. [Google Scholar]
- Iqbal, A.; Razzaq, A.; Hadi, F.; Nisar, M.; Ozturk, M.; Altay, V. Assessment of Genetic Diversity Among Hybrid Pea Lines (Pisum Sativum L.) as Revealed by Random Amplified Polymorphic DNA (RAPD) Markers. Fresenius Environ. Bull. 2018, 27, 6447–6453. [Google Scholar]
- Lázaro, A.; Aguinagalde, I. Genetic variation among Spanish pea landraces revealed by Inter Simple Sequence Repeat (ISSR) markers: Its application to establish a core collection. J. Agric. Sci. 2006, 144, 53–61. [Google Scholar] [CrossRef]
- Adhikari, D.N.; Khanna, V.; Singh, K.N. Crossability and Genetic Diversity Studies in Pea (Pisum sativum). Curr. Investig. Agric. Curr. Res. 2018, 3, 443–452. [Google Scholar]
- Mohamed, A.; García-Martínez, S.; Loumerem, M.; Carbonell, P.; Ruiz, J.J.; Boubaker, M. Assessment of genetic diversity among local pea (Pisum sativum L.) accessions cultivated in the arid regions of Southern Tunisia using agro-morphological and SSR molecular markers. Genet. Resour. Crop Evol. 2019, 66, 1189–1203. [Google Scholar] [CrossRef]
- Smýkal, P.; Hýbl, M.; Corander, J.; Jarkovský, J.; Flavell, A.J.; Griga, M. Genetic diversity and population structure of pea (Pisum sativum L.) varieties derived from combined retrotransposon, microsatellite and morphological marker analysis. Theor. Appl. Genet. 2008, 117, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanz, A.; Caminero, C.; Jing, R.; Flavell, A.J.; de la Vega, M.P. Genetic diversity among Spanish pea (Pisum sativum L.) landraces, pea cultivars and the World Pisum sp. core collection assessed by retrotransposon-based insertion polymorphisms (RBIPs). Span. J. Agric. Res. 2011, 9, 166–178. [Google Scholar] [CrossRef]
- Solberg, S.Ø.; Brantestam, A.K.; Olsson, K.; Leino, M.W.; Weibull, J.; Yndgaard, F. Diversity in local cultivars of Pisum sativum collected from home gardens in Sweden. Biochem. Syst. Ecol. 2015, 62, 194–203. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, A.; Mamidi, S.; McPhee, K. Genetic Diversity and Population Structure Among Pea (Pisum sativum L.) Cultivars as Revealed by Simple Sequence Repeat and Novel Genic Markers. Mol. Biotechnol. 2014, 56, 925–938. [Google Scholar] [CrossRef]
- Siol, M.; Jacquin, F.; Chabert-Martinello, M.; Smýkal, P.; Le Paslier, M.C.; Aubert, G.; Burstin, J. Patterns of genetic structure and linkage disequilibrium in a large collection of pea germplasm. G3 Genes Genomes Genet. 2017, 7, 2461–2471. [Google Scholar] [CrossRef]
- Holdsworth, W.L.; Gazave, E.; Cheng, P.; Myers, J.R.; Gore, M.A.; Coyne, C.J.; McGee, R.J.; Mazourek, M. A community resource for exploring and utilizing genetic diversity in the USDA pea single plant plus collection. Hortic. Res. 2017, 4, 17017. [Google Scholar] [CrossRef] [PubMed]
- CBOL Plant Working Group; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar]
- Gao, T.; Chen, S. Authentication of the Medicinal Plants in Fabaceae by DNA Barcoding Technique. Planta Med. 2009, 75, 417. [Google Scholar] [CrossRef]
- Gao, T.; Sun, Z.; Yao, H.; Song, J.; Zhu, Y.; Ma, X.; Chen, S. Identification of fabaceae plants using the DNA barcode matK. Planta Med. 2011, 77, 92–94. [Google Scholar] [CrossRef]
- Gao, T.; Yao, H.; Song, J.; Liu, C.; Zhu, Y.; Ma, X.; Pang, X.; Xu, H.; Chen, S. Identification of medicinal plants in the family Fabaceae using a potential DNA barcode ITS2. J. Ethnopharmacol. 2010, 130, 116–121. [Google Scholar] [CrossRef]
- Madesis, P.; Ganopoulos, I.; Ralli, P.; Tsaftaris, A. Barcoding the major Mediterranean leguminous crops by combining universal chloroplast and nuclear DNA sequence targets. Genet. Mol. Res. 2012, 11, 2548–2558. [Google Scholar] [CrossRef]
- Jaakola, L.; Suokas, M.; Häggman, H. Novel approaches based on DNA barcoding and high-resolution melting of amplicons for authenticity analyses of berry species. Food Chem. 2010, 123, 494–500. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Madesis, P.; Darzentas, N.; Argiriou, A.; Tsaftaris, A. Barcode High Resolution Melting (Bar-HRM) analysis for detection and quantification of PDO “Fava Santorinis” (Lathyrus clymenum) adulterants. Food Chem. 2012, 133, 505–512. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Madesis, P.; Tsaftaris, A. Universal ITS2 Barcoding DNA Region Coupled with High-Resolution Melting (HRM) Analysis for Seed Authentication and Adulteration Testing in Leguminous Forage and Pasture Species. Plant Mol. Biol. Rep. 2012, 30, 1322–1328. [Google Scholar] [CrossRef]
- Bosmali, I.; Ordoudi, S.A.; Tsimidou, M.Z.; Madesis, P. Greek PDO saffron authentication studies using species specific molecular markers. Food Res. Int. 2017, 100, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Philaniotou, O. Lesser Cyclades. In Archaeology: Agean Islands; Vlachopoulos, A.G., Ed.; Melissa Publishing House: Athens, Greece, 2006; pp. 286–289. ISBN 960-204-272-9. [Google Scholar]
- Chatzilazarou, D.T. An Aegean coastal settlement at the ‘end of Late Antiquity’: The case of Schinoussa near Naxos. In Naxos and the Byzantine Aegean: Insular Responses to Regional Change; Crow, J., Hill, D., Eds.; The Norwegian Institute at Athens: Athens, Greece, 2018; Volume 7, pp. 195–222. ISBN 9789608514577. [Google Scholar]
- De Tournefort, J.P. Relation d’un Voyage du Levant (1700-1702), 1st ed.; Lydaki, E., Ed.; Crete University Press: Crete, Greece, 2003; ISBN 960-524-173-0. [Google Scholar]
- Loupis, D. Piracy in the Ottoman Naval Texts (16th–17th century). In Pirates and Corsairs; Kalliga, C., Malliaris, A., Eds.; Hestia Publishers: Athens, Greece, 2003; p. 294. ISBN 960-05-1106-3. [Google Scholar]
- Ginio, E. Piracy and Redemption in the Aegean Sea during the first half of the Eighteenth century. Turcica 2001, 33, 135–147. [Google Scholar] [CrossRef]
- Sun, S.; Deng, D.; Duan, C.; Zong, X.; Xu, D.; He, Y.; Zhu, Z. Two novel er1 alleles conferring powdery mildew (Erysiphe pisi) resistance identified in a worldwide collection of pea (Pisum sativum L.) germplasms. Int. J. Mol. Sci. 2019, 20, 5071. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Deng, D.; Wang, Z.; Duan, C.; Wu, X.; Wang, X.; Zong, X.; Zhu, Z. A novel er1 allele and the development and validation of its functional marker for breeding pea (Pisum sativum L.) resistance to powdery mildew. Theor. Appl. Genet. 2016, 129, 909–919. [Google Scholar] [CrossRef]
- Barber, R.L.N. The Cyclades in the Bronze Age; Duckworth Publishers: London (Richmond), UK, 1987; ISBN 0715621602. [Google Scholar]
- Treuil, R.; Darcque, P.; Poursat, J.-C.; Touchais, G. Les Civilisations Égéennes du Néolithique et de l’Age du Bronze; Deuxième, É., Ed.; Presses Universitaires de France: Paris, France, 1989. [Google Scholar]
- Li, B.; Zheng, Y. Dynamic evolution and phylogenomic analysis of the chloroplast genome in Schisandraceae. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Whitlock, B.A.; Hale, A.M.; Groff, P.A. Intraspecific inversions pose a challenge for the trnH-psbA plant DNA barcode. PLoS ONE 2010, 5, e11533. [Google Scholar] [CrossRef] [PubMed]
- Baloch, F.S.; Alsaleh, A.; de Miera, L.E.S.; Hatipoğlu, R.; Çiftçi, V.; Karaköy, T.; Yildiz, M.; Özkan, H. DNA based iPBS-retrotransposon markers for investigating the population structure of pea (Pisum sativum) germplasm from Turkey. Biochem. Syst. Ecol. 2015, 61, 244–252. [Google Scholar] [CrossRef]
- Bogdanova, V.S.; Mglinets, A.V.; Shatskaya, N.V.; Kosterin, O.E.; Solovyev, V.I.; Vasiliev, G.V. Cryptic divergences in the genus Pisum L. (peas), as revealed by phylogenetic analysis of plastid genomes. Mol. Phylogenet. Evol. 2018, 129, 280–290. [Google Scholar] [CrossRef]
- Gubae, T.T.; Petros, Y. Genetic diversity of chickpea (Cicer arietinum L.) cultivars from Ethiopia by using ISSR markers. J. Agric. Biotechnol. Sustain. Dev. 2018, 10, 178–184. [Google Scholar]
- Pavan, S.; Schiavulli, A.; Appiano, M.; Miacola, C.; Visser, R.G.F.; Bai, Y.; Lotti, C.; Ricciardi, L. Identification of a complete set of functional markers for the selection of er1 powdery mildew resistance in Pisum sativum L. Mol. Breed. 2013, 31, 247–253. [Google Scholar] [CrossRef]
- Sun, S.; He, Y.; Dai, C.; Duan, C.; Zhu, Z. Two major er1 alleles confer powdery mildew resistance in three pea cultivars bred in Yunnan Province, China. Crop J. 2016, 4, 353–359. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Mylona, P.; Mellidou, I.; Kalivas, A.; Bosmali, I.; Kontzidou, S.; Osathanunkul, M.; Madesis, P. Microsatellite genotyping and molecular screening of pea (Pisum sativum L.) germplasm with high-resolution melting analysis for resistance to powdery mildew. Plant Gene 2018, 15, 1–5. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull 1987, 19, 11–15. [Google Scholar]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P.; et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Gielly, L.; Miquel, C.; Valentini, A.; Vermat, T.; Corthier, G.; Brochmann, C.; Willerslev, E. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res. 2007, 35, e14. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and Diverse Origins of the Polyploid Species. Syst. Bot. 2003, 28, 723–737. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Benson, D.A.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2015, 43, D30–D35. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenALEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]

| Population | Number of Bands | Number of Band Frequency (> = 5%) | Number of Unique Bands |
|---|---|---|---|
| Amorgos | 61 | 59 | 0 |
| Schinoussa | 66 | 66 | 5 |
| Population | N | Na | Ne | I | h | uh | |
|---|---|---|---|---|---|---|---|
| Amorgos | Mean | 39 | 1.742 | 1.403 | 0.373 | 0.244 | 0.250 |
| SE | 0.000 | 0.073 | 0.043 | 0.031 | 0.022 | 0.023 | |
| Schinoussa | Mean | 86 | 2.000 | 1.483 | 0.443 | 0.289 | 0.292 |
| SE | 0.000 | 0.000 | 0.042 | 0.026 | 0.020 | 0.021 |
| Source | Df | SS | MS | Est. Var. | % |
|---|---|---|---|---|---|
| Among populations | 1 | 133.042 | 133.042 | 2.307 | 20 |
| Within populations | 123 | 1133.326 | 9.214 | 9.214 | 80 |
| Total | 124 | 1266.368 | 11.521 | 100 |
| Sample Group | Number of Individuals | Region | Seed Coat Color |
|---|---|---|---|
| A | 13 | Amorgos | Black |
| B | 13 | Amorgos | Brown |
| C | 13 | Amorgos | Green |
| D | 21 | Schinoussa | Black |
| E | 26 | Schinoussa | Brown |
| F | 26 | Schinoussa | Green |
| G | 13 | Schinoussa | Brown-Green |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavridou, E.; Lagiotis, G.; Karapetsi, L.; Osathanunkul, M.; Madesis, P. DNA Fingerprinting and Species Identification Uncovers the Genetic Diversity of Katsouni Pea in the Greek Islands Amorgos and Schinoussa. Plants 2020, 9, 479. https://doi.org/10.3390/plants9040479
Stavridou E, Lagiotis G, Karapetsi L, Osathanunkul M, Madesis P. DNA Fingerprinting and Species Identification Uncovers the Genetic Diversity of Katsouni Pea in the Greek Islands Amorgos and Schinoussa. Plants. 2020; 9(4):479. https://doi.org/10.3390/plants9040479
Chicago/Turabian StyleStavridou, Evangelia, Georgios Lagiotis, Lefkothea Karapetsi, Maslin Osathanunkul, and Panagiotis Madesis. 2020. "DNA Fingerprinting and Species Identification Uncovers the Genetic Diversity of Katsouni Pea in the Greek Islands Amorgos and Schinoussa" Plants 9, no. 4: 479. https://doi.org/10.3390/plants9040479
APA StyleStavridou, E., Lagiotis, G., Karapetsi, L., Osathanunkul, M., & Madesis, P. (2020). DNA Fingerprinting and Species Identification Uncovers the Genetic Diversity of Katsouni Pea in the Greek Islands Amorgos and Schinoussa. Plants, 9(4), 479. https://doi.org/10.3390/plants9040479






