Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl
Abstract
1. Introduction
2. Results
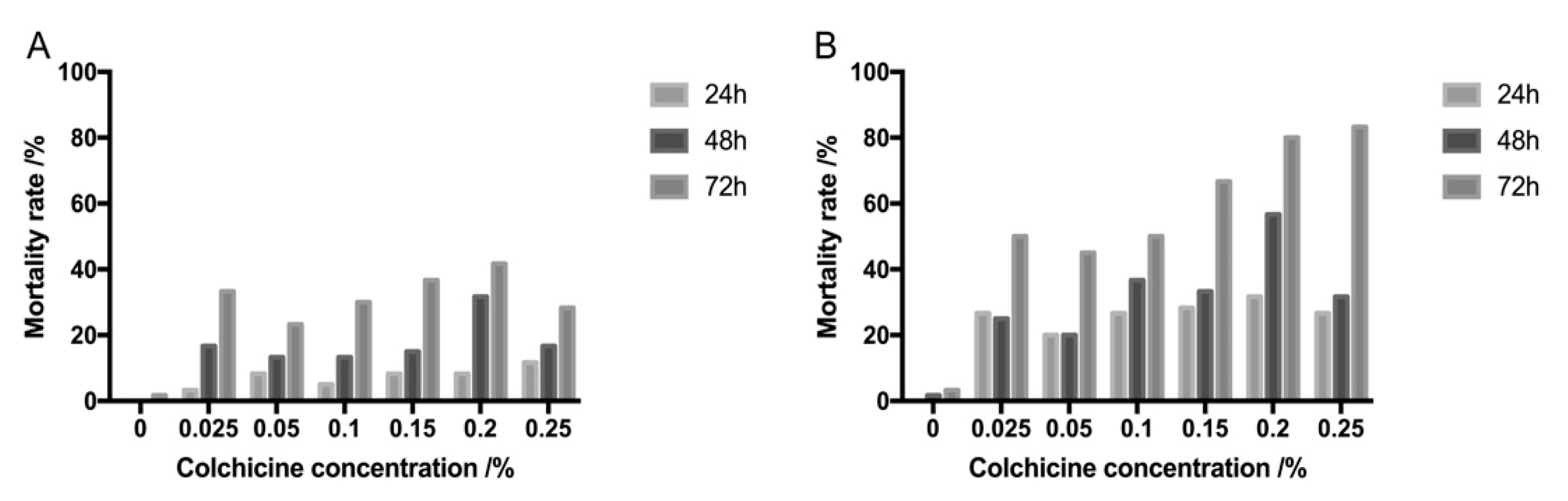
2.1. Effects of Colchicine Treatment on Explants’ Survival
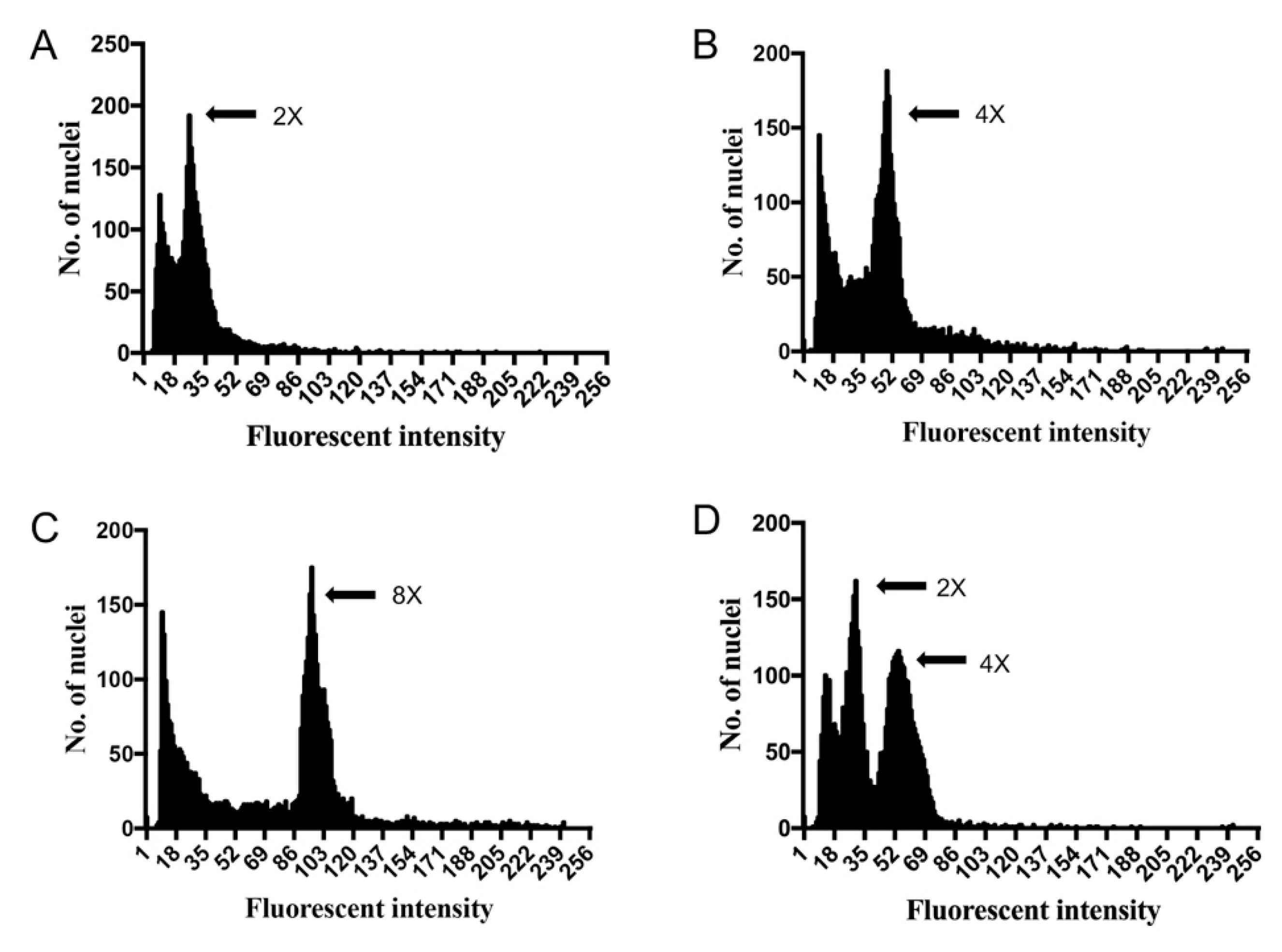
2.2. Polyploid Identification by Flow Cytometry
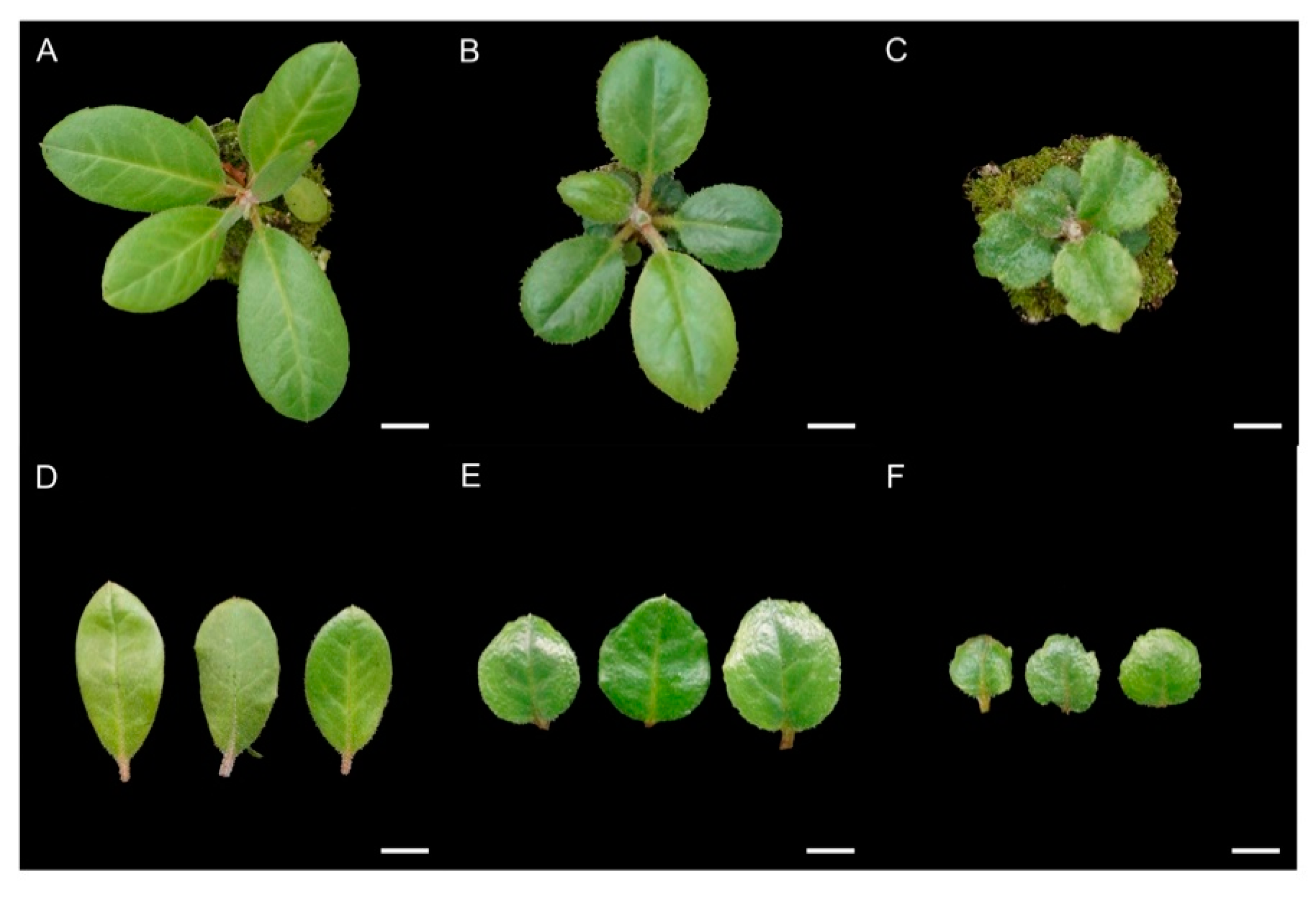
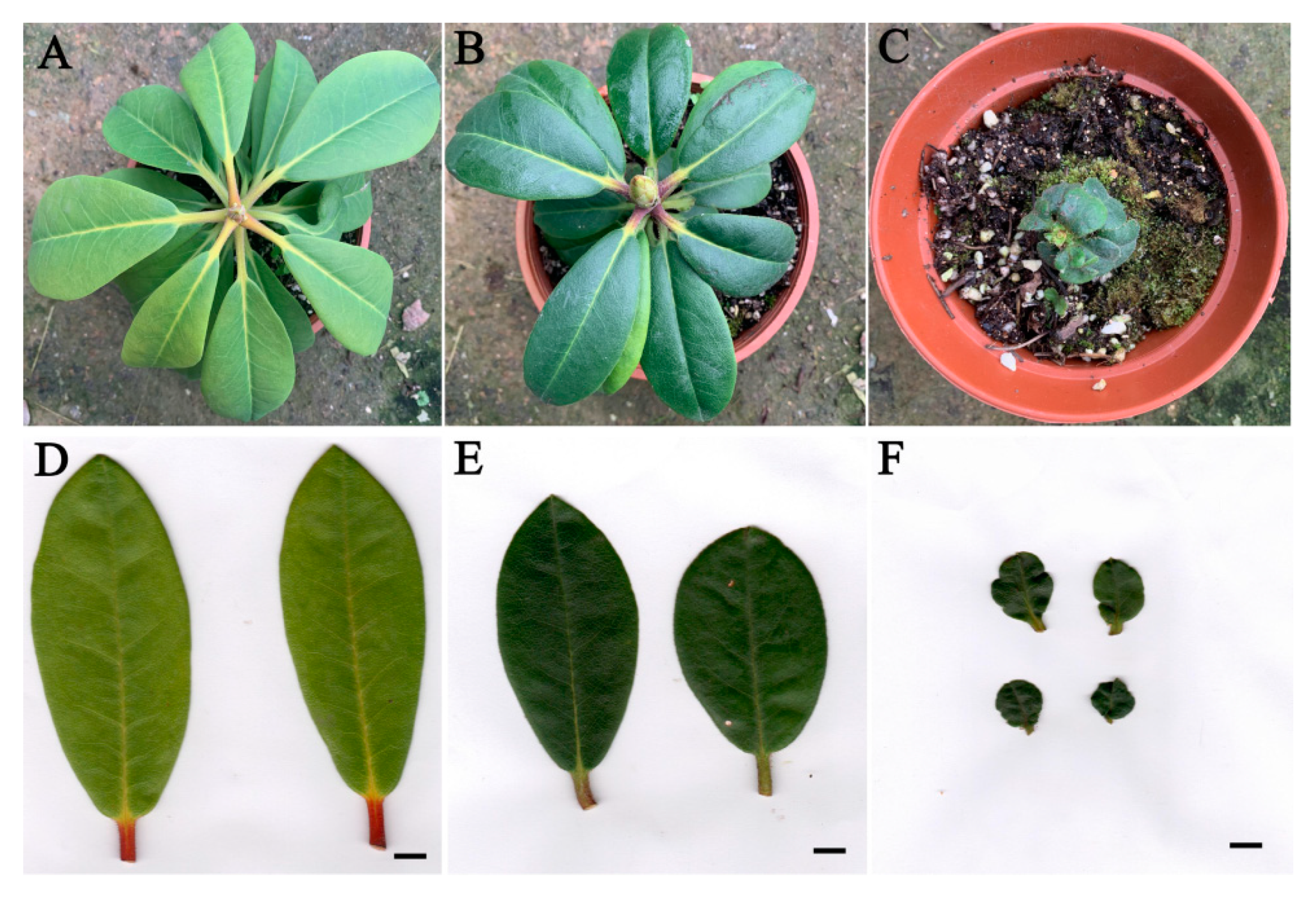
2.3. Morphological Characteristics of R. fortunei Polyploids
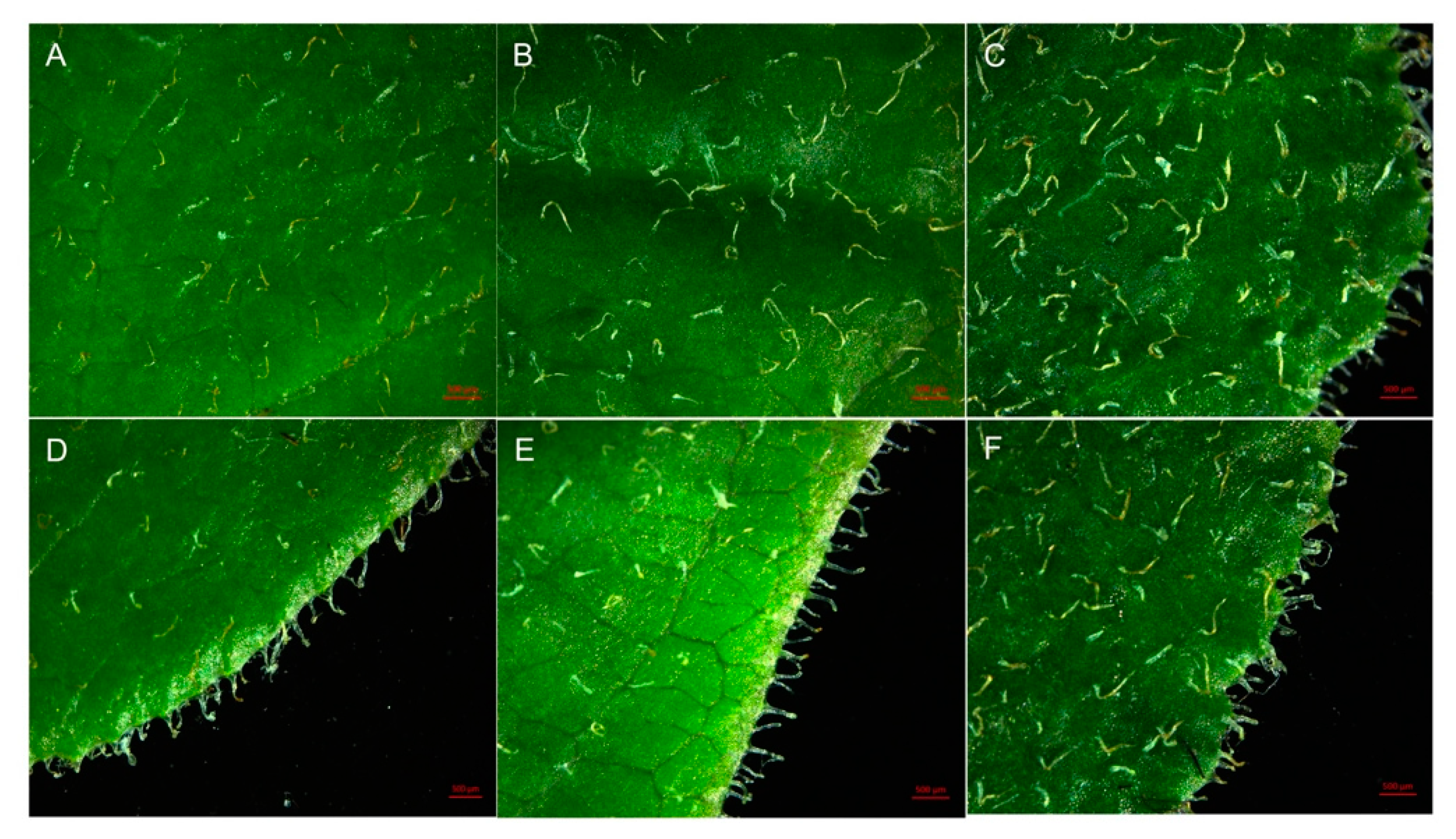
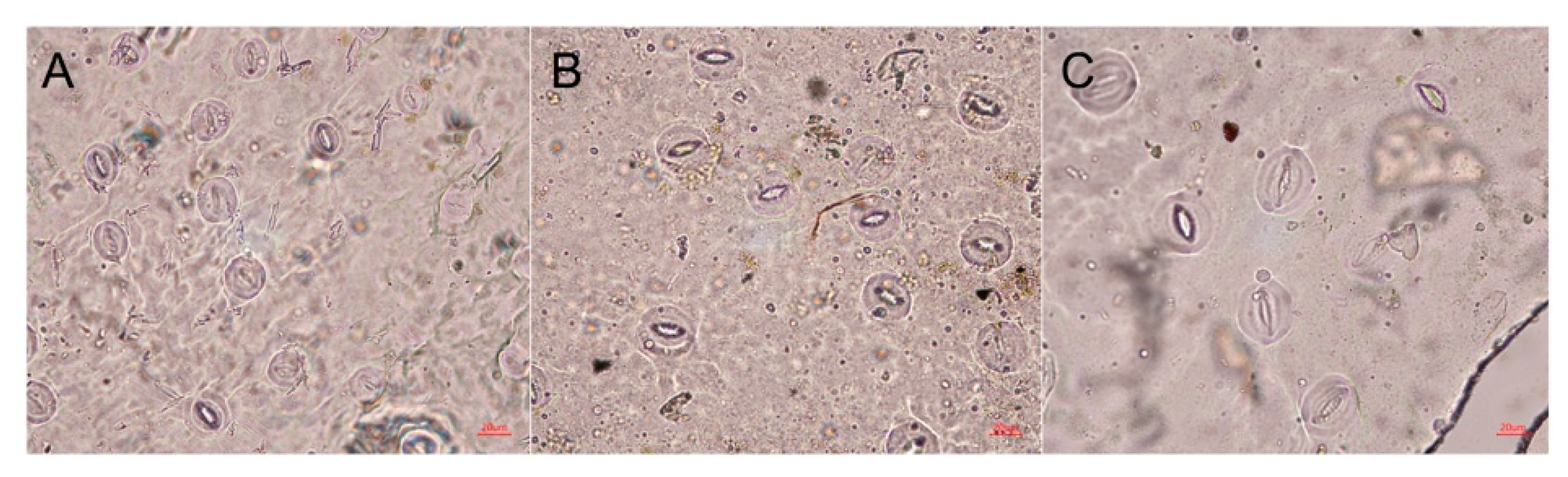
2.4. Stomatal Characteristics of Polyploid R. fortunei
2.5. Pigment Content in Leaves of Polyploid and Diploid R. fortunei
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Colchicine Treatment and Shoot Regeneration
4.3. Mortality Rate Determination
4.4. Flow Cytometry for Ploidy Level Estimation
4.5. Leaf Morphology Measurement
4.6. Determination of Leaf Pigment Content
4.7. Determination of Stomatal Cell Size and Density
4.8. Data Processing
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhengyi, W.; Peter, R.; Deyuan, H. Flora of China. Volume 9: Pittosporaceae through Connaraceae; Science Press: Beijing, China, 2013. [Google Scholar]
- Bian, C.; Jin, Z. The Flowering and Fruit Set Features of Rhododendron fortunei in Tiantai Mountains. Acta Horticulturae Sinica 2006, 33, 101. [Google Scholar]
- Soltis, D.E.; Soltis, P.S.; Tate, J.A. Advances in the study of polyploidy since Plant speciation. New Phytol. 2003, 161, 173–191. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Van Huylenbroek, J.; De Schepper, S.; Van Labeke, M.C. Breeding for polyploidy in Belgian azalea (Rhododendron simsii hybrids). Acta Horticulturae 2006, 714, 113–118. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef]
- Predieri, S. Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Organ Cult. 2001, 64, 185–210. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Madon, M.; Clyde, M.M.; Hashim, H.; Yusuf, Y.M.; Mat, H.; Saratha, S. Polyploidy induction of oil palm through colchicine and oryzalin treatments. J. Oil Palm Res. 2005, 17, 110–123. [Google Scholar]
- Eeckhaut, T.G.R.; Werbrouck, S.P.O.; Leus, L.W.H.; Bockstaele, E.J.V.; Debergh, P.C. Chemically Induced Polyploidization in Spathiphyllum wallisii Regel through Somatic Embryogenesis. Plant Cell Tissue Organ. Cult. 2004, 78, 241–246. [Google Scholar] [CrossRef]
- Väinölä, A. Polyploidization and early screening of Rhododendron hybrids. Euphytica. 2000, 112, 239–244. [Google Scholar] [CrossRef]
- Jones, J.R.; Ranney, G.T.; Eaker, T.A. A novel method for inducing polyploidy in Rhododendron seedlings. J. Am. Rhododendron Soc. 2008, 62, 130–135. [Google Scholar]
- Hua, Y.; Hongxing, L.; Xuzhong, S.; Guojian, D.; Zhuomei, C. Experiment on induction of three species of Rhododendron by colchicines. J. Zhejiang For. Sci. Technol. 2009, 29, 53–56. [Google Scholar]
- De Schepper, S.; Leus, L.; Eeckhaut, T.; Van Bockstaele, E.; Debergh, P.; De Loose, M. Somatic polyploid petals: Regeneration offers new roads for breeding Belgian pot azaleas. Plant Cell Tissue Organ Cult. 2004, 76, 183–188. [Google Scholar] [CrossRef]
- De Schepper, S.; Leus, L.; Mertens, M.; Debergh, P.; Van Bockstaele, E.; De Loose, M. Somatic polyploidy and its consequences for flower coloration and flower morphology in azalea. Plant Cell Rep. 2001, 20, 583–590. [Google Scholar] [CrossRef]
- Yue, F.; Yunhui, R.; Xiuyu, L.; Yuhan, S.; Yongping, D.; Jihong, Z.; Hong, Y.; Yun, L. Artificial Induction of Polyploid Robinia pseudoacacia L. and Identification of Its Ploidy. Mol. Plant Breed. 2018, 16, 7124–7131. [Google Scholar]
- Müntzing, A.; Runquist, E. Note on some colchicine-induced polyploids. Hereditas 2010, 25, 491–495. [Google Scholar] [CrossRef]
- Urwin, N.A.R. Generation and characterisation of colchicine-induced polyploid Lavandula × intermedia. Euphytica 2014, 197, 331–339. [Google Scholar] [CrossRef]
- Zhou, H.; Feng, D.; Yan, H. The Progress of Polyploids Induced In Vitro via Colchicine. J. Nucl. Agric. Sci. 2015, 29, 1307–1315. [Google Scholar]
- De, K.K.; Saha, A.; Tamang, R.; Sharma, B. Investigation on relative genome sizes and ploidy levels of Darjeeling-Himalayan Rhododendron species using flow cytometer. Indian J. Biotechnol. 2010, 9, 64–68. [Google Scholar]
- Väinölä, A.; Repo, T. Polyploidisation of Rhododendron Cultivars In Vitro And How It Affects Cold Hardiness. Acta Horticulturae 2001, 560, 319–322. [Google Scholar] [CrossRef]
- Rounsaville, T.J.; Ranney, T.G. Ploidy Levels and Genome Sizes of Berberis, L. and Mahonia Nutt. Species, Hybrids, and Cultivars. Hortsci. Publ. Am. Soc. Horticult. Sci. 2010, 45, 1029–1033. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Exploitation of flow cytometry for plant breeding. Acta Physiologiae Plantarum 2005, 27, 743–750. [Google Scholar] [CrossRef]
- Lyuchun, P.; Junfeng, T.; Xiu’an, D.; Jie, S.; Weijia, X.; Wenling, G.; Shifeng, L. Polyploidy Induction and Identification of Rhododendron racemosum Franch. J. Nucl. Agric. Sci. 2018, 32, 0257–0265. [Google Scholar]
- Kulkarni, M.; Borse, T. Induced polyploidy with gigas expression for root traits in Capsicum annuum (L.). Plant Breed. 2009, 129, 461–464. [Google Scholar] [CrossRef]
- Miguel, T.P.; Leonhardt, K.W. In Vitro polyploid induction of orchids using oryzalin. Scientia Horticulturae 2011, 130, 314–319. [Google Scholar] [CrossRef]
- Chung, M.Y.; Kim, C.Y.; Min, J.S.; Lee, D.J.; Naing, A.H.; Chung, J.D.; Kim, C.K. In Vitro induction of tetraploids in an interspecific hybrid of Calanthe (Calanthe discolor). Plant Biotechnol. Rep. 2014, 8, 251–257. [Google Scholar] [CrossRef]
- Paniel, S.; Marhold, K.; Filová, B.; Zozomová-Lihová, J. Genetic and morphological variation in the diploid–polyploid Alyssum montanum in Central Europe: Taxonomic and evolutionary considerations. Plant Syst. Evol. 2011, 294, 1–25. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Bao, M. Colchicine-induced chromosome doubling in Platanus acerifolia and its effect on plant morphology. Euphytica 2007, 157, 145–154. [Google Scholar] [CrossRef]
- Ren, J.; Wu, X.; Song, C.; Liang, Y.Y.; Gao, W.; Wang, Y. Induction of polyploid tillered onion using colchicine and pendimethalin. Sains Malaysiana 2018, 47, 2617–2624. [Google Scholar] [CrossRef]
- Eeckhaut, T.; Samyn, G.; Van Bockstaele, E. In Vitro polyploidy induction in Rhododendron simsii hybrids. Acta Horticulturae 2002, 572, 43–49. [Google Scholar] [CrossRef]
- Yingjie, Y.; Beibei, G.; Qian, W.; Junping, G.; Bo, H. Colchicines-induced polyploid plants and identification in Lilium pumilum DC. J. China Agric. Univ. 2013, 18, 128–133. [Google Scholar]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Corneillie, S.; De Storme, N.; Van Acker, R.; Fangel, J.U.; De Bruyne, M.; De Rycke, R.; Geelen, D.; Willats, W.G.T.; Vanholme, B.; Boerjan, W. Polyploidy Affects Plant Growth and Alters Cell Wall Composition. Plant Physiol. 2019, 179, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Duren, M.V.; Morpurgo, R.; Dolezel, J.; Afza, R. Induction and verification of autotetraploids in diploid banana (Musa acuminata) by In Vitro techniques. Euphytica 1996, 88, 25–34. [Google Scholar] [CrossRef]
- Sakhanokho, H.; Rajasekaran, K.; Kelley, R.Y.; Faridi, N.F. Induced polyploidy in diploid ornamental ginger (Hedychium muluense R.M. Smith) using Colchicine and Oryzalin. HortScience 2009, 44, 1809–1814. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Baig, M.M.Q.; Quresh, A.A.; Shah, M.; Nawaz, K.; Hafiz, I.A. Induction and identification of colchicine induced polyploidy in Gladiolus grandiflorus ”White Prosperity”. Folia Horticulturae 2018, 30, 307–319. [Google Scholar] [CrossRef]
- Inceer, H.; Hayirlioglu-Ayaz, S. Chromosome numbers in Tripleurospermum Sch. Bip. (Asteraceae) and closely related genera: Relationships between ploidy level and stomatal length. Plant Syst. Evol. 2010, 285, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Marinho, R.; Mendes-Rodrigues, C.; Bonetti, A.; Oliveira, P. Pollen and stomata morphometrics and polyploidy in Eriotheca (Malvaceae-Bombacoideae). Plant. Biol. 2014, 16, 508–511. [Google Scholar] [CrossRef]
- Smarda, P.; Horova, L.; Knapek, O.; Dieck, H.; Dieck, M.; Ražna, K.; Hrubik, P.; Orloci, L.; Papp, L.F.; Vesela, K. Multiple haploids, triploids, and tetraploids found in modern-day “living fossil” Ginkgo biloba. Horticult. Res. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Gomes, V.; Aud, F.; Santos-Serejo, J.; Oliveira, E. Inducing autotetraploids in cassava using oryzalin and colchicine and their in vitro morphophysiological effects. Genet. Mol. Res. GMR 2016, 15. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Zhang, C.; Wang, Z. In Vitro shoot culture of Rhododendron fortunei: An important plant for bioactive phytochemicals. Ind. Crops Prod. 2018, 126, 459–465. [Google Scholar] [CrossRef]
- Pandey, S.K.; Singh, H.A. Simple, Cost-Effective Method for Leaf Area Estimation. J. Botany 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Ye, Y.; Tong, J.; Shi, X.; Yuan, W.; Li, G. Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Scientia Horticulturae 2010, 124, 95–101. [Google Scholar] [CrossRef]
- Pápista, É.; Ács, É.; Böddi, B. Chlorophyll-a determination with ethanol—A critical test. Hydrobiologia 2002, 485, 191–198. [Google Scholar] [CrossRef]
- Zucchelli, G.; Jennings, R.C.; Garlaschi, F.M.; Cinque, G.; Bassi, T.R.; Cremonesi, O. The Calculated In Vitro and In Vivo Chlorophyll a Absorption Bandshape. Biophys. J. 2002, 82, 378–390. [Google Scholar] [CrossRef]
- Makeen, K.; Babu, G.S.; Lavanya, G.R.; Abraham, G. Studies of Chlorophyll Content by Different Methods in Black Gram (Vigna mungo L.). Int. J. Agric. Res. 2007, 2, 651–654. [Google Scholar]
- Rego, M.M.D.; Rego, E.R.D.; Bruckner, C.H.; Finger, F.L.; Otoni, W.C. In Vitro induction of autotetraploids from diploid yellow passion fruit mediated by colchicine and oryzalin. Plant Cell Tissue Organ Cult. 2011, 107, 451–459. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Mirzaee, M.; Hassani, M.E.; Moghadam, M.S. Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. Int. J. Plant. Prod. 2010, 4, 87–98. [Google Scholar]
- Mishra, M.K. Tomatal characteristics at different ploidy levels in Coffea L. Ann. Botany 1997, 80, 689–692. [Google Scholar] [CrossRef]
- Yang, X.; Cao, Z.Y.; An, L.; Wang, Y.M.; Fang, X. In Vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica 2006, 152, 217–224. [Google Scholar] [CrossRef]
| Ploidy Level | No. of Evaluated Plants | Peak Value |
|---|---|---|
| Diploid | 405 | 25.26 ± 1.08 |
| Tetraploid | 69 | 48.92 ± 1.81 |
| Octoploid | 29 | 94.94 ± 2.44 |
| Chimera | 37 | 25.25 ± 1.33, 52.51 ± 2.01 a |
| Colchicine Concentration /% | Treatment Time /h | Tetraploids Frequency | Octoploids Frequency | Chimeras Frequency | Polyploids Frequency | ||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot Apex | Stem Base | Shoot Apex | Stem Base | Shoot Apex | Stem Base | Shoot Apex | Stem Base | ||
| 0 | 24 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 |
| 48 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | |
| 72 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | |
| 0.025 | 24 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 |
| 48 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | |
| 72 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | |
| 0.05 | 24 | 1/30 | 3/30 | 0/30 | 0/30 | 0/30 | 0/30 | 1/30 | 3/30 |
| 48 | 1/30 | 3/30 | 0/30 | 2/30 | 1/30 | 2/30 | 2/30 | 7/30 | |
| 72 | 1/30 | 4/30 | 1/30 | 2/30 | 1/30 | 1/30 | 3/30 | 7/30 | |
| 0.1 | 24 | 4/30 | 8/30 | 1/30 | 3/30 | 2/30 | 2/30 | 7/30 | 13/30 |
| 48 | 1/30 | 4/30 | 0/30 | 1/30 | 2/30 | 1/30 | 3/30 | 6/30 | |
| 72 | 2/30 | 3/30 | 1/30 | 2/30 | 1/30 | 2/30 | 4/30 | 7/30 | |
| 0.15 | 24 | 1/30 | 3/30 | 1/30 | 3/30 | 0/30 | 0/30 | 2/30 | 6/30 |
| 48 | 1/30 | 4/30 | 0/30 | 2/30 | 1/30 | 3/30 | 2/30 | 9/30 | |
| 72 | 2/30 | 2/30 | 0/30 | 1/30 | 1/30 | 2/30 | 3/30 | 5/30 | |
| 0.2 | 24 | 1/30 | 2/30 | 0/30 | 1/30 | 2/30 | 3/30 | 3/30 | 6/30 |
| 48 | 2/30 | 3/30 | 1/30 | 1/30 | 1/30 | 2/30 | 4/30 | 6/30 | |
| 72 | 2/30 | 2/30 | 0/30 | 0/30 | 1/30 | 1/30 | 3/30 | 3/30 | |
| 0.25 | 24 | 1/30 | 3/30 | 0/30 | 2/30 | 0/30 | 0/30 | 1/30 | 5/30 |
| 48 | 1/30 | 2/30 | 0/30 | 1/30 | 2/30 | 1/30 | 3/30 | 4/30 | |
| 72 | 1/30 | 1/30 | 1/30 | 2/30 | 1/30 | 1/30 | 3/30 | 4/30 | |
| Total | 22/540 | 47/540 | 6/540 | 23/540 | 16/540 | 21/540 | 44/540 | 91/540 | |
| Plant Age | Ploidy Level | No. of Evaluated Plants | Leaf Length (cm) | Leaf Width (cm) | Leaf Index | Leaf Area (cm2) | Thickness (mm) |
|---|---|---|---|---|---|---|---|
| 6 months | Diploid | 10 | 4.34 ± 1.00 a | 3.20± 0.51 a | 1.35 ± 0.32 a | 10.81 ± 5.10 a | 0.338 ± 0.027 c |
| Tetraploid | 10 | 2.34 ± 0.50 b | 2.04 ±0.22 b | 1.14 ± 0.20 b | 3.74 ± 1.22 b | 0.547 ± 0.021 b | |
| Octoploid | 10 | 1.37 ± 0.47 c | 1.29 ± 0.35 c | 1.06 ± 0.14 b | 1.37 ± 1.35 c | 0.753 ±0.031 a | |
| 15 months | Diploid | 10 | 9.63 ± 0.78 a | 3.55 ± 0.39 a | 2.71 ± 0.27 a | 26.91 ± 4.03 a | 0.458 ± 0.047 c |
| Tetraploid | 10 | 6.57 ± 1.03 b | 3.26 ±0.35 b | 2.04 ± 0.30 b | 17.05 ± 5.12 b | 0.675 ± 0.043 b | |
| Octoploid | 10 | 2.43 ± 0.30 c | 1.75 ± 0.24 c | 1.39 ± 0.11 c | 3.72 ± 0.41 c | 0.905 ±0.053 a |
| Ploidy Level | No of Evaluated Stomata | Stomata Length (μm) | Stomata Width (μm) | Stomata Index (Length/Width) | Stoma Area (μm2) | Stoma Density (mm−2) |
|---|---|---|---|---|---|---|
| Diploid | 30 | 27.30 ± 0.66 c | 21.19 ± 1.18 c | 1.30 ± 0.07 a | 454.02 ± 27.83 c | 164.79±11.85 a |
| Tetraploid | 30 | 34.07 ± 1.93 b | 30.41 ±1.87 b | 1.12 ± 0.06 b | 814.77 ± 82.39 b | 78.93±7.72 b |
| Octoploid | 30 | 42.67 ± 2.99 a | 38.89 ± 3.00 a | 1.10 ± 0.07 b | 1307.44 ± 176.26 a | 52.40±5.38 c |
| Ploidy Level | No. of Evaluated Plants | Total Chlorophyll (mg/g) | Chlorophyll A (mg/g) | Chlorophyll B (mg/g) | Carotenoid (mg/g) |
|---|---|---|---|---|---|
| Diploid | 10 | 41.19 ± 1.29 c | 28.55 ± 1.89 c | 12.64 ± 2.33 c | 17.83 ± 1.47 a |
| Tetraploid | 10 | 76.37 ± 3.58 b | 48.57 ± 2.15 b | 27.80 ± 2.47 b | 14.71 ± 0.73 b |
| Octoploid | 10 | 117.80 ± 6.63 a | 76.42 ± 4.50 a | 41.38 ± 3.57 a | 14.70 ± 2.12 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl. Plants 2020, 9, 424. https://doi.org/10.3390/plants9040424
Mo L, Chen J, Lou X, Xu Q, Dong R, Tong Z, Huang H, Lin E. Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl. Plants. 2020; 9(4):424. https://doi.org/10.3390/plants9040424
Chicago/Turabian StyleMo, Lan, Junhao Chen, Xiongzhen Lou, Qiangwei Xu, Renhui Dong, Zaikang Tong, Huahong Huang, and Erpei Lin. 2020. "Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl" Plants 9, no. 4: 424. https://doi.org/10.3390/plants9040424
APA StyleMo, L., Chen, J., Lou, X., Xu, Q., Dong, R., Tong, Z., Huang, H., & Lin, E. (2020). Colchicine-Induced Polyploidy in Rhododendron fortunei Lindl. Plants, 9(4), 424. https://doi.org/10.3390/plants9040424




