Effects of Exogenous Abscisic Acid (ABA) on Carotenoids and Petal Color in Osmanthus fragrans ‘Yanhonggui’
Abstract
1. Introduction
2. Results
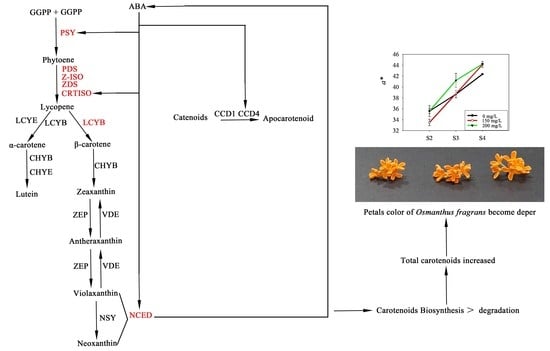
2.1. Effect of ABA Treatment on the Petal Color
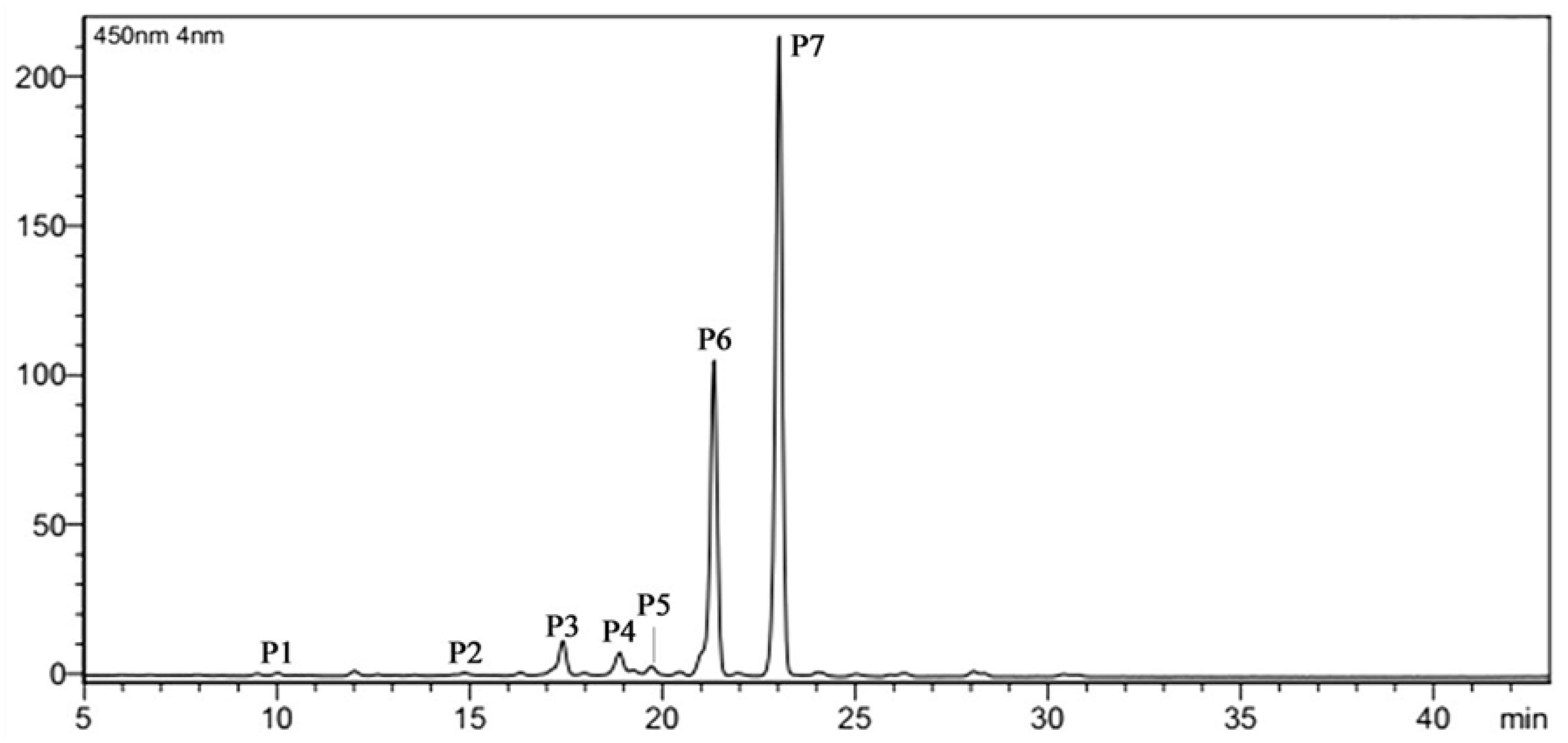
2.2. Effect of ABA Treatment on Carotenoid Composition of the Petals
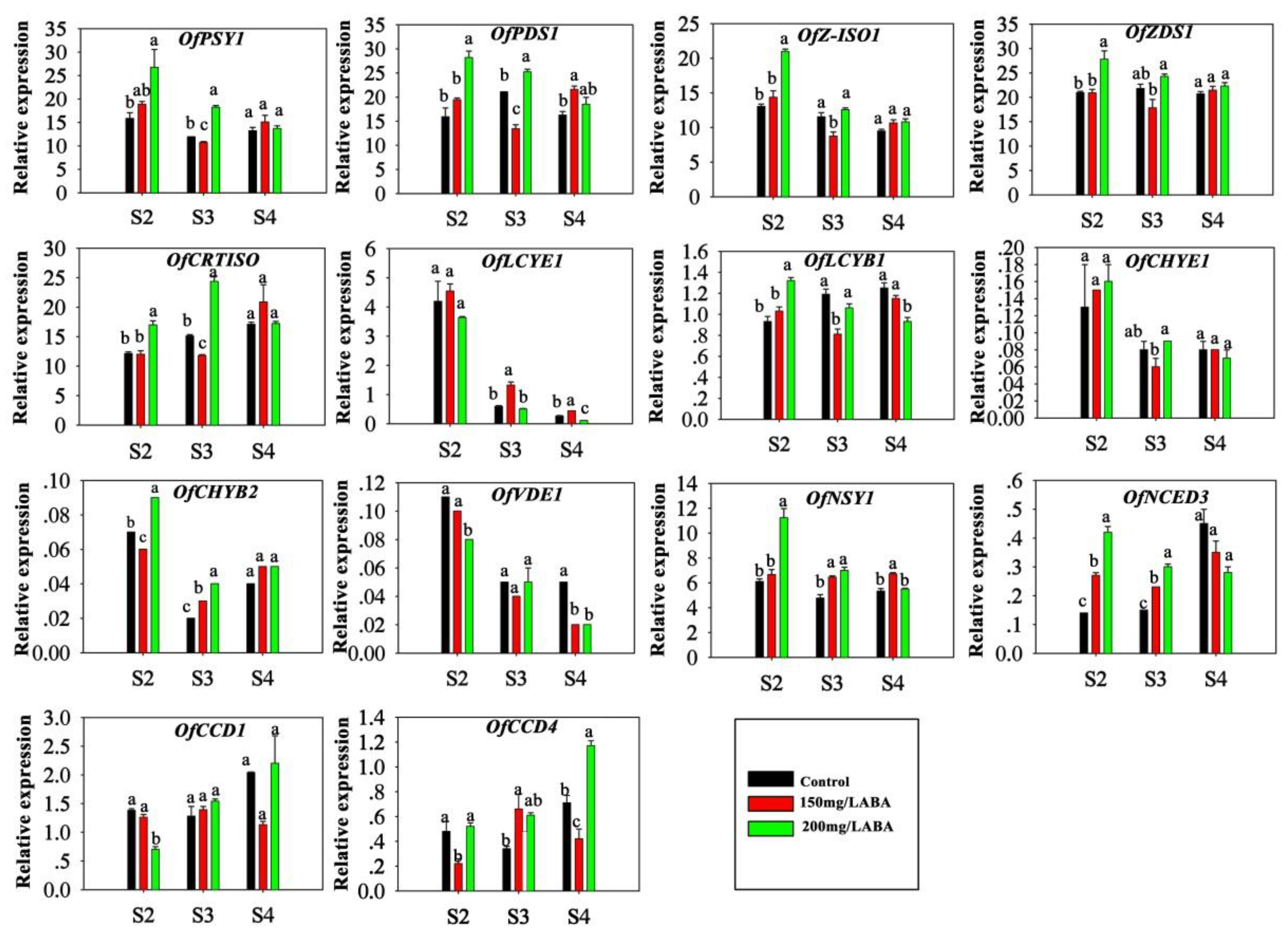
2.3. Effect of ABA Treatment on the Expression of Genes Involved in Carotenoid Metabolism in the Petals
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. Measurement of Flower Color
4.3. Carotenoids Extraction and Analysis
4.4. Analysis of Carotenoids
4.5. RNA Extraction and cDNA Synthesis
4.6. Expression Analysis by Real-Time PCR
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Han, Y.; Wang, X.; Chen, W.; Dong, M.; Yuan, W.; Liu, X.; Shang, F. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genomes 2014, 10, 329–338. [Google Scholar] [CrossRef]
- Naznin, M.; Lefsrud, M.; Gravel, V.; Azad, M. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-deLeón, E.; Jiménez-Halla, J.O.; Báez, J.E.; Bah, M.M. A Simple and Efficient Method for the Partial Synthesis of Pure (3R,3′S)-Astaxanthin from (3R,3′R,6′R)-Lutein and Lutein Esters via (3R,3′S)-Zeaxanthin and Theoretical Study of Their Formation Mechanisms. Molecules 2019, 24, 1386. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Moehs, C.P.; Tian, L.; Osteryoung, K.W.; Dellapenna, D. Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol. Biol. 2001, 45, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Ohmiya, A. Qualitative and quantitative control of carotenoid accumulation in flower petals. Sci. Hortic. 2013, 163, 10–19. [Google Scholar] [CrossRef]
- Zhu, C.; Bai, C.; Sanahuja, G.; Yuan, D.; Farre, G.; Naqvi, S.; Shi, L.; Capell, T.; Christou, P. The regulation of carotenoid pigmentation in flowers. Arch. Biochem. Biophys. 2010, 504, 132–141. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Zacarias, L. Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biol. Technol. 2007, 43, 14–22. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Postharvest Biol. Technol. 2015, 99, 99–104. [Google Scholar] [CrossRef]
- Barreto, G.P.M.; Fabi, J.P.; Rosso, V.V.D.; Cordenunsi, B.R.; Lajolo, F.M.; Nascimento, J.R.O.D.; Mercadante, A.Z. Influence of ethylene on carotenoid biosynthesis during papaya postharvesting ripening. J. Food Compos. Anal. 2011, 24, 620–624. [Google Scholar] [CrossRef]
- Su, L.; Diretto, G.; Purgatto, E.; Danoun, S.; Zouine, M.; Li, Z.; Roustan, J.P.; Bouzayen, M.; Giuliano, G.; Chervin, C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Divya, P.; Puthusseri, B.; Savanur, M.A.; Lokesh, V.; Neelwarne, B. Effects of methyl jasmonate and carotenogenic inhibitors on gene expression and carotenoid accumulation in coriander (Coriandrum sativum L.) foliage. Food Res. Int. 2018, 111, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prashanth, K.V.H.; Shetty, N.P.; Giridhar, P. Elicitors, SA and MJ enhance carotenoids and tocopherol biosynthesis and expression of antioxidant related genes in Moringa oleifera Lam. leaves. Acta Physiol. Plant. 2014, 36, 2695–2704. [Google Scholar] [CrossRef]
- Karppinen, K.; Hirvelä, E.; Nevala, T.; Sipari, N.; Suokas, M.; Jaakola, L. Changes in the abscisic acid levels and related gene expression during fruit development and ripening in bilberry (Vaccinium myrtillus L.). Phytochemistry 2013, 95, 127–134. [Google Scholar] [CrossRef]
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010, 20, 55–67. [Google Scholar] [CrossRef]
- Neves, D.M.; Filho, M.A.; Bellete, B.S.; Silva, M.F.; Souza, D.T.; Dos, S.S.F.W.; Costa, M.G.; Gesteira, A.S. Comparative study of putative 9-cis-epoxycarotenoid dioxygenase and abscisic acid accumulation in the responses of Sunki mandarin and Rangpur lime to water deficit. Mol. Biol. Rep. 2013, 40, 5339–5349. [Google Scholar] [CrossRef]
- Xia, H.; Wu, S.; Ma, F. Cloning and expression of two 9-cis-epoxycarotenoid dioxygenase genes during fruit development and under stress conditions from Malus. Mol. Biol. Rep. 2014, 41, 6795–6802. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid impacts tomato carotenoids, soluble sugars, and organic acids. HortScience 2016, 51, 370–376. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic Acid Increases Carotenoid and Chlorophyll Concentrations in Leaves and Fruit of Two Tomato Genotypes. J. Am. Soc. Hortic. Sci. 2014, 139, 261–266. [Google Scholar] [CrossRef]
- Welsch, R.; Wüst, F.; Bär, C.; Al-Babili, S.; Beyer, P. A Third Phytoene Synthase Is Devoted to Abiotic Stress-Induced Abscisic Acid Formation in Rice and Defines Functional Diversification of Phytoene Synthase Genes. Plant Physiol. 2008, 147, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Dong, B.; Fu, J.; Hu, S.; Zhao, H. Carotenoid Accumulation and Its Contribution to Flower Coloration of Osmanthus fragrans. Front. Plant Sci. 2018, 9, 1499. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Hou, D.; Zhang, C.; Bao, Z.; Zhao, H.; Hu, S. The Emission of the Floral Scent of Four Osmanthus fragrans Cultivars in Response to Different Temperatures. Molecules 2017, 22, 430. [Google Scholar]
- Baldermann, S.; Kato, M.; Fleischmann, P.; Watanabe, N. Biosynthesis of α- and β-ionone, prominent scent compounds, in flowers of Osmanthus fragrans. Acta Biochim. Pol. 2012, 59, 79–81. [Google Scholar] [CrossRef]
- Huang, F.C.; Molnar, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef]
- Hatmi, S. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J. Exp. Biol. 2015, 66, 775. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, H.; You, S.; Xu, Y.; Ran, K.; Fan, S. Cloning, characterization and functional analysis of the role MhNCED3, a gene encoding 9-cis-epoxycarotenoid dioxygenase in Malus hupehensis Rehd., plays in plant tolerance to osmotic and Cd2+ stresses. Plant Soil 2014, 381, 143–160. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Fu, J.; Bao, Z.; Zhao, H. Transcriptomic analysis and carotenogenic gene expression related to petal coloration in Osmanthus fragrans ‘Yanhong Gui’. Trees 2016, 30, 1207–1223. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Baskar, V.; Kim, S.H.; Chung, I.M. Effects of abscisic acid, jasmonic acid and salicylic acid on the content of phytochemicals and their gene expression profiles and biological activity in turnip (Brassica rapa ssp. rapa). Plant Growth Regul. 2016, 80, 1–14. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.H.; Chung, I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Tian, S.L.; Li, L.; Tian, Y.Q.; Shah, S.N.M.; Gong, Z.H. Effects of Abscisic Acid on Capsanthin Levels in Pepper Fruit. J. Am. Soc. Hortic. Sci. 2016, 141, 609–616. [Google Scholar] [CrossRef]
- Baldermann, S.; Yang, Z.; Sakai, M.; Fleischmann, P.; Morita, A.; Todoroki, Y.; Watanabe, N. Influence of exogenously applied abscisic acid on carotenoid content and water uptake in flowers of the tea plant (Camellia sinensis). J. Sci. Food Agric. 2013, 93, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Kim, J.K.; Lee, S.; Chae, S.C.; Park, S.U. Molecular characterization of carotenoid cleavage dioxygenases and the effect of gibberellin, abscisic acid, and sodium chloride on the expression of genes involved in the carotenoid biosynthetic pathway and carotenoid accumulation in the callus of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 2013, 61, 5565–5572. [Google Scholar] [PubMed]
- Kim, Y.; Hwang, I.; Jung, H.J.; Park, J.I.; Kang, J.G.; Nou, I.S. Genome-Wide Classification and Abiotic Stress-Responsive Expression Profiling of Carotenoid Oxygenase Genes in Brassica rapa and Brassica oleracea. J. Plant Growth Regul. 2016, 35, 202–214. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, X.-L.; Zhang, Q.; Cao, Z.-Y.; Zhao, Y.-X.; Zhang, H. Isolation and characterization of carotenoid cleavage dioxygenase genein halophyte Suaeda salsa. Plant Growth Regul. 2005, 46, 61–67. [Google Scholar] [CrossRef]
- Wang, R.K.; Wang, C.E.; Fei, Y.Y.; Gai, J.Y.; Zhao, T.J. Genome-wide identification and transcription analysis of soybean carotenoid oxygenase genes during abiotic stress treatments. Mol. Biol. Rep. 2013, 40, 4737–4745. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Tzfadia, O.; Vallabhaneni, R.; Gehring, C.; Wurtzel, E.T. A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana. BMC Syst. Biol. 2011, 5, 77. [Google Scholar] [CrossRef]
- Wan, X.R.; Mo, A.Q.; Liu, S.; Liang, J.H.; Li, L.; Yu, S.Y.; Zheng, Y.X. Molecular cloning and Gus-aided activity assaying of promoter sequence of AhNCED1 gene from Arachis hypogaea L. cv. Yueyou 7. J. Nucl. Agric. Sci. 2011, 25, 692–699. [Google Scholar]
- Liu, Y.C.; Wang, Y.G.; Zhang, C.; Dong, B.; Fu, J.X.; Hu, S.Q.; Zhao, H.B. Cloning and transient expression assay of OfCCD1 gene promotersfrom Osmanthus fragrans. J. Zhejiang A F Univ. 2018, 35, 596–603. [Google Scholar]
- Wan, X.R.; Mo, A.Q.; Guo, X.J.; Yang, M.X.; Yu, S.Y.; Cao, J.P. Osmotic Stress Tolerance Improvement of Arabidopsis Plants Ectopically Expressing Peanut AhNCED1 Gene. Acta Agron. Sin. 2010, 36, 1440–1449. [Google Scholar] [CrossRef]
- Wan, X.R.; Mo, A.Q.; Pan, J.H.; Liang, C.Y. Molecular cloning and Gus-aided activity analysis of promoter region sequence of AtNCED2 gene from Arabidopsis thaliana L. Biotechnology 2010, 20, 18–23. [Google Scholar]
- Bhalothia, P.; Sangwan, C.; Alok, A.; Mehrotra, S.; Mehrotra, R. PP2C-like Promoter and Its Deletion Variants Are Induced by ABA but Not by MeJA and SA in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 547. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Mehrotra, S. Promoter activation by ACGT in response to salicylic and abscisic acids is differentially regulated by the spacing between two copies of the motif. J. Plant Physiol. 2010, 167, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Argyris, J.; Truco, M.J.; Ochoa, O.; McHale, L.; Dahal, P.; Deynze, A.V.; Michelmore, R.W.; Bradford, K.J. A gene encoding an abscisic acid biosynthetic enzyme (LsNCED4) collocates with the high temperature germination locus Htg6.1 in lettuce (Lactuca sp.). Theor. Appl. Genet. 2011, 122, 95–108. [Google Scholar] [CrossRef]
- Lindgren LO, S.K. Höglund AS, Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results indelayed germination and increased levels of carotenoids, chlorophyll, and abscisic acid. Plant Physiol. 2003, 132, 779–785. [Google Scholar] [CrossRef]
- Prital, O.; Shaarmoshe, L.; Wiseglass, G.; Peleg, Z.; Mosquna, A. Non-redundant functions of the dimeric ABA receptor BdPYL1 in the grass Brachypodium. Plant J. 2017, 92, 774–786. [Google Scholar] [CrossRef]
- Melcher, K.; Ng, L.M.; Zhou, X.E.; Soon, F.F.; Xu, Y.; Suinopowell, K.M.; Park, S.Y.; Weiner, J.J.; Fujii, H.; Chinnusamy, V. A Gate-Latch-Lock Mechanism for Hormone Signaling by Abscisic Acid Receptors. Nature 2009, 462, 602–608. [Google Scholar] [CrossRef]
- Choi, H.; Hong, J.; Ha, J.; Kang, J.; Kim, S.Y. ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 2000, 275, 1723–1730. [Google Scholar] [CrossRef]
- Ureshino, K.; Nakayama, M.; Miyajima, I. Contribution made by the carotenoid cleavage dioxygenase 4 gene to yellow colour fade in azalea petals. Euphytica 2016, 207, 401–417. [Google Scholar] [CrossRef]
- Delpinorius, A.; Eras, J.; Marsolvall, A.; Vilaró, F.; Balcells, M.; Canelagarayoa, R. Ultra performance liquid chromatography analysis to study the changes in the carotenoid profile of commercial monovarietal fruit juices. J. Chromatogr. A 2014, 1331, 90–99. [Google Scholar] [CrossRef]
- Baldermann, S.; Kato, M.; Kurosawa, M.; Kurobayashi, Y.; Fujita, A.; Fleischmann, P.; Watanabe, N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010, 61, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, J.; Wang, Y.; Bao, Z.; Zhao, H. Identification of Suitable Reference Genes for Gene Expression Normalization in the Quantitative Real-Time PCR Analysis of Sweet Osmanthus (Osmanthus fragrans Lour). PLoS ONE 2015, 10, e0136355. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| Stage | Treatment | a* | b* | L* | C* | h* |
|---|---|---|---|---|---|---|
| S2 | 0 mg/L | 0 | 35.60 ± 0.98a | 52.35 ± 0.03a | 63.00 ± 0.23b | 63.32 ± 0.58a |
| 150 mg/L | 150 | 33.50 ± 0.60a | 54.77 ± 1.21a | 67.33 ± 0.60a | 64.20 ± 1.31a | |
| 200 mg/L | 200 | 35.57 ± 0.45a | 51.93 ± 2.52a | 63.13 ± 1.27b | 62.96 ± 2.30a | |
| S3 | 0 mg/L | 0 | 38.70 ± 0.69a | 47.95 ± 2.45a | 61.75 ± 0.43a | 61.64 ± 2.34a |
| 150 mg/L | 150 | 38.80 ± 0.06a | 50.55 ± 0.89a | 60.00 ± 0.52a | 63.73 ± 0.67a | |
| 200 mg/L | 200 | 41.20 ± 1.31a | 49.20 ± 2.02a | 58.67 ± 1.10b | 64.17 ± 2.38a | |
| S4 | 0 mg/L | 0 | 42.35 ± 0.03b | 48.80 ± 1.73a | 59.15 ± 0.55ab | 64.63 ± 1.29b |
| 150 mg/L | 150 | 44.15 ± 0.55a | 49.40 ± 1.33a | 56.55 ± 0.61b | 66.26 ± 1.36ab | |
| 200 mg/L | 200 | 44.20 ± 0.17a | 55.43 ± 1.79a | 60.83 ± 1.39a | 70.91 ± 1.48a |
| Content | 0 mg/L ABA | 150 mg/L ABA | 200 mg/L ABA |
|---|---|---|---|
| P1 | 44.81 ± 0.91b | 53.90 ± 2.53a | 59.17 ± 1.15a |
| P2 | 33.53 ± 0.55c | 53.92 ± 0.29a | 43.96 ± 0.64b |
| P4 | 376.97 ± 0.36b | 364.53 ± 0.38b | 404.89 ± 7.80a |
| P5 | 185.05 ± 2.56a | 150.90 ± 1.03b | 192.67 ± 2.90a |
| P6 | 4779.50 ± 15.97c | 5089.04 ± 64.07b | 5740.65 ± 72.36a |
| P7 | 8627.15 ± 1.05b | 8476.13 ± 98.27b | 8887.31 ± 27.09a |
| TC | 14,047 ± 13.07b | 14,188.43 ± 165.24b | 15,328.64 ± 95.05a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Dong, B.; Zhang, C.; Yang, L.; Wang, Y.; Zhao, H. Effects of Exogenous Abscisic Acid (ABA) on Carotenoids and Petal Color in Osmanthus fragrans ‘Yanhonggui’. Plants 2020, 9, 454. https://doi.org/10.3390/plants9040454
Liu Y, Dong B, Zhang C, Yang L, Wang Y, Zhao H. Effects of Exogenous Abscisic Acid (ABA) on Carotenoids and Petal Color in Osmanthus fragrans ‘Yanhonggui’. Plants. 2020; 9(4):454. https://doi.org/10.3390/plants9040454
Chicago/Turabian StyleLiu, Yucheng, Bin Dong, Chao Zhang, Liyuan Yang, Yiguang Wang, and Hongbo Zhao. 2020. "Effects of Exogenous Abscisic Acid (ABA) on Carotenoids and Petal Color in Osmanthus fragrans ‘Yanhonggui’" Plants 9, no. 4: 454. https://doi.org/10.3390/plants9040454
APA StyleLiu, Y., Dong, B., Zhang, C., Yang, L., Wang, Y., & Zhao, H. (2020). Effects of Exogenous Abscisic Acid (ABA) on Carotenoids and Petal Color in Osmanthus fragrans ‘Yanhonggui’. Plants, 9(4), 454. https://doi.org/10.3390/plants9040454