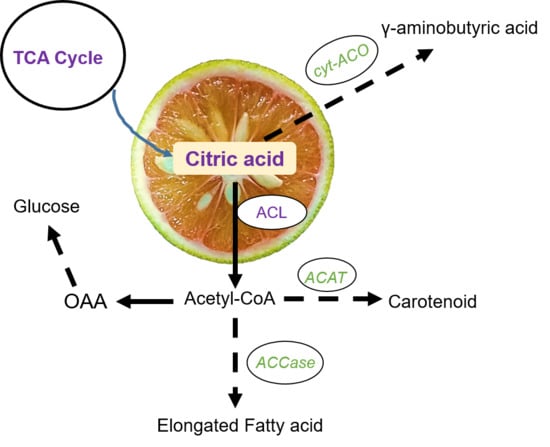
Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions
Abstract
1. Introduction
2. Results
2.1. Changes in Soluble Sugar Concentration during Fruit Development and Ripening
2.2. Changes in Organic Acids Concentration during Fruit Development and Ripening
2.3. Changes in Cytosolic Aconitase (cyt-ACO) Activity and γ-Aminobutyric Acid (GABA) Concentration during Fruit Development and Ripening
2.4. Changes in ACL Activity and Acetyl-CoA Concentration during Fruit Development and Ripening
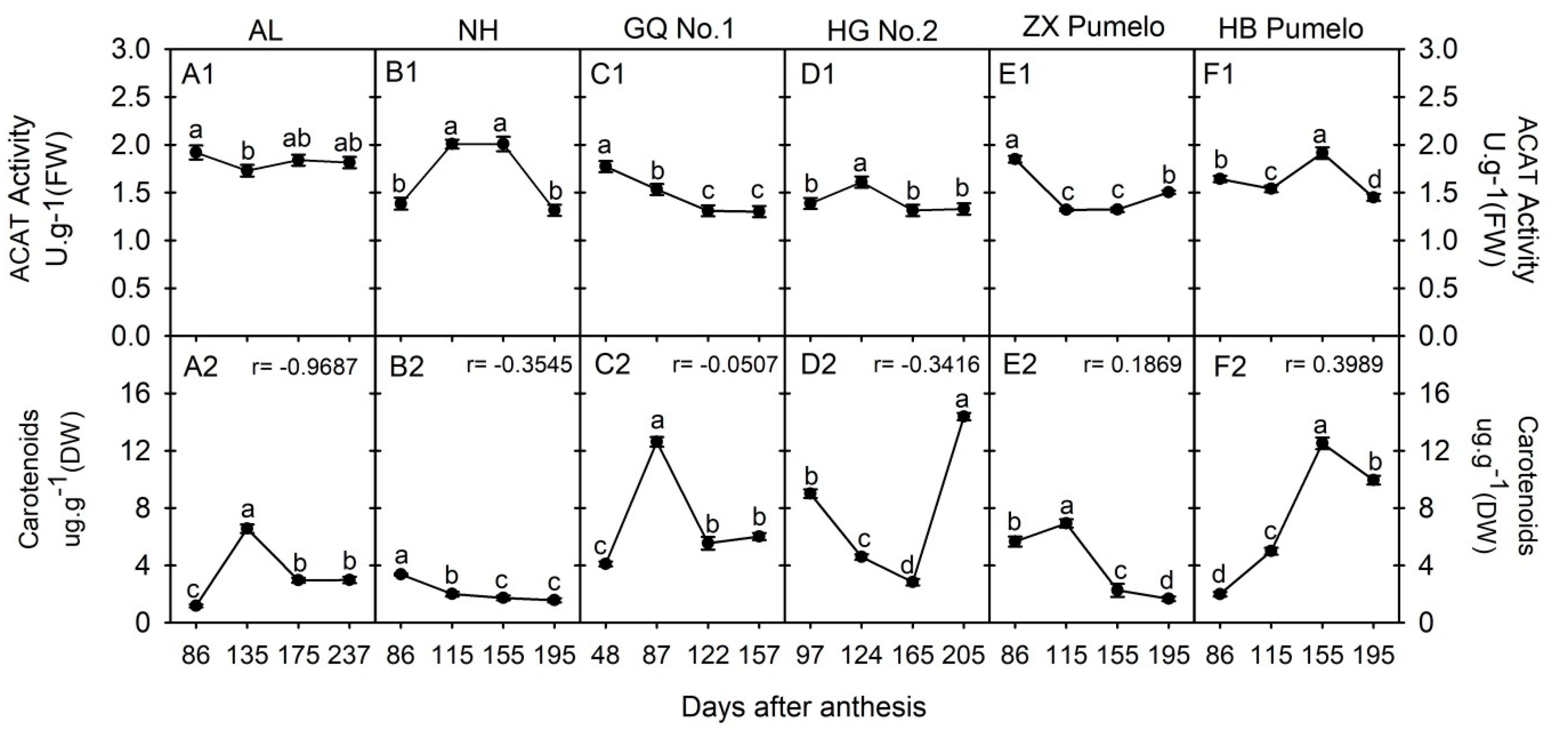
2.5. Changes in Acetyl-CoA C-Acetyltransferase (ACAT) and Carotenoids Concentration during Fruit Development and Ripening
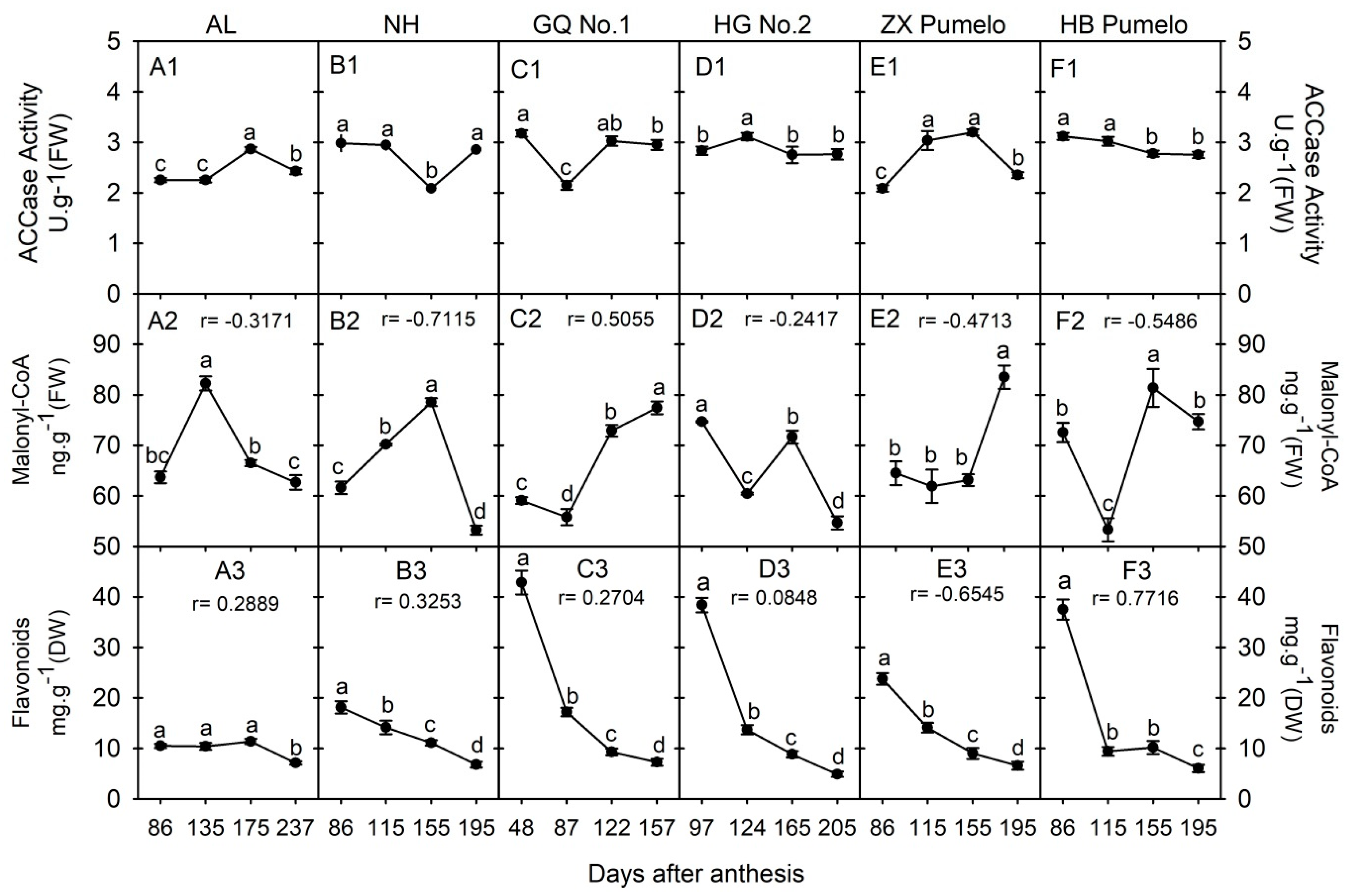
2.6. Changes in Acetyl-CoA Carboxylase Activity (ACCase), Malonyl-CoA and Flavonoids Concentration during Fruit Development and Ripening
2.7. Change in ACL Activity Could Alter the Cyt-ACO Activity as well as Citrate, Acetyl-CoA, GABA, Total Flavonoids, and Carotenoids Concentrations
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Soluble Sugars, Organic Acids, γ-Aminobutyric Acid (GABA), Acetyl-CoA, Malonyl-CoA, Total Flavonoids, and Carotenoids
4.3. Determination of ATP-Citrate Lyase, Cyt-Aconitase, Acetyl-CoA Carboxylase, and Acetyl-CoA Acetyltransferase Activities
4.4. Vector Construction and Transformation
4.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, T.; Burri, B.J. Potential Nutritional Benefits of Current Citrus Consumption. Agriculture 2013, 3, 170–187. [Google Scholar] [CrossRef]
- Baldwin, E.A. Citrus Fruit. In Biochemistry of Fruit Ripening, 3rd ed.; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Chapman and Hall: London, UK, 1993; pp. 107–149. [Google Scholar]
- White, P.J. Recent advances in fruit development and ripening: An overview. J. Exp. Bot. 2002, 53, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gierson, D.; Kader, A.A. Fruit ripening and quality. In The Tomato Crop, 2nd ed.; Atherton, J.G., Rudich, J., Eds.; Chapman and Hall: London, UK, 1986; pp. 167–200. [Google Scholar]
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Shackel, K.A.; Matthews, M.A. Fruit ripening in Vitis vinifera: Spatiotemporal relationships among turgor, sugar accumulation, and anthocyanin biosynthesis. J. Exp. Bot. 2011, 62, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit Development and Ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef]
- Bogin, E.; Wallace, A. Organic acid synthesis and accumulation in sweet and sour lemon fruit. J. Am. Soc. Hort. Sci. 1966, 89, 182–194. [Google Scholar]
- Sadka, A.; Dahan, E.; Cohen, L.; Marsh, K.B. Aconitase activity and expression during the development of lemon fruit. Physiol. Plantarum 2000, 108, 255–262. [Google Scholar] [CrossRef]
- Cercós, M.; Soler, G.; Iglesias, D.; Gadea, J.; Forment, J.; Talón, M. Global analysis of gene expression during development and ripening of citrus fruit flesh. A proposed mechanism for citric acid utilization. Plant Mol. Biol. 2006, 62, 513–527. [Google Scholar]
- Degu, A.; Hatew, B.; Nunes-Nesi, A.; Shlizerman, L.; Zur, N.; Katz, E.; Fernie, A.R.; Blumwald, E.; Sadka, A. Inhibition of aconitase in citrus fruit callus results in a metabolic shift towards amino acid biosynthesis. Planta 2011, 234, 501–513. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef]
- Fatland, B.L.; Ke, J.; Anderson, M.D.; Mentzen, W.I.; Cui, L.W.; Allred, C.C.; Johnston, J.L.; Nikolau, B.J.; Wurtele, E.S. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol. 2002, 130, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Beard, K.F.M.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; van Deenen, N.; Magliano, P.; Frahm, L.; Forestier, E.; Nawrath, C.; Schaller, H.; Gronover, C.S.; Prüfer, D.; Poirier, Y. ATP citrate lyase activity is post-translationally regulated by sink strength and impacts the wax, cutin and rubber biosynthetic pathways. Plant J. 2014, 79, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Chypre, M.; Zaidi, N.; Smans, K. ATP-citrate lyase: A mini-review. Biochem. Biophys. Res. Commun. 2012, 422, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fatland, B.L.; Nikolau, B.J.; Wurtele, E.S. Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell 2005, 17, 182–203. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.Y.; Guo, L.X.; Kamran, H.M.; Sadka, A.; Liu, Y.Z. Recent Advances in the Regulation of Citric Acid Metabolism in Citrus Fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Rangasamy, D.; Ratledge, C. Compartmentation of ATP-Citrate Lyase in Plants. Plant Physiol. 2000, 122, 1225–1230. [Google Scholar] [CrossRef]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Wang, S.; Tu, H.; Wan, J.; Chen, W.; Liu, X.; Luo, J.; Xu, J.; Zhang, H. Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Perez-Cacho, P.R.; Jabalpurwala, F. Historical Review of Citrus Flavor Research during the Past 100 Years. J. Agric. Food Chem. 2009, 57, 8115–8124. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.V.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.; Berti, L. Changes in Organic Acids and Sugars during Early Stages of Development of Acidic and Acidless Citrus Fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, X.M.; Jin, L.F.; Shi, C.Y.; Liu, Y.Z.; Peng, S.A. Identification and transcript analysis of two glutamate decarboxylase genes, CsGAD1 and CsGAD2, reveal the strong relationship between CsGAD1 and citrate utilization in citrus fruit. Mol. Biol. Rep. 2014, 41, 6253–6262. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xie, J.X.; Wang, F.F.; Zhong, J.; Liu, Y.Z.; Li, G.H.; Peng, S.A. Comparison of ascorbate metabolism in fruits of two citrus species with obvious difference in ascorbate content in pulp. J. Plant Physiol. 2011, 168, 2196–2205. [Google Scholar] [CrossRef]
- Tadeo, F.R.; Cercós, M.; ColmeneroFlores, J.M.; Iglesias, D.J.; Naranjo, M.A.; Ríos, G.; Carrera, E.; RuizRivero, O.; Lliso, I.; Morillon, R.; et al. Molecular Physiology of Development and Quality of Citrus. Adv. Bot. Res. 2008, 47, 147–223. [Google Scholar]
- Cano, A.; Medina, A.; Bermejo, A. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal. 2008, 21, 377–381. [Google Scholar] [CrossRef]
- John, W.P.; Frikkie, C.B.; Robert, G.B. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar]
- Hu, X.M.; Shi, C.Y.; Liu, X.; Jin, L.F.; Liu, Y.Z.; Peng, S.A. Genome-wide identification of citrus ATP-citrate lyase genes and their transcript analysis in fruits reveals their possible role in citrate utilization. Mol. Genet. Genomics 2015, 290, 29–38. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Tadeo, F.R.; Legaz, F.; Primo-Millo, E.; Talon, M. In vivo sucrose stimulation of colour change in citrus fruit epicarps: Interactions between nutritional and hormonal signals. Physiol. Plant. 2001, 112, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Telef, N.; Stammitti-Bert, L.; Mortain-Bertrand, A.; Maucourt, M.; Carde, J.; Rolin, D.; Gallusci, P. Sucrose deficiency delays lycopene accumulation in tomato fruit pericarp discs. Plant Mol. Biol. 2006, 62, 453–469. [Google Scholar] [CrossRef]
- Metallo, C.M.; Vander Heiden, M.G. Metabolism strikes back: Metabolic flux regulates cell signaling. Genes Dev. 2010, 24, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.X.; Shi, C.Y.; Liu, X.; Ning, D.Y.; Jing, L.F.; Yang, H.; Liu, Y.Z. Citrate Accumulation-Related Gene Expression and/or Enzyme Activity Analysis Combined With Metabolomics Provide a Novel Insight for an Orange Mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef] [PubMed]
- Crifo, T.; Petrone, G.; Lo Cicero, L.; Lo Piero, A.R. Short cold storage enhances the anthocyanin contents and level of transcripts related to their biosynthesis in blood oranges. J. Agric. Food Chem. 2012, 60, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar]
- Rodríguez-Concepción, M.; Gruissem, W. Arachidonic Acid Alters Tomato HMG Expression and Fruit Growth and Induces 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase-Independent Lycopene Accumulation. Plant Physiol. 1999, 119, 41–48. [Google Scholar]
- Bartolozzi, F.; Bertazza, G.; Bass, D.; Cristoferi, G. Simultaneous determination of soluble sugars and organic acids as their trimethylsilyl derivatives in apricot fruits by gas-liquid chromatography. J. Chromatogr. A 1997, 758, 99–107. [Google Scholar] [CrossRef]
- Zhang, G.; Bown, A.W. The rapid determination of γ-aminobutyric acid. Phytochemistry 1997, 44, 1007–1009. [Google Scholar] [CrossRef]
- Langlade, N.B.; Messerli, G.; Weisskopf, L.; Plaza, S.; Tomasi, N.; Smutny, J.; Neumann, G.; Martinoia, E.; Massonneau, A. ATP citrate lyase: Cloning, heterologous expression and possible implication in root organic acid metabolism and excretion. Plant Cell Environ. 2002, 25, 1561–1569. [Google Scholar] [CrossRef]
- Hirai, M.; Ueno, I. Development of citrus fruits: Fruit development and enzymatic changes in juice vesicle tissue. Plant Cell Physiol. 1977, 18, 791–799. [Google Scholar]
- Luo, A.; Yang, X.; Deng, Y.; Li, C.; Xiang, K.; Daogao, L. Organic Acid Concentrations and the Relative Enzymatic Changes During the Development of Citrus Fruits. Agric. Sci. China 2003, 36, 941–944. [Google Scholar]
- Wang, X.Y.; Wang, P.; Qi, Y.P.; Zhou, C.P.; Yang, L.T.; Liao, X.Y.; Wang, L.Q.; Zhu, D.H.; Chen, L.S. Effects of granulation on organic acid metabolism and its relation to mineral elements in Citrus grandis juice sacs. Food Chem. 2014, 145, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Zheng, X.; Zhu, K.; Xu, Q.; Deng, X. Isolation and Functional Characterization of a Lycopene beta-cyclase Gene Promoter from Citrus. Front. Plant Sci. 2016, 7, 1367. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Shi, W.; Deng, X.X. Agrobacterium-mediated transformation of embryogenic calluses of Ponkan mandarin and the regeneration of plants containing the chimeric ribonuclease gene. Plant Cell Rep. 2002, 21, 153–156. [Google Scholar]
- Cao, H.; Zhang, J.; Xu, J.; Ye, J.; Yun, Z.; Xu, Q.; Xu, J.; Deng, X. Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus. J. Exp. Bot. 2012, 63, 4403–4417. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittigen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Soluble Sugar | Cultivar | Sampling Time | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| Sucrose, mg·g−1 (FW) | Anliu | 15.8 ± 0.45c | 39.5 ± 1.61b | 46.6 ± 0.99a | 47.6 ± 2.53a |
| Newhall | 28.0 ± 1.22c | 45.2 ± 1.27b | 57.7 ± 1.32a | 62.6 ± 2.78a | |
| Guoqing No. 1 | 16.4 ± 0.62d | 35.5 ± 1.09c | 56.9 ± 1.91b | 64.3 ± 1.76a | |
| Huagan No. 2 | 11.2 ± 0.35c | 12.1 ± 1.10c | 20.0 ± 1.21b | 40.4 ± 1.85a | |
| Zaoxiang | 36.7 ± 1.99c | 43.7 ± 4.63b | 55.8 ± 3.21a | 62.9 ± 4.05a | |
| HB | 15.4 ± 1.17c | 37.7 ± 1.16b | 40.4 ± 2.25ab | 45.8 ± 3.94a | |
| Glucose, mg·g−1 (FW) | Anliu | 15.0 ± 1.20d | 18.3 ± 1.73c | 25.2 ± 1.64b | 39.9 ± 1.21a |
| Newhall | 9.0 ± 0.62d | 12.6 ± 1.12c | 36.9 ± 1.76b | 41.8 ± 1.46a | |
| Guoqing No. 1 | 10.9 ± 1.46c | 11.3 ± 1.19c | 26.3 ± 2.35b | 35.6 ± 2.71a | |
| Huagan No. 2 | 6.7 ± 0.58a | 6.1 ± 0.72ab | 5.6 ± 0.48b | 4.7 ± 0.63b | |
| Zaoxiang | 25.6 ± 1.12b | 20.4 ± 1.03c | 29.1 ± 1.34a | 18.2 ± 1.06d | |
| HB | 34.8 ± 2.21b | 19.2 ± 1.56d | 24.9 ± 1.41c | 38.9 ± 1.59a | |
| Fructose, mg·g−1 (FW) | Anliu | 19.9 ± 1.31c | 21.5 ± 1.15c | 28.1 ± 1.53b | 45.9 ± 1.52a |
| Newhall | 24.6 ± 1.11d | 28.2 ± 1.28c | 44.3 ± 2.84b | 50.4 ± 1.83a | |
| Guoqing No. 1 | 14.7 ± 1.13c | 15.6 ± 1.48c | 34.7 ± 1.55b | 44.5 ± 1.83a | |
| Huagan No. 2 | 5.5 ± 0.37a | 5.3 ± 0.26a | 4.8 ± 0.18b | 3.6 ± 0.29c | |
| Zaoxiang | 19.5 ± 1.53b | 20.0 ± 1.24b | 28.0 ± 2.87a | 21.9 ± 1.51b | |
| HB | 20.9 ± 1.46bc | 17.2 ± 1.98c | 23.4 ± 1.75b | 40.2 ± 1.88a | |
| Soluble Sugar | Cultivar | Sampling Time | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| Citric acid, mg·g−1 (FW) | Anliu | 1.8 ± 0.15c | 8.2 ± 0.18a | 7.7 ± 0.15b | 6.6 ± 0.20b |
| Newhall | 15.0 ± 0.49a | 15.7 ± 0.45a | 12.7 ± 0.32b | 9.1 ± 0.19c | |
| Guoqing No. 1 | 16.5 ± 1.24b | 21.0 ± 1.27a | 9.8 ± 0.16c | 9.1 ± 0.55c | |
| Huagan No. 2 | 28.7 ± 1.63a | 16.6 ± 1.51b | 10.8 ± 1.31c | 5.0 ± 0.59d | |
| Zaoxiang | 0.7 ± 0.10d | 3.3 ± 0.12c | 4.9 ± 0.14b | 5.7 ± 0.38a | |
| HB | 0.6 ± 0.11c | 7.3 ± 0.18b | 10.6 ± 0.48a | 9.4 ± 0.92a | |
| Malic acid, mg·g−1 (FW) | Anliu | 1.3 ± 0.13ab | 1.4 ± 0.08a | 0.9 ± 0.04c | 1.1 ± 0.14bc |
| Newhall | 2.0 ± 0.12a | 0.6 ± 0.07b | 0.5 ± 0.03b | 0.6 ± 0.07b | |
| Guoqing No. 1 | 4.1 ± 0.25a | 2.2 ± 0.04c | 2.9 ± 0.18b | 1.8 ± 0.16d | |
| Huagan No. 2 | 4.8 ± 0.22a | 1.0 ± 0.06b | 0.5 ± 0.06c | 0.5 ± 0.02c | |
| Zaoxiang | 0.4 ± 0.02a | 0.4 ± 0.01a | 0.1 ± 0.01c | 0.3 ± 0.02b | |
| HB | 0.7 ± 0.05a | 0.5 ± 0.03b | 0.1 ± 0.01c | 0.1 ± 0.01c | |
| Quinic acid, mg·g−1 (FW) | Anliu | 2.7 ± 0.20a | 1.6 ± 0.15b | 1.0 ± 0.10c | 0.6 ± 0.01d |
| Newhall | 2.3 ± 0.14a | 1.2 ± 0.07b | 0.5 ± 0.02c | 0.3 ± 0.01d | |
| Guoqing No. 1 | 4.0 ± 0.10a | 1.0 ± 0.06b | 0.6 ± 0.02c | 0.4 ± 0.01d | |
| Huagan No. 2 | 1.5 ± 0.09a | 0.6 ± 0.01b | 0.2 ± 0.01c | 0.1 ± 0.00d | |
| Zaoxiang | 1.6 ± 0.07a | 0.6 ± 0.01b | 0.4 ± 0.02c | 0.0 ± 0.00d | |
| HB | 2.7 ± 0.04a | 0.5 ± 0.00b | 0.5 ± 0.01b | 0.0 ± 0.00c | |
| Citrus cultivars | ||||||
|---|---|---|---|---|---|---|
| Anliu | Newhall | Guoqing No.1 | Huagan No.2 | Zaoxiang Pumelo | HB Pumelo | |
| ACL | −0.8718 | −0.6597 | 0.2552 | 0.1199 | −0.0597 | 0.9583 |
| cyt-ACO | −0.0275 | −0.9224 | −0.8170 | −0.8953 | 0.8944 | −0.4035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Liu, Y.; Luo, L.; Hussain, S.B.; Bai, Y.; Alam, S.M. Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions. Plants 2020, 9, 350. https://doi.org/10.3390/plants9030350
Guo L, Liu Y, Luo L, Hussain SB, Bai Y, Alam SM. Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions. Plants. 2020; 9(3):350. https://doi.org/10.3390/plants9030350
Chicago/Turabian StyleGuo, Lingxia, Yongzhong Liu, Lijuan Luo, Syed Bilal Hussain, Yingxin Bai, and Shariq Mahmood Alam. 2020. "Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions" Plants 9, no. 3: 350. https://doi.org/10.3390/plants9030350
APA StyleGuo, L., Liu, Y., Luo, L., Hussain, S. B., Bai, Y., & Alam, S. M. (2020). Comparative Metabolites and Citrate-Degrading Enzymes Activities in Citrus Fruits Reveal the Role of Balance between ACL and Cyt-ACO in Metabolite Conversions. Plants, 9(3), 350. https://doi.org/10.3390/plants9030350