Dynamic of Phenolic Compounds, Antioxidant Activity, and Yield of Rhubarb under Chemical, Organic and Biological Fertilization
Abstract
1. Introduction
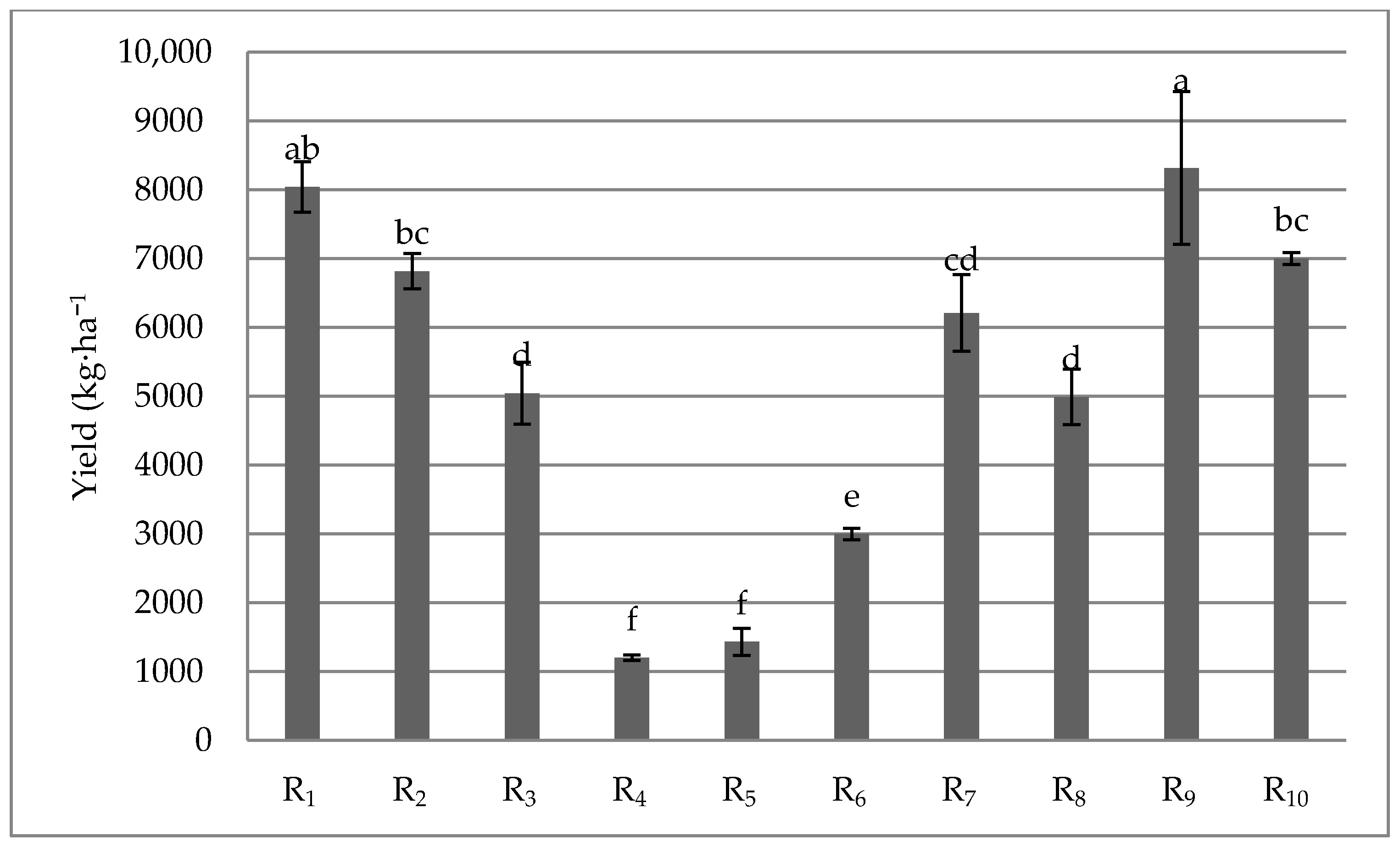
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Experimental Protocol
3.3. Biometric Measurements
3.4. Samples Preparation
3.5. HPLC-MS Analysis of Phenolic Compounds
3.6. Antioxidant Activity Test
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jintao, X.; Yongli, S.; Liming, Y.; Quanwei, Y.; Chunyan, L.; Xingyy, C.; Yun, J. Near-infrared spectroscopy for rapid and simultaneous determination offive main active components in rhubarb of different geographical originsand processing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Han, J.; Chen, H.; Zheng, J.; Guo, D. Analysis of phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Heaney, R.P.; Nickel, K.P.; Packard, P.I. Calcium bioavailability from high oxalate vegetables: Chinese vegetables, sweet potatoes and rhubarb. J. Food Sci. 1997, 62, 524–525. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Total phenolic content and antioxidant properties of commonly consumed vegetables grown in California. Lwt-Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.; Harden, L.; Pantoja, A.; Kuhl, J.C. Antioxidant activity, phenolic and anthocyanin contents of various rhubarb (Rheum spp.) varieties. Int. J. Food Sci. Technol. 2012, 48, 172–178. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, G.; Shen, H.M.; Chung, M.C.M.; Ong, C.N. Anti-cancer properties of anthraquinones from rhubarb. Med. Res. Rev. 2017, 27, 609–630. [Google Scholar] [CrossRef]
- Raudsepp, P.; Koskar, J.; Anton, D.; Meremäea, K.; Kapp, K.; Laurson, P.; Bleive, U.; Kaldmäe, H.; Roasto, M.; Püssa, T. Antibacterial and antioxidative properties of different parts of garden rhubarb, black currant, chokeberry and blue honeysuckle. J. Sci. Food Agric. 2019, 99, 2311–2320. [Google Scholar] [CrossRef]
- Mi, W.H.; Wu, L.H.; Brookes, P.C.; Liu, Y.L.; Zhang, X.; Yang, X. Changes in soil organic carbon fractions under integrated management systems in a low-productivity paddy soil given different organic amendments and chemical fertilizers. Soil Till. Res. 2016, 163, 64–70. [Google Scholar] [CrossRef]
- Nicoulaud, B.A.L.; Bloom, A.J. Absorption and assimilation of foliarly applied urea in tomato. J. Am. Soc. Hortic. Sci. 1996, 121, 1117–1121. [Google Scholar] [CrossRef]
- Cojocaru, A.; Munteanu, N.; Petre, B.A.; Stan, T.; Teliban, G.C.; Vintu, C.; Stoleru, V. Biochemical and production of rhubarb under growing technological factors. Rev. Chim.-Bucharest. 2019, 70, 2000–2003. [Google Scholar] [CrossRef]
- Stoleru, V.; Munteanu, N.; Istrate, A. Perception towards organic vs. conventional products in Romania. Sustainability 2019, 11, 2394. [Google Scholar] [CrossRef]
- Conti, S.; Villari, G.; Faugno, S.; Melchionna, G.; Somma, S.; Caruso, G. Effects of organic vs. conventional farming system on yield and quality of strawberry grown as an annual or biennial crop in southern Italy. Sci. Hortic. 2014, 180, 63–71. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). Lwt-Food Sci. Technol. 2020, 118, 108775. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; He, M.F.; Ma, S.C.; But, P.P.H. Anti-angiogenic effects of rhubarb and its anthraquinone derivatives. J. Ethnopharmacol. 2009, 121, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Etxeberria, U.; Taminiau, B.; Daube, G.; Van, H.M.; Everard, A.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol. Nutr. Food Res. 2016, 61, 1500899. [Google Scholar] [CrossRef] [PubMed]
- Püssa, T.; Raudsepp, P.; Kuzina, K.; Raal, A. Polyphenolic composition of roots and petioles of Rheum rhaponticum L. Phytochem. Anal. 2009, 20, 98–103. [Google Scholar] [CrossRef]
- Dregus, M.; Engel, K.H. Volatile constituents of uncooked rhubarb (Rheum rhabarbarum L.) stalks. J. Agric. Food Chem. 2003, 51, 6530–6536. [Google Scholar] [CrossRef]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2001, 59, 12361–12367. [Google Scholar] [CrossRef]
- Santos, E.R.M.; Oliveira, H.N.M.; Oliveira, E.J.; Azevedo, S.H.G.; Jesus, A.A.; Medeiros, A.M.; Dariva, C.; Sousa, E.M.B.D. Supercritical fluid extraction of Rumex acetosa L. roots: Yield, composition, kinetics, bioactive evaluation and comparison with conventional techniques. J. Supercrit. Fluid. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Tolra, R.P.; Poschenrieder, C.; Luppi, B.; Barcelo, J. Aluminium-induced changes in the profiles of both organic acids and phenolic substances underlie Al tolerance in Rumex acetosa L. Environ. Exp. Bot. 2005, 54, 231–238. [Google Scholar] [CrossRef]
- Kucekova, Z.; Mlcek, J.; Humpolicek, P.; Rop, O.; Valasek, P.; Saha, P. Phenolic compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and their antiproliferative effects. Molecules 2011, 16, 9207–9217. [Google Scholar] [CrossRef]
- Savran, A.; Zengin, G.; Aktumsek, A.; Mocan, A.; Glamoclija, J.; Ciric, A.; Sokovic, M. Phenolic compounds and biological effects of edible Rumex scutatus and Pseudosempervivum sempervivum: Potential sources of natural agents with health benefits. Food Funct. 2016, 7, 3252–3262. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; Gonzalez, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Coruh, I.; Gormez, A.A.; Ercisli, S.; Sengul, M. Total phenolic content, antioxidant, and antibacterial activity of Rumex crispus grown wild in Turkey. Pharm. Biol. 2008, 46, 634–638. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Antioxidant and antibacterial activities of Rumex japonicus Houtt. aerial parts. Biol. Pharm. Bull. 2005, 28, 2225–2230. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. leaves. J. Med. Plant Res. 2011, 5, 2755–2765. [Google Scholar]
- Ahmad, S.; Ullah, F.; Ayaz, M.; Sadiq, A.; Imran, M. Antioxidant and anticholinesterase investigations of Rumex hastatus D. Don: Potential effectiveness in oxidative stress and neurological disorders. Biol. Res. 2015, 48, 20. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Singh, B.; Vats, S.K.; Sood, R.P. Total phenols, tannins and condensed tannins in different parts of Rumex hastatus. Bioresour. Technol. 1993, 45, 69–71. [Google Scholar] [CrossRef]
- Jimoh, F.O.; Adedapo, A.A.; Aliero, A.A.; Afolayan, A.J. Polyphenolic contents and biological activities of Rumex ecklonianus. Pharm Biol. 2008, 46, 333–340. [Google Scholar] [CrossRef]
- Mhalla, D.; Bouaziz, A.; Ennouri, K.; Chawech, R.; Smaoui, S.; Jarraya, R.; Tounsi, S.; Trigui, M. Antimicrobial activity and bioguided fractionation of Rumex tingitanus extracts for meat preservation. Meat Sci. 2017, 125, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Isbilir, S.S.; Sagiroglu, A. Total phenolic content, antiradical and antioxidant activities of wild and cultivated Rumex acetosella L. extracts. Biol. Agric. Hortic. 2013, 29, 219–226. [Google Scholar] [CrossRef]
- Spinola, V.; Llorent-Martinez, E.J.; Castilho, P.C. Antioxidant polyphenols of Madeira sorrel (Rumex maderensis): How do they survive to in vitro simulated gastrointestinal digestion? Food Chem. 2018, 259, 105–112. [Google Scholar] [CrossRef]
- Feduraev, P.; Chupakhina, G.; Maslennikov, P.; Tacenko, N.; Skrypnik, L. Variation in phenolic compounds content and antioxidant activity of different plant organs from Rumex crispus L. and Rumex obtusifolius L. at different growth stages. Antioxidants 2019, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Celebi, S.Z.; Demir, S.; Celebi, R.; Durak, E.D.; Yilmaz, I.H. The effect of Arbuscular Mycorrhizal Fungi (AMF) applications on the silage maize (Zea mays L.) yield in different irrigation regimes. Eur. J. Soil Biol. 2010, 46, 302–305. [Google Scholar] [CrossRef]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Bello, O.M.; Fasinu, P.S.; Bello, O.E.; Ogbesejana, A.B.; Adetunji, C.O.; Dada, A.O.; Ibitoye, O.S.; Aloko, S.; Oguntoye, O.S. Wild vegetable Rumex acetosa Linn.: Its ethnobotany, pharmacology and phytochemistry—A review. S. Afr. J. Bot. 2019, 125, 149–160. [Google Scholar] [CrossRef]
- Christian, K.R.; Jackson, J.C. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J. Food Compos. Anal. 2009, 22, 663–667. [Google Scholar] [CrossRef]
- Ferrol, N.; Pérez-Tienda, J. Coordinated Nutrient Exchange in Arbuscular Mycorrhiza. In Mycorrhizas—Functional Processes and Ecological Impact; Azcón-Aguilar, C., Barea, J., Gianinazzi, S., Gianinazzi-Pearson, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 73–87. [Google Scholar]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant. Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef]
- Popovic, B.M.; Stajner, D.; Zdero-Pavlovic, R.; Tumbas-Saponjac, V.; Canadanovic-Brunet, J.; Orlovic, S. Water stress induces changes in polyphenol profile and antioxidant capacity in poplar plants (Populus spp.). Plant. Physiol. Biochem. 2016, 105, 242–250. [Google Scholar] [CrossRef]
- Dimitriu, D.C.; Stoleru, V.; Corciova, A.; Vlase, L.; Stan, T.; Jitareanu, A.; Munteanu, N.; Rotaru, L.; Patras, A. P-coumaric acid content in sweet pepper under farming methods. Environ. Eng. Manag. J. 2016, 15, 1841–1848. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and nutritional quality of vesuvian piennolo tomato pdo as affected by farming system and biostimulant application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Chanthini, K.M.P.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Palanikani, R.; Sundar, N.S.; Sivanesh, H.; Soranam, R.; Senthil-Nathan, S. Sustainable agronomic strategies for enhancing the yield and nutritional quality of wild tomato, Solanum Lycopersicum (l) var Cerasiforme Mill. Agronomy 2019, 9, 311. [Google Scholar] [CrossRef]
- Kim, G.D.; Lee, Y.S.; Cho, J.Y.; Lee, Y.H.; Choi, K.J.; Lee, Y.; Han, T.H.; Lee, S.H.; Park, K.H.; Moon, J.H. Comparison of the content of bioactive substances and the inhibitory effects against rat plasma oxidation of conventional and organic hot peppers (Capsicum annuum L.). J. Agric. Food Chem. 2010, 58, 12300–12306. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, C.Y.; Farmer, J.; Sun, J.D. Effects of bio-organic fertilizer on pepper growth and Fusarium wilt biocontrol. Sci. Hortic. 2015, 193, 114–120. [Google Scholar] [CrossRef]
- Aliyu, L. Effect of organic and mineral fertilizers on growth, yield and composition of pepper (Capsicum annuum L.). Biol. Agric. Hortic. 2000, 18, 29–36. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodriguez, M.A.; Vazquez-Oderiz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria x ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Rawat, S.; Jugran, A.K.; Bhatt, I.D.; Rawal, R.S. Influence of the growth phenophases on the phenolic composition and anti-oxidant properties of Roscoea procera Wall. in western Himalaya. J. Food Sci. Technol. 2018, 55, 578–585. [Google Scholar] [CrossRef]
- Francaviglia, R.; Bruno, A.; Falcucci, M.; Farina, R.; Renzi, G.; Russo, D.E.; Sepe, L.; Neri, U. Yields and quality of Cynara cardunculus L. wild and cultivated cardoon genotypes. A case study from a marginal land in Central Italy. Eur. J. Agron. 2016, 72, 10–19. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and bioactive compounds characterization of plant parts from Cynara cardunculus L. (Asteraceae) cultivated in central Greece. Front. Plant. Sci. 2018, 9, 459. [Google Scholar] [CrossRef]
- Farhat, M.B.; Jordan, M.J.; Chaouch-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Phenophase effects on sage (Salvia officinalis L.) yield and composition of essential oil. J. Appl. Res. Med. Aromat. Plants. 2016, 3, 87–93. [Google Scholar] [CrossRef]
- Stoleru, V.; Munteanu, N.; Stan, T.; Ipatioaie, C.; Cojocaru, A.; Butnariu, M. Effects of production system on the content of organic acids in Bio rhubarb (Rheum rhabarbarum L.). Rom. Biotechnol. Lett. 2019, 24, 184–192. [Google Scholar] [CrossRef]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crișan, O.; Gheldiu, A.-M.; Crișan, G. Phytochemical Characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and Their Antioxidant and Antimicrobial Properties. Int. J. Mol. Sci. 2015, 16, 21109–21127. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activitiesof Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi–Dumitrescu, R.; Tilea, I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Putnoky, S.; Caunii, A.; Butnariu, M. Study on the stability and antioxidant effect of the Allium ursinum watery extract. Chem. Cent. J. 2013, 7, 21. [Google Scholar] [CrossRef]
- Lotfi, S.; Kordsardouei, H.; Oloumi, H. Study of total phenolic content and antioxidant capacity of the ethanolic extracts of two medicinal plants, Hibiscus sabdariffa L. and Amaranthus caudatus L. Banat’s J. Biotechnol. 2019, 10, 66–74. [Google Scholar] [CrossRef]
- Butnariu, M.; Caunii, A.; Putnoky, S. Reverse phase chromatographic behaviour of major components in Capsicum Annuum extract. Chem. Cent. J. 2012, 6, 146. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved Abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Caruso, G.; Conti, S.; Villari, G.; Borrelli, C.; Melchionna, G.; Minutolo, M.; Russo, G.; Amalfitano, C. Effects of transplanting time and plant density on yield, quality and antioxidant content of onion (Allium cepa L.) in southern Italy. Sci. Hortic. 2014, 166, 111–120. [Google Scholar] [CrossRef]
- Ouis, N.; Hariri, A. Phytochemical analysis and antioxidant activity of the flavonoids extracts from pods of Ceratonia siliqua L. Banat’s J. Biotechnol. 2017, 8, 93–104. [Google Scholar] [CrossRef]
| Treatment | Total Phenols Content—TPC (mg GAE·g−1 d.w.) | Antioxidant Activity—AC (mmol Trolox·g−1 d.w.) |
|---|---|---|
| Fertilization | ||
| Ch | 1320.62 ± 111.71 b | 478.48 ± 41.43 c |
| Mo | 1605.03 ± 154.45 a | 877.07 ± 86.31 a |
| Og | 1477.95 ± 102.31 ab | 728.05 ± 50.68 b |
| C | 992.74 ± 87.35 c | 313.17 ± 26.78 d |
| Harvest time | ||
| R1 | 1345.63 ± 62.52 c | 582.73 ± 51.14 |
| R2 | 1364.14 ± 47.86 c | 589.75 ± 48.89 c |
| R3 | 1315.55 ± 80.32 cd | 565.12 ± 48.75 c |
| R4 | 2450.83 ± 287.23 a | 1079.76 ± 165.65 a |
| R5 | 1819.34 ± 213.24 b | 834.44 ± 147.00 b |
| R6 | 1259.78 ± 240.31 cd | 586.83 ± 147.35 c |
| R7 | 1164.37 ± 144.00 cd | 515.47 ± 69.14 c |
| R8 | 1015.14 ± 109.14 d | 467.39 ± 73.83 c |
| R9 | 1085.13 ± 77.13 cd | 477.34 ± 52.5 c |
| R10 | 670.92 ± 50.3 e | 293.09 ± 31.19 d |
| Harvest Time | Treatment | |||
|---|---|---|---|---|
| Ch | Mo | Og | C | |
| R1 | 1431.34 ± 119.09 ab | 1427.73 ± 125.68 cd | 1332.73 ± 104.3 bc | 1190.73 ± 161.28 b |
| R2 | 1449.88 ± 81.00 ab | 1450.65 ± 122.27 cd | 1324.53 ± 90.65 bc | 1231.48 ± 60.55 b |
| R3 | 1610.11 ± 186.78 a | 1329.76 ± 161.31 cd | 1229.71 ± 69.75 bcd | 1092.62 ± 64.38 bc |
| R4 | 1738.26 ± 144.62 a | 1915.12 ± 168.59 bc | 3966.56 ± 310.41 a | 2183.38 ± 295.73 a |
| R5 | 1521.36 ± 85.00 a | 2954.52 ± 249.02 a | 1627.58 ± 111.39 b | 1173.89 ± 57.72 b |
| R6 | 901.42 ± 104.57 bc | 2569.88 ± 311.75 b | 687.72 ± 39.01 d | 880.10 ± 51.86 bcd |
| R7 | 1704.53 ± 141.82 a | 1285.07 ± 113.13 | 1218.81 ± 95.38 bcd | 449.08 ± 60.82 d |
| R8 | 829.51 ± 46.34 c | 1364.39 ± 115.00 cd | 1324.28 ± 90.63 bc | 542.37 ± 26.67 cd |
| R9 | 1188.14 ± 137.83 abc | 1092.31 ± 132.51 cd | 1310.19 ± 74.32 bcd | 749.87 ± 44.18 bcd |
| R10 | 831.59 ± 70.09 c | 660.88 ± 45.23 d | 757.35 ± 37.24 cd | 433.86 ± 50.33 d |
| Harvest Time | Treatment | |||
|---|---|---|---|---|
| Ch | Mo | Og | C | |
| R1 | 518.60 ± 43.15 bc | 780.18 ± 68.68 cd | 656.52 ± 51.38 bc | 375.62 ± 50.88 b |
| R2 | 525.32 ± 29.35 b | 792.70 ± 66.81 cd | 652.48 ± 44.66 bc | 388.48 ± 19.10 b |
| R3 | 583.37 ± 67.67 a | 726.64 ± 88.15 cd | 605.77 ± 34.36 bcd | 344.68 ± 20.31 bc |
| R4 | 629.80 ± 52.40 a | 1046.51 ± 92.13 bc | 1953.97 ± 152.91 a | 688.76 ± 93.29 a |
| R5 | 551.22 ± 30.80 a | 1614.49 ± 136.08 a | 801.76 ± 54.87 b | 370.31 ± 18.21 b |
| R6 | 326.60 ± 37.89 bc | 1404.31 ± 170.36 ab | 338.78 ± 19.22 d | 277.63 ± 16.36 bcd |
| R7 | 617.58 ± 51.38 a | 702.22 ± 61.82 cd | 600.40 ± 46.99 bcd | 141.67 ± 19.19 d |
| R8 | 300.55 ± 16.79 c | 745.57 ± 62.84 cd | 652.35 ± 44.65 bc | 171.09 ± 8.41 cd |
| R9 | 430.49 ± 49.94 bc | 596.89 ± 72.41 cd | 645.41 ± 36.61 bcd | 236.55 ± 13.94 bcd |
| R10 | 301.30 ± 34.95 c | 361.14 ± 43.81 d | 373.08 ± 21.16 cd | 136.86 ± 8.06 d |
| Treatment | P-Coumaric Acid (µg·g−1) | Ferulic Acid (µg·g−1) | Isoquercitrin (µg·g−1) | Rutozid (µg·g−1) | Quercetrol (µg·g−1) |
|---|---|---|---|---|---|
| Fertilization | |||||
| Ch | 9.86 ± 0.87 c | 21.67 ± 1.76 c | 37.04 ± 3.44 c | 473.2 ± 37.97 a | 16.55 ± 1.28 b |
| Mo | 15.46 ± 1.37 a | 31.45 ± 2.98 a | 78.65 ± 5.58 a | 479.08 ± 39.48 a | 20.20 ± 1.87 a |
| Og | 11.03 ± 0.84 bc | 22.23 ± 1.71 c | 61.82 ± 4.30 b | 490.97 ± 42.42 a | 15.94 ± 1.41 b |
| C | 11.58 ± 0.98 b | 27.06 ± 2.51 b | 68.2 ± 6.22 b | 372.57 ± 37.89 b | 11.17 ± 0.96 c |
| Harvest time | |||||
| R1 | 6.43 ± 0.41 e | 16.46 ± 0.68 d | 47.26 ± 6.48 b | 491.69 ± 21.68 cd | 11.22 ± 0.78 c |
| R2 | 6.72 ± 0.36 e | 16.76 ± 0.81 d | 48.66 ± 6.39 b | 497.92 ± 19.07 c | 11.38 ± 0.70 c |
| R3 | 9.12 ± 1.11 cde | 25.55 ± 3.89 bc | 50.93 ± 6.58 b | 462.48 ± 31.60 cd | 11.73 ± 0.69 bc |
| R4 | 18.45 ± 2.52 b | 24.53 ± 1.88 c | 75.58 ± 9.41 a | 904.12 ± 110.43 a | 21.09 ± 1.20 a |
| R5 | 8.22 ± 0.92 de | 15.69 ± 0.88 d | 56.32 ± 6.51 b | 666.61 ± 65.75 b | 12.28 ± 1.13 bc |
| R6 | 10.32 ± 1.32 cd | 31.37 ± 1.82 b | 84.83 ± 16.41 a | 382.32 ± 76.61 de | 15.31 ± 1.76 b |
| R7 | 23.57 ± 4.04 a | 38.44 ± 3.56 a | 58.63 ± 8.76 b | 344.47 ± 54.88 e | 19.71 ± 3.00 a |
| R8 | 7.61 ± 0.95 de | 39.96 ± 3.20 a | 79.44 ± 11.55 a | 281.38 ± 30.47 ef | 13.94 ± 1.61 bc |
| R9 | 17.25 ± 1.55 b | 26.30 ± 1.53 bc | 80.21 ± 7.03 a | 312.55 ± 20.48 e | 21.64 ± 1.51 a |
| R10 | 12.13 ± 1.24 c | 20.99 ± 0.93 cd | 32.43 ± 1.36 c | 196.02 ± 16.67 f | 21.37 ± 2.63 a |
| Treatment | P-Coumaric Acid (µg·g−1) | Ferulic Acid (µg·g−1) | Isoquercitrin (µg·g−1) | Rutozid (µg·g−1) | Quercetrol (µg·g−1) |
|---|---|---|---|---|---|
| Interaction of factors | |||||
| Ch × R1 | 5.84 ± 0.49 ns | 14.02 ± 0.78 ns | 26.12 ± 2.17 b | 547.04 ± 42.81 ns | 12.12 ± 1.64 ab |
| Mo × R1 | 8.22 ± 0.73 ns | 18.50 ± 1.56 ns | 62.36 ± 5.49 a | 454.58 ± 61.57 ns | 12.16 ± 0.68 ab |
| Og × R1 | 5.96 ± 0.47 ns | 16.44 ± 1.12 ns | 29.22 ± 2.29 b | 478.24 ± 26.72 ns | 12.98 ± 1.09 a |
| C × R1 | 5.68 ± 0.77 ns | 16.86 ± 0.83 ns | 71.34 ± 9.66 a | 486.90 ± 41.04 ns | 7.64 ± 0.52 b |
| Ch × R2 | 6.58 ± 0.37 ab | 14.52 ± 1.69 ns | 26.28 ± 1.47 b | 553.40 ± 37.88 ns | 12.20 ± 0.60 a |
| Mo × R2 | 8.40 ± 0.71 a | 18.80 ± 2.28 ns | 64.02 ± 5.40 a | 461.20 ± 22.68 ns | 12.32 ± 1.02 a |
| Og × R2 | 6.06 ± 0.42 b | 16.68 ± 0.95 ns | 30.54 ± 2.09 b | 473.26 ± 39.38 ns | 12.96 ± 1.14 a |
| C × R2 | 5.86 ± 0.29 b | 17.02 ± 1.00 ns | 73.80 ± 3.63 a | 503.84 ± 44.35 ns | 8.04 ± 0.63 b |
| Ch × R3 | 7.62 ± 0.43 bc | 24.28 ± 1.90 b | 28.58 ± 3.32 b | 607.96 ± 47.58 a | 12.28 ± 1.66 ns |
| Mo × R3 | 14.84 ± 0.87 a | 45.52 ± 6.17 a | 68.66 ± 5.37 a | 376.38 ± 50.98 b | 12.28 ± 0.69 ns |
| Og × R3 | 8.82 ± 0.78 b | 17.20 ± 0.96 b | 31.66 ± 4.29 b | 429.82 ± 24.01 ab | 13.38 ± 1.13 ns |
| C × R3 | 5.20 ± 0.41 c | 15.18 ± 1.28 b | 74.82 ± 4.18 a | 435.76 ± 36.73 ab | 8.98 ± 0.61 ns |
| Ch × R4 | 12.42 ± 1.68 bc | 19.22 ± 1.32 b | 37.82 ± 3.19 c | 646.56 ± 44.25 b | 18.88 ± 0.93 ns |
| Mo × R4 | 22.06 ± 1.23 ab | 33.38 ± 1.64 a | 65.56 ± 4.49 b | 599.06 ± 29.45 b | 25.50 ± 2.96 ns |
| Og × R4 | 10.02 ± 0.78 c | 21.26 ± 2.47 b | 121.04 ± 5.95 a | 1442.24 ± 167.31 a | 21.08 ± 2.56 ns |
| C × R4 | 29.28 ± 3.97 a | 24.28 ± 2.95 ab | 77.90 ± 9.04 b | 928.62 ± 112.65 b | 18.88 ± 1.07 ns |
| Ch × R5 | 8.82 ± 0.49 ab | 12.16 ± 0.69 b | 31.66 ± 3.84 c | 578.28 ± 32.80 b | 12.28 ± 0.72 b |
| Mo × R5 | 12.42 ± 1.68 a | 15.18 ± 1.26 ab | 87.14 ± 4.94 a | 1017.68 ± 69.65 a | 17.78 ± 1.57 a |
| Og × R5 | 6.42 ± 0.36 b | 18.22 ± 1.61 a | 44.00 ± 2.59 c | 584.22 ± 28.72 b | 10.08 ± 0.79 b |
| C × R5 | 5.20 ± 0.44 b | 17.20 ± 1.34 ab | 62.48 ± 3.49 b | 486.24 ± 56.41 b | 8.98 ± 1.21 b |
| Ch × R6 | 5.20 ± 0.46 b | 24.28 ± 3.29 b | 40.92 ± 3.45 b | 296.22 ± 35.93 b | 14.48 ± 1.21 b |
| Mo × R6 | 7.62 ± 0.60 b | 37.44 ± 2.09 a | 124.12 ± 8.50 a | 806.88 ± 67.13 a | 24.40 ± 2.15 a |
| Og × R6 | 14.84 ± 2.01 a | 30.36 ± 2.56 ab | 25.50 ± 1.25 b | 198.24 ± 17.45 b | 11.18 ± 0.88 b |
| C × R6 | 13.64 ± 1.2 a | 33.38 ± 2.28 ab | 148.78 ± 17.26 a | 227.94 ± 17.84 b | 11.18 ± 1.51 b |
| Ch × R7 | 17.24 ± 0.96 b | 30.36 ± 1.49 b | 37.82 ± 4.59 bc | 602.02 ± 81.54 a | 33.20 ± 1.85 a |
| Mo × R7 | 46.14 ± 3.89 a | 51.58 ± 5.99 a | 56.32 ± 3.20 b | 322.94 ± 18.04 b | 23.30 ± 1.96 b |
| Og × R7 | 16.04 ± 1.10 b | 27.32 ± 2.14 b | 105.64 ± 6.22 a | 331.86 ± 27.97 b | 15.58 ± 1.06 c |
| C × R7 | 14.84 ± 0.73 b | 44.50 ± 6.03 ab | 34.74 ± 2.72 c | 121.06 ± 8.29 c | 6.78 ± 0.34 d |
| Ch × R8 | 4.00 ± 0.22 b | 27.32 ± 2.27 b | 31.66 ± 4.29 c | 275.44 ± 13.54 ab | 12.28 ± 1.43 b |
| Mo × R8 | 5.20 ± 0.44 b | 47.54 ± 4.18 a | 130.30 ± 7.28 a | 325.92 ± 37.81 a | 22.20 ± 2.69 a |
| Og × R8 | 11.22 ± 0.77 a | 36.42 ± 2.85 ab | 96.38 ± 8.12 b | 385.30 ± 46.74 a | 10.08 ± 0.57 b |
| C × R8 | 10.02 ± 0.49 a | 48.56 ± 6.58 a | 59.40 ± 4.07 c | 138.86 ± 7.88 b | 11.18 ± 0.66 b |
| Ch × R9 | 24.46 ± 2.84 a | 28.32 ± 1.58 a | 71.74 ± 5.97 ab | 355.60 ± 20.95 ns | 22.20 ± 1.74 ns |
| Mo × R9 | 17.24 ± 2.09 ab | 26.30 ± 2.22 ab | 96.38 ± 8.48 a | 257.62 ± 22.68 ns | 27.70 ± 3.75 ns |
| Og × R9 | 13.64 ± 0.78 b | 19.22 ± 1.32 b | 102.56 ± 8.03 a | 379.36 ± 29.69 ns | 18.88 ± 1.06 ns |
| C × R9 | 13.64 ± 0.80 b | 31.36 ± 1.54 a | 50.16 ± 6.79 b | 257.62 ± 34.89 ns | 17.78 ± 1.50 ns |
| Ch × R10 | 6.42 ± 0.75 c | 22.26 ± 2.58 ns | 37.82 ± 2.11 ns | 269.50 ± 22.42 a | 15.58 ± 1.06 bc |
| Mo × R10 | 12.42 ± 1.51 b | 20.24 ± 2.46 ns | 31.66 ± 2.67 ns | 168.56 ± 14.84 b | 24.40 ± 1.20 ab |
| Og × R10 | 17.24 ± 0.98 a | 19.22 ± 1.09 ns | 31.66 ± 2.17 ns | 207.16 ± 16.21 ab | 33.20 ± 3.85 a |
| C × R10 | 12.42 ± 0.73 b | 22.26 ± 1.31 ns | 28.58 ± 1.41 ns | 138.86 ± 18.81 b | 12.28 ± 1.49 c |
| Fertilization | Average Number of Stalks per Plant | Average Weight per Plant (g) | Average Weight of Stalks per Plant (g) | Yield (t·ha−1) |
|---|---|---|---|---|
| ns | ns | ns | ns | |
| Ch | 11.14 ± 0.53 | 443.8 ± 10.77 | 41.07 ± 1.43 | 59.16 ± 5.21 |
| Mo | 9.32 ± 0.32 | 368.80 ± 13.82 | 42.92 ± 1.42 | 49.16 ± 3.85 |
| Og | 10.22 ± 0.06 | 395.20 ± 19.14 | 40.31 ± 1.17 | 52.68 ± 7.13 |
| C | 9.22 ± 0.47 | 354.60 ± 6.86 | 39.11 ± 1.05 | 47.27 ± 2.64 |
| Harvest Time | Treatment | |||
|---|---|---|---|---|
| Ch | Mo | Og | C | |
| R1 | 7278 ± 498.1 b–g | 7838 ± 385.37 b–e | 8665 ± 1005.19 bc | 8398 ± 1018.76 bd |
| R2 | 5865 ± 332.67 c–i | 5865 ± 487.97 c–i | 7438 ± 654.77 b–f | 8105 ± 634.26 b–e |
| R3 | 5092 ± 689.69 e–l | 5519 ± 308.34 c–j | 4212 ± 355.01 g–n | 5359 ± 366.77 d–k |
| R4 | 800 ± 39.33 o–p | 2106 ± 244.31 l–p | 1333 ± 161.71 n–p | 560 ± 31.76 p |
| R5 | 1013 ± 79.27 o–p | 2266 ± 306.92 k–p | 1600 ± 89.39 m–p | 853 ± 71.89 op |
| R6 | 3066 ± 209.84 i–p | 2453 ± 120.61 j–p | 3946 ± 328.31 h–o | 2533 ± 222.98 j–p |
| R7 | 11864 ± 928.43 a | 2853 ± 386.42 i–p | 5199 ± 290.46 e–l | 4932 ± 415.7 e–l |
| R8 | 6612 ± 452.52 b–h | 5305 ± 260.83 d–k | 2800 ± 324.82 i–p | 5252 ± 637.12 d–l |
| R9 | 11997 ± 1624.93 a | 6985 ± 390.24 b–h | 9758 ± 822.45 ab | 4532 ± 310.17 f–m |
| R10 | 5572 ± 273.96 c–j | 7971 ± 924.68 b–e | 7731 ± 937.84 b–e | 6745 ± 382.59 b–h |
| Month/Year | Temperature Average (°C) | Total Rainfall (mm) | Relative Humidity (%) | Sunlight (hours) |
|---|---|---|---|---|
| April/2017 | 10.3 | 89.1 | 67 | 205.5 |
| May/2017 | 16.5 | 71.0 | 68 | 278.4 |
| June/2017 | 21.7 | 46.6 | 65 | 293.4 |
| July/2017 | 22.0 | 47.8 | 68 | 291.0 |
| August/2017 | 22.2 | 39.0 | 66 | 290.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, A.; Vlase, L.; Munteanu, N.; Stan, T.; Teliban, G.C.; Burducea, M.; Stoleru, V. Dynamic of Phenolic Compounds, Antioxidant Activity, and Yield of Rhubarb under Chemical, Organic and Biological Fertilization. Plants 2020, 9, 355. https://doi.org/10.3390/plants9030355
Cojocaru A, Vlase L, Munteanu N, Stan T, Teliban GC, Burducea M, Stoleru V. Dynamic of Phenolic Compounds, Antioxidant Activity, and Yield of Rhubarb under Chemical, Organic and Biological Fertilization. Plants. 2020; 9(3):355. https://doi.org/10.3390/plants9030355
Chicago/Turabian StyleCojocaru, Alexandru, Laurian Vlase, Neculai Munteanu, Teodor Stan, Gabriel Ciprian Teliban, Marian Burducea, and Vasile Stoleru. 2020. "Dynamic of Phenolic Compounds, Antioxidant Activity, and Yield of Rhubarb under Chemical, Organic and Biological Fertilization" Plants 9, no. 3: 355. https://doi.org/10.3390/plants9030355
APA StyleCojocaru, A., Vlase, L., Munteanu, N., Stan, T., Teliban, G. C., Burducea, M., & Stoleru, V. (2020). Dynamic of Phenolic Compounds, Antioxidant Activity, and Yield of Rhubarb under Chemical, Organic and Biological Fertilization. Plants, 9(3), 355. https://doi.org/10.3390/plants9030355







