Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress
Abstract
1. Introduction
2. Results
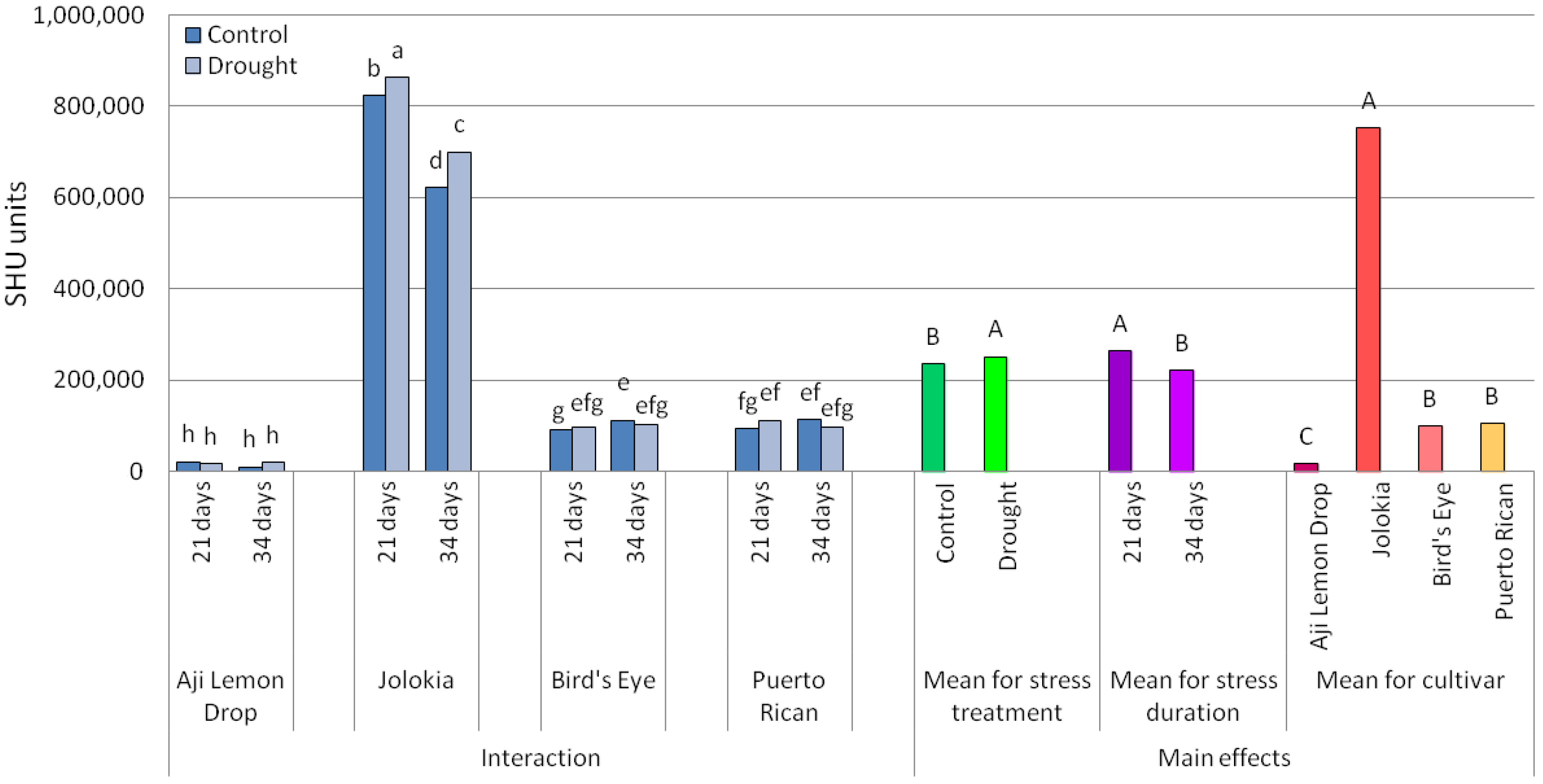
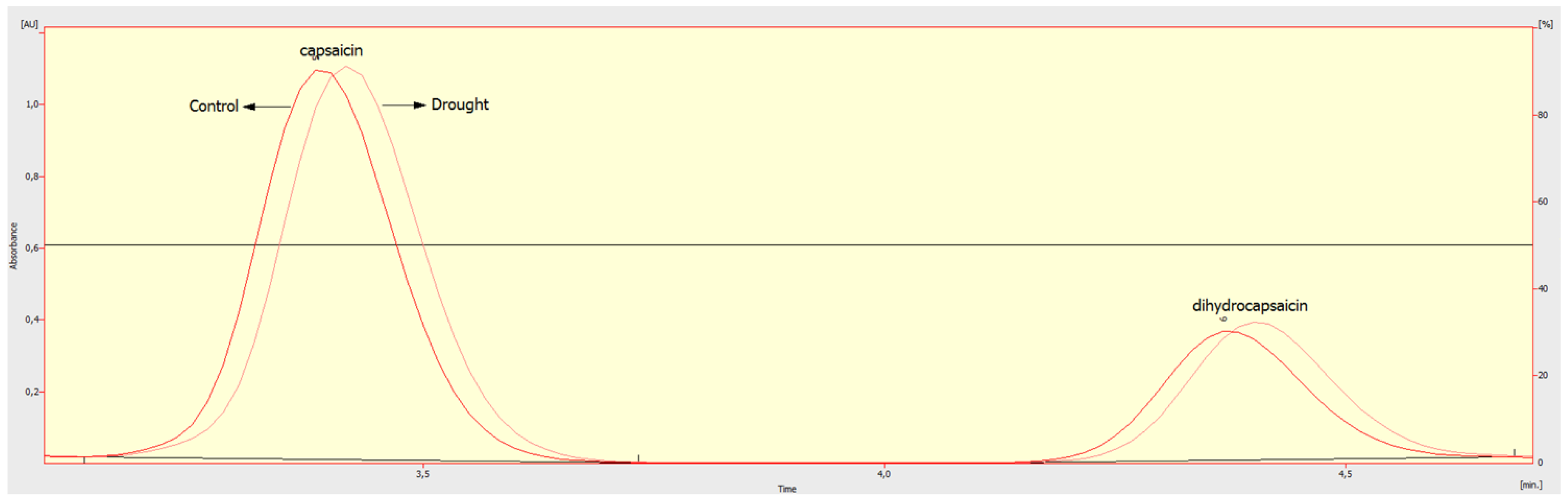
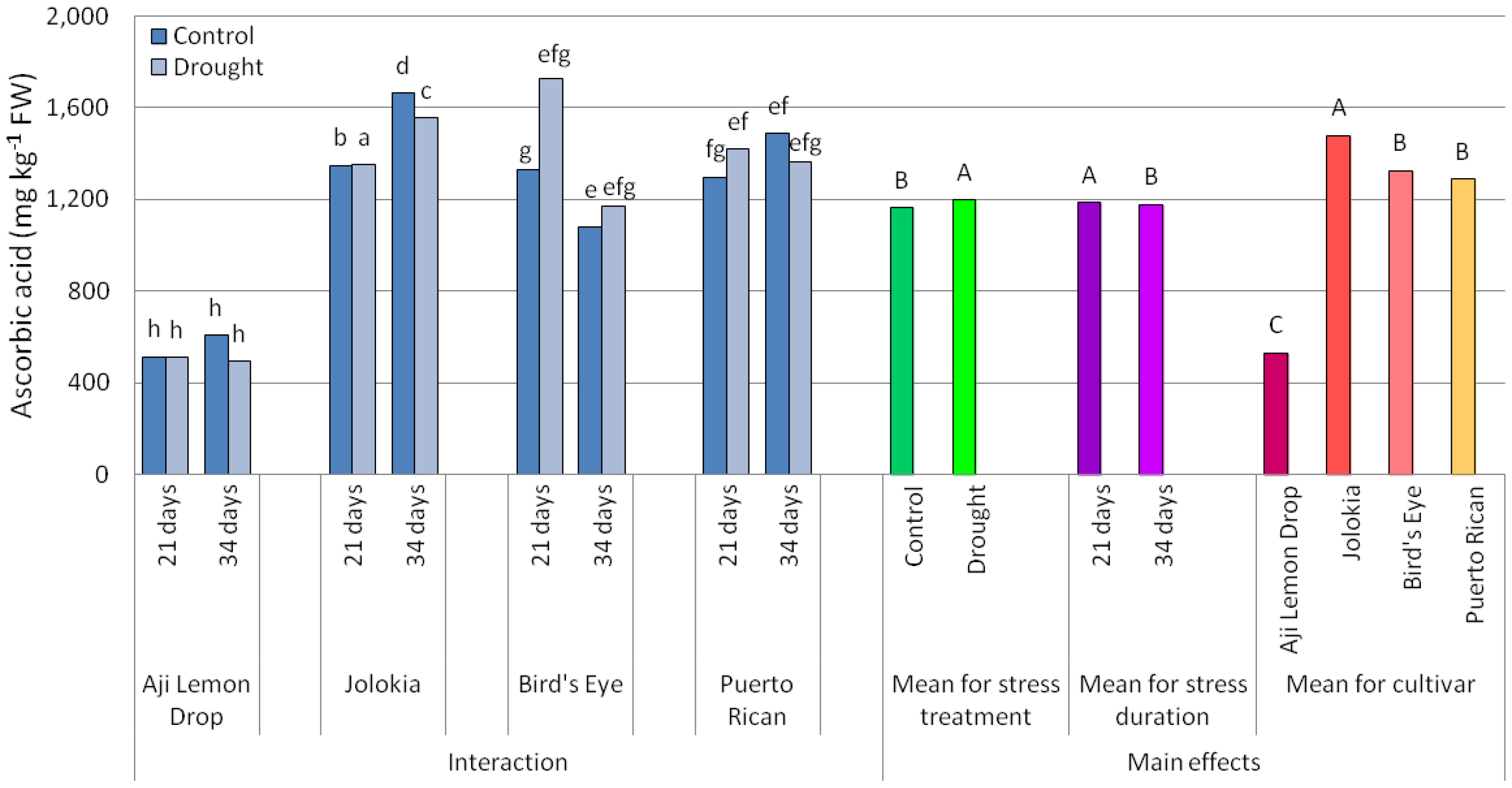
2.1. Scoville Heat Units (SHU) Values and Ascorbic Acid (AsA) Content in Chilli Pepper Fruits
2.2. Stress Parameters of Chilli Pepper Fruits and Leaves
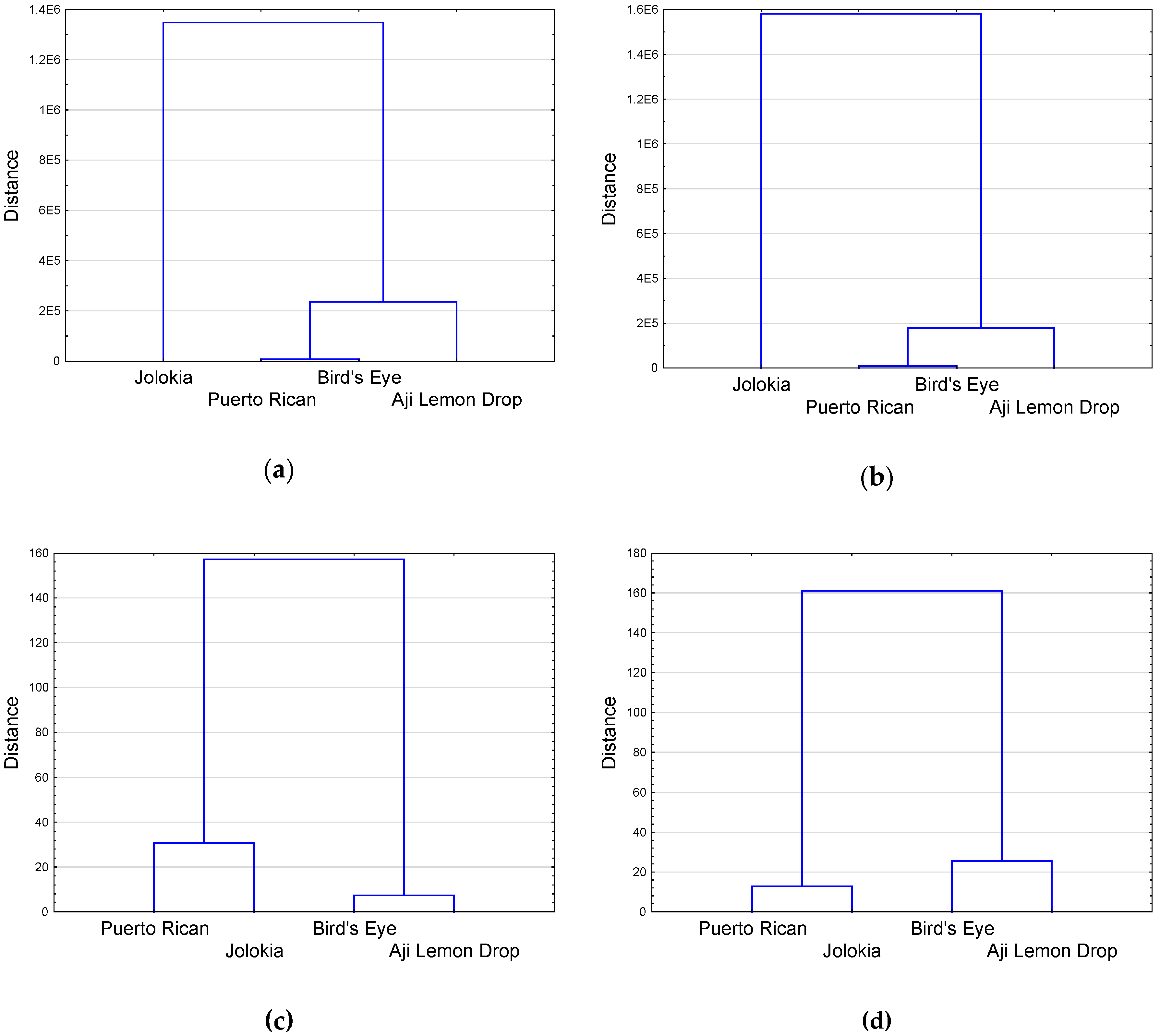
2.3. Analysis of Correlation Coefficients, Principal Component Analysis and Hierarchical Cluster Analysis of Chilli Pepper Fruit and Leaf Parameters
3. Discussion
3.1. Scoville Heat Units (SHU) Values and Ascorbic Acid (AsA) Content in Chilli Pepper Fruits
3.2. Stress Parameters of Chilli Pepper Fruits and Leaves
4. Materials and Methods
4.1. Experimental Design
4.2. Ascorbic Acid (AsA) and Scoville Heat Unit (SHU) Evaluation
4.3. Total Phenolics
4.4. Soluble Carbohydrates
4.5. DPPH˙ Radical Scavenging Activity
4.6. Malondialdehyde
4.7. Antioxidant Enzyme Assays
4.8. Statistical Data Evaluation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruiz-Lau, N.; Medina-Lara, F.; Minero-García, Y.; Zamudio-Moreno, E.; Guzmán-Antonio, A.; Echevarría-Machado, I.; Martínez-Estévez, M. Water Deficit Affects the Accumulation of Capsaicinoids in Fruits of Capsicum chinense Jacq. HortScience 2011, 46, 487–492. [Google Scholar] [CrossRef]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Primed plants do not forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch fossils and the domestication and dispersal of chilli peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Ambroszczyk, A.; Cebula, S.; Sekara, A. The effect of plant pruning on the light conditions and vegetative development of eggplant (Solanum melongena L.) in greenhouse cultivation. Veg. Crop. Res. Bull. 2008, 68, 57–70. [Google Scholar] [CrossRef]
- Ambroszczyk, A.M.; Cebula, S.; Sekara, A. The effect of shoot training on yield, fruit quality and leaf chemical composition of eggplant in greenhouse cultivation. Folia Hortic. 2008, 20, 3–15. [Google Scholar] [CrossRef]
- Perez-Grajales, M.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Content of capsaicinoids and physicochemical characteristics of Manzano hot pepper grown in greenhouse. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 119–127. [Google Scholar] [CrossRef]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of different capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography, determination of pungency and effect of high temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive characteristics and antioxidant activities of nine peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, G.A. Secondary metabolites of Capsicum species and their importance in the human diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Barbero, G.F.; de Aguiar, A.C.; Carrera, C.; Olachea, Á.; Ferreiro-González, M.; Martínez, J.; Palma, M.; Barroso, C.G. Evolution of capsaicinoids in peter pepper (Capsicum annuum var. annuum) during fruit ripening. Chem. Biodivers. 2016, 13, 1068–1075. [Google Scholar] [CrossRef]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Mardani, S.; Tabatabaei, S.H.; Pessarakli, M.; Zareabyaneh, H. Physiological responses of pepper plant (Capsicum annuum L.) to drought stress. J. Plant Nutr. 2017, 40, 1453–1464. [Google Scholar] [CrossRef]
- Ozkur, O.; Ozdemir, F.; Bor, M.; Turkan, I. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environ. Exp. Bot. 2009, 66, 487–492. [Google Scholar] [CrossRef]
- Hu, W.H.; Xiao, Y.A.; Zeng, J.J.; Hu, X.H. Photosynthesis, respiration and antioxidant enzymes in pepper leaves under drought and heat stresses. Biol. Plant. 2010, 54, 761–765. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereir, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Sziderics, A.H.; Oufir, M.; Trognitz, F.; Kopecky, D.; Matušíková, I.; Hausman, J.F.; Wilhelm, E. Organ-specific defence strategies of pepper (Capsicum annuum L.) during early phase of water deficit. Plant Cell Rep. 2010, 29, 295–305. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Aswathanarayana, R.G. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Phimchan, P.; Techawongstien, S.; Chanthai, S.; Bosland, P.W. Impact of drought stress on the accumulation of capsaicinoids in Capsicum cultivars with different initial capsaicinoid levels. HortScience 2012, 47, 1204–1209. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Chanthai, S.; Bosland, P.W. Influence of water stresses on capsaicinoid production in hot pepper (Capsicum chinense Jacq.) cultivars with different pungency levels. Food Chem. 2018, 245, 792–797. [Google Scholar] [CrossRef]
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205. [Google Scholar] [CrossRef]
- Sung, Y.; Yu-Yun, C.; Ni-Lun, T. Capsaicin biosynthesis in water-stressed hot pepper fruits. Bot. Bull. Acad. Sin. 2005, 46, 35–42. [Google Scholar]
- Guzmán, I.; Bosland, P.W. Sensory properties of chilli pepper heat—And its importance to food quality and cultural preference. Appetite 2017, 117, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Dewitt, D.; Bosland, P.W. The Complete Chile Pepper Book: A Gardener’s Guide to Choosing, Growing, Preserving, and Cooking; Timber Press: Portland, OR, USA, 2009. [Google Scholar]
- Kantar, M.B.; Anderson, J.E.; Lucht, S.A.; Mercer, K.; Bernau, V.; Case, K.A.; Le, N.C.; Frederiksen, M.K.; DeKeyser, H.C.; Wong, Z.-Z.; et al. Vitamin variation in Capsicum spp. provides opportunities to improve nutritional value of human diets. PLoS ONE 2016, 11, e0161464. [Google Scholar] [CrossRef]
- Maguire, K. RHS Red Hot Chilli Grower: The Complete Guide to Planting, Picking and Preserving Chillies; Octopus Publishing Group Ltd.: London, UK, 2015; p. 144. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Impact of environments on the accumulation of capsaicinoids in Capsicum spp. HortScience 2011, 46, 1576–1581. [Google Scholar] [CrossRef]
- Othman, Z.A.; Ahmed, Y.B.; Habila, M.A.; Ghafar, A.A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules 2011, 10, 8919–8929. [Google Scholar] [CrossRef]
- Kopta, T.; Šlosár, M.; Andrejiová, A.; Jurica, M.; Pokluda, R. The influence of genotype and season on the biological potential of chilli pepper cultivars. Folia Hortic. 2019, 31, 121–126. [Google Scholar] [CrossRef]
- Vaishnavi, B.A.; Bhoomika, H.R.; Raviraj Shetty, G.; Naveen, N.E.; Khanm, H.; Mohankumar, H.D. Evaluation of bird’s eye chilli (Capsicum frutescens L.) accessions for quality traits. J. Pharm. Phytochem. 2018, 7, 170–172. [Google Scholar]
- Tuteja, N.; Gill, S.S.; Tiburcio, A.F.; Tuteja, R. Improving Crop Resistance to Abiotic Stress; Wiley-VCH Verlag GmbH & Company KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Kaur, R.; Nayyar, H. Ascorbic acid: A potent defender against environmental stresses. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 235–287. [Google Scholar]
- Munné-Bosch, S.; Alegre, L. Drought-induced changes in the redox state of alpha-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol. 2003, 131, 1816–1825. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Guiamet, J.J.; Kiddle, G.; Pastori, G.M.; Di Cagno, R.; Theodoulou, F.L.; Foyer, C.H. Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ. 2005, 28, 1073–1081. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, S., Jr.; Libardi, S.H.; Dias, F.F.; Coutinho, J.P.; Bochi, V.C.; Rodrigues, D.; Melo, A.M.T.; Godoy, H.T. Brazilian Capsicum peppers: Capsaicinoid content and antioxidant activity. J. Sci. Food Agric. 2018, 98, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Asnin, L.; Park, S.W. Isolation and analysis of bioactive compounds in capsicum peppers. Crit. Rev. Food Sci. Nutr. 2015, 55, 254–289. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Xie, X.Y.; Liu, X.J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Vachun, M. Meteorological Data 2019, Station Lednice. University Information System of Mendel University in Brno. Available online: http://is.mendelu.cz/2019 (accessed on 6 November 2019).
- Sawant, L.; Prabhakar, B.; Pandita, N. Quantitative HPLC analysis of ascorbic acid and gallic acid in Phyllanthus emblica. J. Anal. Bioanal. Tech. 2010, 1, 111. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Method 995.03; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compound. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Bartosz, G. Druga Twarz Tlenu. Wolne Rodniki w Przyrodzie Free Radicals in Nature, 2nd ed.; PWN: Warszawa, Poland, 2009. [Google Scholar]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defense against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Zhang, Z.; Pang, X.; Duan, X.; Ji, Z.L.; Jiang, Y. Role of peroxidase in anthocyanine degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]

| ANOVA Source of Variation | SHU | Ascorbic Acid |
|---|---|---|
| Cultivar (C) | *** | *** |
| Drought stress (DS) | *** | *** |
| Drought duration (DD) | *** | *** |
| C × DS | *** | *** |
| C × DD | *** | *** |
| DS × DD | ns | *** |
| C × DS × DD | *** | *** |
| Interaction | Total Phenols (mg GAE g−1 FW) | Soluble Carbohydrates (g 100 g−1 FW) | DPPH (% Scavenging of DPPH˙) | ||||
|---|---|---|---|---|---|---|---|
| Cultivar | Irrigation Regime | Fruits | Leaves | Fruits | Leaves | Fruits | Leaves |
| Aji Lemon Drop | Control | 1.81c 1 | 0.60f | 14.63b | 3.77b | 43.4e | 34.9d |
| Drought | 1.81c | 0.82e | 12.75c | 4.04ab | 39.3e | 36.6d | |
| Jolokia | Control | 3.03a | 1.19c | 14.09b | 5.31ab | 71.9b | 90.3a |
| Drought | 3.18a | 1.39a | 15.84a | 5.98a | 90.8a | 89.8a | |
| Bird’s Eye | Control | 2.65b | 0.69f | 8.50d | 3.36b | 64.8c | 34.9d |
| Drought | 2.71b | 0.98d | 9.25d | 4.94ab | 72.6b | 50.2c | |
| Puerto Rican | Control | 2.47b | 1.30ab | 6.28e | 4.57ab | 52.2d | 78.9b |
| Drought | 2.52b | 1.18c | 8.33e | 5.21ab | 84.6a | 87.7a | |
| Main Effects | |||||||
| Irrigation regime: Control Drought | 2.49 2.55 | 0.91B 1.09A | 11.29 11.54 | 4.22 5.00 | 58.62B 71.83A | 58.0B 66.1A | |
| ns | ns | ns | |||||
| Cultivar: Aji Lemon Drop Jolokia Bird’s Eye Puerto Rican | 1.81D 3.11A 2.68B 2.49C | 0.71D 1.29A 0.83C 1.23B | 7.51D 14.96A 8.87C 13.70B | 3.90ns 5.65ns 4.15ns 4.96ns | 41.4C 81.35A 68.7B 68.7B | 35.8D 90.0A 42.5C 84.2B | |
| Interaction | Catalase (μmol H2O2 min−1 g−1 FW) | Ascorbate Peroxidase (µg AsA min−1 g−1 FW) | Guaiacol Peroxidase (μmol of tetraguaiacol min−1 g−1 FW) | Malondialdehyde (μmol g−1 FW) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cultivar | Irrigation Regime | Fruits | Leaves | Fruits | Leaves | Fruits | Leaves | Fruits | Leaves |
| Aji Lemon Drop | Control | 6.006b 1 | 7.19d | 0.173b | 0.251c | 0.472a | 1.285c | 51.7b | 53.9e |
| Drought | 9.294a | 9.95c | 0.221a | 0.361a | 0.526a | 1.739b | 61.4a | 87.3b | |
| Jolokia | Control | 0.558c | 8.17d | 0.127c | 0.202d | 0.337b | 0.998c | 41.9de | 53.2e |
| Drought | 0.641c | 10.30b | 0.146c | 0.267c | 0.472a | 1.179c | 44.7c | 68.7c | |
| Bird’s Eye | Control | 0.850c | 6.84d | 0.030e | 0.077f | 0.129d | 3.165b | 38.9e | 50.5e |
| Drought | 1.971c | 9.40c | 0.168b | 0.184e | 0.233c | 1.200c | 43.2d | 92.3a | |
| Puerto Rican | Control | 0.644c | 10.03b | 0.030e | 0.201d | 0.117d | 1.216c | 43.4d | 39.5f |
| Drought | 2.228c | 12.11a | 0.079d | 0.294b | 0.270c | 3.165a | 46.9c | 62.1d | |
| Main Effects | |||||||||
| Irrigation regime: Control Drought | 2.014B 3.533A | 8.06B 10.4A | 0.090B 0.153A | 0.183B 0.277A | 0.264B 0.375A | 1.170B 1.824A | 44.0B 49.1A | 49.3B 77.6A | |
| Cultivar: Aji Lemon Drop Jolokia Bird’s Eye Puerto Rican | 7.650A 0.599B 1.411B 1.436B | 8.57B 9.24B 8.12C 11.07A | 0.197A 0.137B 0.099C 0.054D | 0.306A 0.235B 0.131C 0.248B | 0.499A 0.404B 0.193C 0.181C | 1.512B 1.089C 2.182A 1.205C | 56.6A 43.3C 41.1D 45.2B | 70.58A 60.96B 71.45A 50.81C | |
| SHU | AsA | TP | SC | DPPH | APX | GPX | CAT | MDA | |
|---|---|---|---|---|---|---|---|---|---|
| SHU | 1.000 | ||||||||
| AsA | 0.706 ***,1 | 1.000 | |||||||
| TP | 0.800 *** | 0.886 *** | 1.000 | ||||||
| SC | 0.550 ** | −0.153 | 0.037 | 1.000 | |||||
| DPPH | 0.634 *** | 0.750 *** | 0.842 *** | 0.036 | 1.000 | ||||
| APX | 0.022 | −0.530 ** | −0.350 | 0.678 *** | −0.248 | 1.000 | |||
| GPX | 0.213 | −0.444 * | −0.317 | 0.864 *** | −0.170 | 0.841 *** | 1.000 | ||
| CAT | −0.531 ** | −0.894 *** | −0.854 *** | 0.293 | −0.701 *** | 0.694 *** | 0.654 ** | 1.000 | |
| MDA | −0.386 | −0.748 *** | −0.763 *** | 0.339 | −0.588 ** | 0.706 *** | 0.941 *** | 0.283 | 1.000 |
| TP | SC | DPPH | APX | GPX | CAT | MDA | |
|---|---|---|---|---|---|---|---|
| TP | 1.000 | ||||||
| SC | 0.793 ***,1 | 1.000 | |||||
| DPPH | 0.933 *** | 0.774 *** | 1.000 | ||||
| APX | 0.167 | 0.236 | 0.138 | 1.000 | |||
| GPX | −0.567 ** | −0.615 ** | −0.594 ** | −0.503 * | 1.000 | ||
| CAT | 0.636 ** | 0.555 ** | 0.563 ** | 0.556 ** | −0.476 * | 1.000 | |
| MDA | −0.123 | 0.105 | −0.277 | 0.412 ** | −0.124 | 0.283 | 1.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopta, T.; Sekara, A.; Pokluda, R.; Ferby, V.; Caruso, G. Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress. Plants 2020, 9, 364. https://doi.org/10.3390/plants9030364
Kopta T, Sekara A, Pokluda R, Ferby V, Caruso G. Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress. Plants. 2020; 9(3):364. https://doi.org/10.3390/plants9030364
Chicago/Turabian StyleKopta, Tomas, Agnieszka Sekara, Robert Pokluda, Vojtech Ferby, and Gianluca Caruso. 2020. "Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress" Plants 9, no. 3: 364. https://doi.org/10.3390/plants9030364
APA StyleKopta, T., Sekara, A., Pokluda, R., Ferby, V., & Caruso, G. (2020). Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress. Plants, 9(3), 364. https://doi.org/10.3390/plants9030364







