Endemic Plant Species Conservation: Biotechnological Approaches
Abstract
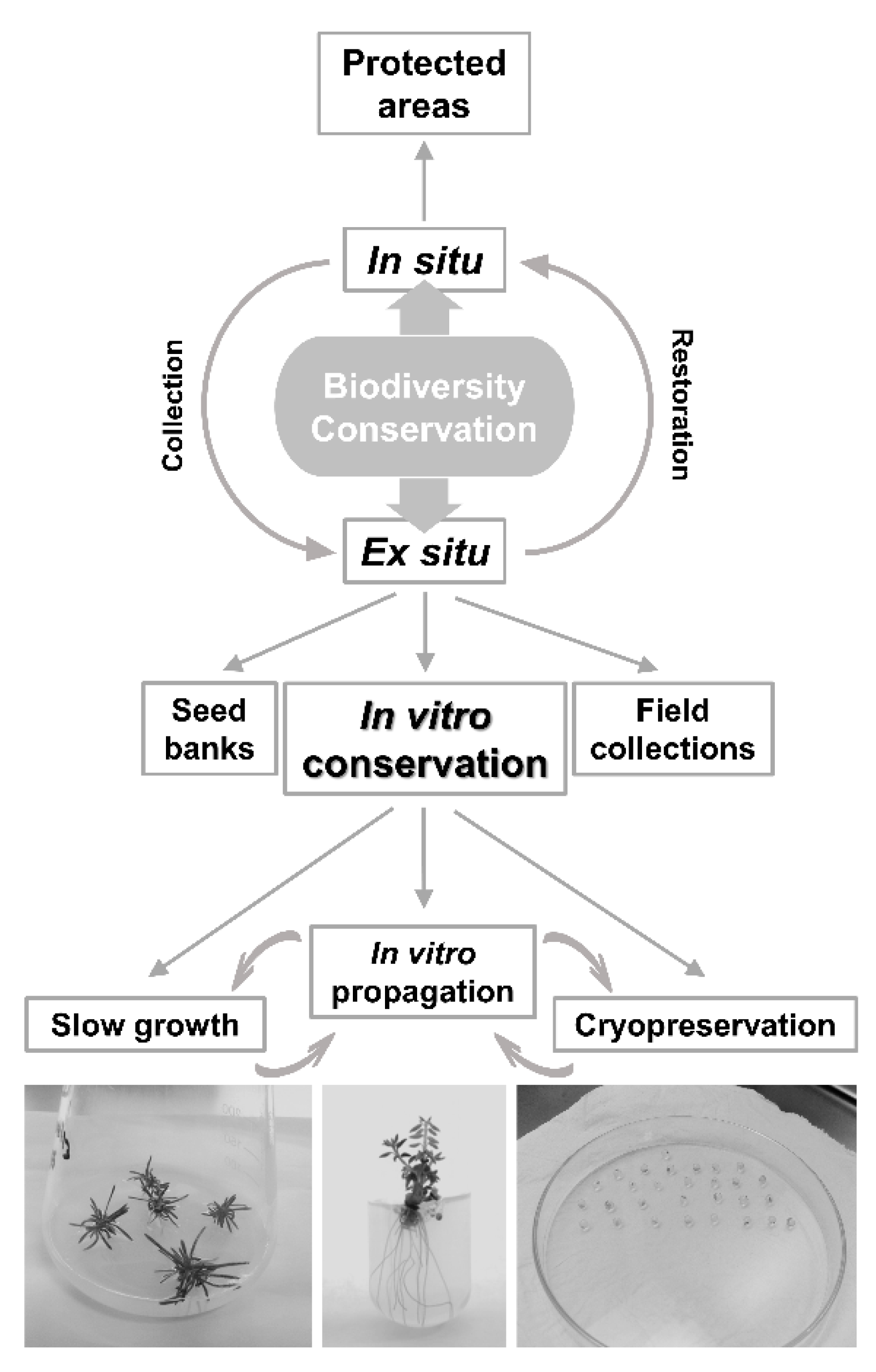
1. The Conservation of Endemic Plant Species
2. Biotechnological Approaches
3. Short- (In Vitro Propagation) and Medium-Term Preservation (Slow Growth)
4. Cryopreservation
4.1. Explant Type
4.2. Cryopreservation Techniques
4.2.1. Vitrification, Encapsulation-Dehydration, and Encapsulation-Vitrification
4.2.2. Droplet-Vitrification
4.2.3. Cryo-Plate Methods
4.2.4. Vacuum-Infiltration Vitrification (VIV)
4.3. Studies to Evaluate Cryogenic Effects
5. Synopsis and Future Perspectives
- -
- Unpublished data: there could be a reasonable number of protocols that were developed at cryobanks or botanic gardens for specific endemic species that are been used but have not been published;
- -
- Low funding: the investment in the conservation of wild plants is not retrieved as the investment on crop, medicinal and ornamental species. Their economic value makes these last species more engaging for research and therefore more accessible to funds, not only from funding agencies at an academic level but also from private investors, leaving few resources for the conservation of endemic and rare species. In addition, there are considerably more funding and resources for the conservation of animals rather than plants [114,115];
- -
- Scarce plant material: endemic plant species frequently occur in small populations in the wild, occasionally with difficult access, and thus the plant material available is very limited. In addition, a considerable number of endemic species are legally protected and, therefore, subjected to restrictions for their use and collection [115];
- -
- No standard protocols: protocol development and optimization is the most labor-intensive phase of cryopreservation [56]. In the case of endemic species, their unique nature and often unknown biology and physiology makes it even harder to determine the best conditions for cryopreservation and almost impossible to establish standard protocols.
- -
- Cryobank facilities: although to develop a cryopreservation protocol, with the vitrification-based techniques, only a standard tissue culture lab is necessary, to effectively store germplasm under cryogenic conditions more complex and costly facilities are required. Not always laboratories that develop the cryopreservation protocols are prepared to maintain germplasm for long periods of time. The scale-up of the germplasm and cryopreservation procedures requires the establishment of specific measures for their management [14]. Priority is given once again to species economically more desirable.
- -
- High number: presently it is not possible to extend conservation efforts to all endemic species. There are just too many and too less resources to cover all species.
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The State of the World’s Biodiversity for Food and Agriculture. In FAO Commission on Genetic Resources for Food and Agriculture Assessments; Bélanger, J., Pilling, D., Eds.; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/CA3129EN/CA3129EN.pdf (accessed on 18 January 2020).
- Corlett, R.T. A Bigger Toolbox: Biotechnology in Biodiversity Conservation. Trends Biotechnol. 2017, 35, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Cuttelod, A.; García, N.; Abdul Malak, D.; Temple, H.; Katariya, V. The 2008 Review of the IUCN Red List of Threatened Species; Vié, J.-C., Hilton-Taylor, C., Stuart, S.N., Eds.; IUCN: Gland, Switzerland, 2008; p. 1. [Google Scholar]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Sarasan, V.; Cripps, R.; Ramsay, M.M.; Atherton, C.; Mcmichen, M.; Prendergast, G.; Rowntree, J.K. Conservation in vitro of threatened plants—Progress in the past decade. In Vitro Cell. Dev. Biol.-Plant 2006, 42, 206–214. [Google Scholar] [CrossRef]
- Anderson, S. Area and endemism. Q. Rev. Biol. 1994, 69, 451–471. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A.; May, M.E.; Bennett, D.L.; Cook, M.J. Endemic species: Contribution to community uniqueness, effect of habitat alteration, and conservation priorities. Biol. Conserv. 2011, 144, 155–165. [Google Scholar] [CrossRef]
- Foggi, B.; Viciani, D.; Baldini, R.M.; Carta, A.; Guidi, T. Conservation assessment of the endemic plants of the Tuscan Archipelago, Italy. Orix 2014, 49, 118–126. [Google Scholar] [CrossRef]
- Işik, K. Rare and endemic species: Why are they prone to extinction? Turk. J. Bot. 2011, 35, 411–417. [Google Scholar] [CrossRef]
- Ladle, R.J.; Whittaker, R.J. Conservation Biogeography; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2019-2. Available online: http://www.iucnredlist.org (accessed on 20 January 2020).
- Marchese, C. Biodiversity hotspots: A shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Maxted, N.; Ford-Lloyd, B.V.; Hawkes, J.G. Complementary conservation strategies. In Plant Genetic Conservation: The In Situ Approach; Maxted, N., Ford-Lloyd, B.V., Hawkes, J.G., Eds.; Chapman and Hall: London, UK, 1997; Chapter 2; p. 15. [Google Scholar]
- Pritchard, H.W. Cryopreservation of desiccation tolerant seeds. In Cryopreservation and Freeze-Drying Protocols; Day, J.G., Stacey, G.N., Eds.; Humana Press Inc: Totowa, NJ, USA, 2007; Chapter 13; p. 183. [Google Scholar]
- González-Benito, M.E.; Clavero-Ramírez, I.; López-Aranda, J.M. Review. The use of cryopreservation for germplasm conservation of vegetatively propagated crops. Span. J. Agric. Res. 2004, 2, 341–351. [Google Scholar] [CrossRef]
- Benson, E.E. An Introduction to Plant Conservation Biotechnology. In Plant Conservation Biotechnology; Benson, E.E., Ed.; Taylor & Francis: London, UK, 1999; Part 1; Chapter 1; p. 3. [Google Scholar]
- González-Benito, M.E.; Martín, C. In Vitro Preservation of Spanish Biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 417, 46–54. [Google Scholar] [CrossRef]
- Streczynski, R.; Clark, H.; Whelehan, L.; Ang, S.-T.; Hardstaff, L.; Funnekotter, B.; Bunn, E.; Offord, C.A.; Sommerville, K.; Mancera, R. Current issues in plant cryopreservation and importance for ex situ conservation of threatened Australian native species. Aust. J. Bot. 2019, 67, 1–15. [Google Scholar] [CrossRef]
- Barnicoat, H.; Cripps, R.; Kendon, J.; Sarasan, V. Conservation in vitro of rare and threatened ferns—Case studies of biodiversity hotspot and island species. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 37–45. [Google Scholar] [CrossRef]
- Bowes, B.G. A Colour Atlas of Plant Propagation and Conservation; Manson Publishing Ltd.: London, UK, 1999. [Google Scholar]
- Kartha, K.K. Cryopreservation of Plant Cells and Organs; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Ayuso, M.; García-Pérez, P.; Ramil-Rego, P.; Gallego, P.P.; Barreal, M.E. In vitro culture of the endangered plant Eryngium viviparum as dual strategy for its ex situ conservation and source of bioactive compounds. Plant Cell Tissue Organ Cult. 2019, 138, 427–435. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Faria, D.V.; Simão, M.J.; Cipriano, R.; Werner, E.T.; Soares, T.C.B.; Aoyama, E.M.; Lima-Gontijo, A.B.P. In vitro morphogenesis and micropropagation of Aechmea ramosa var. ramosa Mart. ex Schult. f. (Bromeliaceae) from leaf explants. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 530–536. [Google Scholar] [CrossRef]
- Regalado, J.J.; Carmona-Martín, E.; López-Granero, M.; Jiménez, A.; Castro, P.; Encina, C.L. Micropropagation of Asparagus macrorrhizus, a Spanish endemic species in extreme extinction risk. Plant Cell Tissue Organ Cult. 2018, 132, 573–578. [Google Scholar] [CrossRef]
- Lakshmi, S.R.; Parthibhan, S.; Sherif, N.A.; Kumar, T.S.; Rao, M.V. Micropropagation, in vitro flowering, and tuberization in Brachystelma glabrum Hook.f., an endemic species. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 64–72. [Google Scholar] [CrossRef]
- Nair, L.G.; Seeni, S. In vitro multiplication of Calophyllum apetalum (Clusiaceae), an endemic medicinal tree of the Western Ghats. Plant Cell Tissue Organ Cult. 2003, 75, 169–174. [Google Scholar] [CrossRef]
- Chavan, J.J.; Nalawade, A.S.; Gaikwad, N.B.; Gurav, R.V.; Dixit, G.B.; Yadav, S.R. An efficient in vitro regeneration of Ceropegia noorjahaniae: An endemic and critically endangered medicinal herb of the Western Ghats. Physiol. Mol. Biol. Plants 2014, 20, 405–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abraham, S.; Augustine, J.; Thomas, T.D. Asymbiotic seed germination and in vitro conservation of Coelogyne nervosa A. Rich. an endemic orchid to Western Ghats. Physiol. Mol. Biol. Plants 2012, 18, 245–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arrabal, R.; Amancio, F.; Carneiro, L.A.; Neves, L.J.; Mansur, E. Micropropagation of endangered endemic Brazilian bromeliad Cryptanthus sinuosus (L.B. Smith) for in vitro preservation. Biodivers. Conserv. 2002, 11, 1081–1089. [Google Scholar] [CrossRef]
- Pence, V.C.; Finke, L.R.; Chaiken, M.F. Tools for the ex situ conservation of the threatened species, Cycladenia humilis var. jonesii. Conserv. Physiol. 2017, 5, cox053. [Google Scholar] [CrossRef]
- Jarda, L.; Cristea, V.; Halmagyi, A.; Palada, M. In vitro culture initiation and cryopreservation of endemic taxa Dianthus giganteus ssp. banaticus. Acta Hortic. 2011, 918, 153–159. [Google Scholar] [CrossRef]
- Markovic, M.; Grbić, M.; Djukic, M. Micropropagation of Endangered and Decorative Species Dianthus pinifolius Sibth. et Sm. Braz. Arch. Biol. Technol. 2016, 59, e16150320. [Google Scholar] [CrossRef][Green Version]
- Prameela, J.; Ramakrishnaiah, H.; Deepalakshmi, A.P.; Kumar, N.N.; Radhika, R.N. Micropropagation and assessment of genetic fidelity of Henckelia incana: An endemic and medicinal Gesneriad of South India. Physiol. Mol. Biol. Plants 2015, 21, 441–446. [Google Scholar] [CrossRef]
- Ambrožič-Dolinšek, J.; Ciringer, T.; Kaligarič, M. Micropropagation of the Narrow Endemic Hladnikia pastinacifolia (Apiaceae). Acta Bot. Croat. 2016, 75, 244–252. [Google Scholar] [CrossRef]
- Coste, A.; Halmagyi, A.; Keul, A.; Deliu, C.; Coldea, G.; Hurdu, B. In vitro propagation and cryopreservation of Romanian endemic and rare Hypericum species. Plant Cell. Tissue Organ Cult. 2012, 110, 213–226. [Google Scholar] [CrossRef]
- Castro, M.; Belo, A.; Afonso, A.; Zavattieri, A. Micropropagation of Juniperus navicularis, an endemic and rare species from Portugal SW coast. Plant Growth Regul. 2011, 65, 223–230. [Google Scholar] [CrossRef]
- Khater, N.; Benbouza, H. Preservation of Juniperus thurifera L.: A rare endangered species in Algeria through in vitro regeneration. J. For. Res. 2019, 30, 77–86. [Google Scholar] [CrossRef]
- Mata-Rosas, M.; Jiménez-Rodríguez, A. Somatic embryogenesis and organogenesis in Magnolia dealbata Zucc. (Magnoliaceae), an endangered, endemic Mexican species. HortScience 2006, 41, 1329. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Cruz-Cruz, C.A.; Atlahua-Temoxtle, J.; Bello-Bello, J.J. In vitro conservation and regeneration of Laelia anceps Lindl. S. Afr. J. Bot. 2019, 121, 219–223. [Google Scholar] [CrossRef]
- Alfonso, D.; Cicatelli, A.; Guarino, F.; Rodríguez, D.; Castiglione, S. In vitro propagation of Leucocroton havanensis Borhidi (Euphorbiaceae): A rare serpentine-endemic species of Cuba. Plant Biosyst. 2018, 152, 649–656. [Google Scholar] [CrossRef]
- Mendonça, D.; Luna, S.; Bettencourt, S.; Lopes, M.S.; Monteiro, L.; Neves, J.D.; Monjardino, P.; Machado, A.C. In vitro propagation of Picconia azorica (Tutin) Knobl. (Oleaceae) an Azorean endangered endemic plant species. Acta Physiol. Plant. 2015, 37, 47. [Google Scholar] [CrossRef]
- Gonçalves, S.; Martins, N.; Romano, A. Micropropagation and conservation of endangered species Plantago algarbiensis and P. almogravensis. Biol. Plantarum 2009, 53, 774–778. [Google Scholar] [CrossRef]
- Kartsonas, E.; Papafotiou, M. Mother plant age and seasonal influence on in vitro propagation of Quercus euboica Pap., an endemic, rare and endangered oak species of Greece. Plant Cell Tissue Organ Cult. 2007, 90, 111–116. [Google Scholar] [CrossRef]
- San José, M.C.; Santiago, M.T.; Cernadas, M.J.; Montenegro, R.; Mosteiro, F.; Corredoira, E. Biotechnological efforts for the propagation of Quercus lusitanica Lam., an endangered species. Trees-Struct. Funct. 2017, 31, 1571–1581. [Google Scholar] [CrossRef]
- Al-Qurainy, F.; Mohammad, N.; Khan, S.; Alansi, S.; Tarroum, M.; Alameri, A.A.; Gaafar, A.-R.Z.; Al-Shameri, A. Rapid plant regeneration, validation of genetic integrity by ISSR markers and conservation of Reseda pentagyna an endemic plant growing in Saudi Arabia. Saudi J. Biol. Sci. 2017, 25, 111–116. [Google Scholar] [CrossRef]
- Cuenca, S.; Amo-Marco, J.B. In vitro propagation of two Spanish endemic species of Salvia through bud proliferation. In Vitro Cell. Dev. Biol.-Plant 2000, 36, 225–229. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; González-Benito, M.E.; Romano, A. Establishment of an in vitro propagation protocol for Thymus lotocephalus, a rare aromatic species of the Algarve (Portugal). Plant Growth Regul. 2012, 66, 69–74. [Google Scholar] [CrossRef]
- Gonçalves, S.; Fernandes, L.; Romano, A. High-frequency in vitro propagation of the endangered species Tuberaria major. Plant Cell Tissue Organ Cult. 2010, 101, 359–363. [Google Scholar] [CrossRef]
- Carra, A.; Catalano, C.; Badalamenti, O.; Carimi, F.; Pasta, S.; Motisi, A.; Abbate, L.; Bella, F.; Fazan, L.; Kozlowski, G.; et al. Overcoming sexual sterility in conservation of endangered species: The prominent role of biotechnology in multiplication of Zelkova sicula (Ulmaceae), a relict tree at the brink of extinction. Plant Cell Tissue Organ Cult. 2019, 137, 139–148. [Google Scholar] [CrossRef]
- Reed, B.M.; Gupta, S.; Uchendu, E.E. In Vitro Genebanks for Preserving Tropical Biodiversity. In Conservation of Tropical Plant Species; Normah, M., Chin, H., Reed, B., Eds.; Springer: New York, NY, USA, 2013; Chapter 5; p. 77. [Google Scholar]
- Iriondo, J.M.; Pérez, C. Micropropagation and in vitro storage of Centaurium rigualii Esteve (Gentianaceae). Isr. J. Plant Sci. 1996, 44, 115–123. [Google Scholar] [CrossRef]
- Martin, K.P.; Pradeep, A.K. Simple strategy for the in vitro conservation of Ipsea malabarica an endemic and endangered orchid of the Western Ghats of Kerala, India. Plant Cell Tissue Organ Cult. 2003, 74, 197–200. [Google Scholar] [CrossRef]
- Benson, E.E. Cryopreservation of Phytodiversity: A Critical Appraisal of Theory & Practice. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar] [CrossRef]
- Matsumoto, T. Cryopreservation of plant genetic resources: Conventional and new methods. Rev. Agric. Sci. 2017, 5, 13–20. [Google Scholar] [CrossRef]
- Harding, K. Genetic integrity of cryopreserved plant cells: A review. CryoLetters 2004, 25, 3–22. [Google Scholar]
- Walters, C.; Wheeler, L.; Stanwood, P.C. Longevity of cryogenically stored seeds. Cryobiology 2004, 48, 229–244. [Google Scholar] [CrossRef]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. CryoLetters 2004, 25, 375–388. [Google Scholar]
- Reed, B.M. Plant Cryopreservation: A Practical Guide; Springer: New York, NY, USA, 2008. [Google Scholar]
- Ciringer, T.; Martín, C.; Šajna, N.; Kaligarič, M.; Ambrožič-Dolinšek, J. Cryopreservation of an endangered Hladnikia pastinacifolia Rchb. by shoot tip encapsulation-dehydration and encapsulation-vitrification. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 565–575. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; González-Benito, M.E.; Romano, A. Germination and cryopreservation tolerance of seeds from the rare aromatic species Thymus lotocephalus. Sci. Hortic. 2012, 145, 84–86. [Google Scholar] [CrossRef]
- Gonçalves, S.; Fernandes, L.; Pérez-García, F.; González-Benito, M.E.; Romano, A. Germination requirements and cryopreservation tolerance of seeds of the endangered species Tuberaria major. Seed Sci. Technol. 2009, 37, 480–484. [Google Scholar] [CrossRef]
- Hmeljevski, K.V.; Freitas, L.; Domingues, R.; Pereira, A.R.; Cancio, A.S.; Andrade, A.C.S.; Machado, M.A.; Viccini, L.F.; Forzza, R.C. Conservation assessment of an extremely restricted bromeliad highlights the need for population-based conservation on granitic inselbergs of the Brazilian Atlantic Forest. Flora 2014, 209, 250–259. [Google Scholar] [CrossRef]
- Voronkova, N.M.; Kholina, A.B. Conservation of Endemic Species from the Russian Far East Using Seed Cryopreservation. Biol. Bull. 2010, 37, 496–501. [Google Scholar] [CrossRef]
- Ferrari, E.; Colombo, R.; Faria, R.; Takane, R. Cryopreservation of seeds of Encholirium spectabile Martius ex Schultes f. by the vitrification method. Rev. Cienc. Agron. 2016, 47, 172–177. [Google Scholar] [CrossRef]
- Almerekova, S.; Mukhitdinova, Z.; Ydyrys, A.; Mukhitdinov, N.; Курманбаева, М.; Tynybekov, B.; Inelova, Z.; Akhmetova, A. The effect of cryopreservation on seed germination of the endangered, rare, endemic and medicinal plant Ferula iliensis Krasn. ex Korov. J. Biotechnol. 2016, 231, S40. [Google Scholar] [CrossRef]
- Civatti, L.M.; Marchi, M.N.G.; Bellintani, M.C. Cryopreservation of cacti seeds of three ornamental species endemic to the state of Bahia, Brazil. Seed Sci. Technol. 2015, 43, 284–290. [Google Scholar] [CrossRef]
- Coelho, N.; González-Benito, M.E.; Romano, A. Approaches for the cryopreservation of Plantago algarbiensis, a rare endemic species of the Algarve. CryoLetters 2014, 35, 521–529. [Google Scholar]
- Dodonova, A.S.; Gavrilkova, H.A.; Ishmuratova, M.; Tleukenova, S.U.; Pogossyan, G.P. Analysis of the effects of cryopreservation conditions on germination ability of Tanacetum ulutavicum seeds. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 698–703. [Google Scholar]
- Walters, C.; Wesley-Smith, J.; Crane, J.; Hill, L.M.; Chmielarz, P.; Pammenter, N.W.; Berjak, P. Cryopreservation of Recalcitrant (i.e. Desiccation-Sensitive) Seeds. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; Section II; Chapter 18; p. 465. [Google Scholar]
- Wesley-Smith, J.; Berjak, P.; Pammenter, N.W.; Walters, C. Intracellular ice and cell survival in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum: An ultrastructural study of factors affecting cell and ice structures. Ann. Bot. 2014, 113, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Radha, R.K.; Decruse, S.W.; Krishnan, P.N. Cryopreservation of excised embryonic axes of Nothapodytes nimmoniana (Graham) Mebberly—A vulnerable medicinal tree species of the Western Ghats. Indian J. Biotechnol. 2010, 9, 435–437. [Google Scholar] [CrossRef]
- Ajeeshkumar, S.; Decruse, S.W. Fertilizing Ability of Cryopreserved Pollinia of Luisia macrantha, an Endemic Orchid of Western Ghats. CryoLetters 2013, 34, 20–29. [Google Scholar] [PubMed]
- Martín, C.; Iriondo, J.; González-Benito, M.E.; Pérez, C. The use of tissue culture techniques in the conservation of plant biodiversity. Agro Food Ind. Hi-Tech 1998, 9, 37–40. [Google Scholar]
- Kaczmarczyk, A.; Funnekotter, B.; Menon, A.; Phang, P.; Al-Hanbali, A.; Bunn, E.; Mancera, R. Current Issues in Plant Cryopreservation. In Current Frontiers in Cryobiology; Katkov, I., Ed.; IntechOpen Ltd.: London, UK, 2012; Chapter 14; p. 417. [Google Scholar]
- Kaczmarczyk, A.; Funnekotter, B.; Turner, S.; Bunn, E.; Bryant, G.; Hunt, T.; Mancera, R. Development of cryopreservation for Loxocarya cinerea—An endemic Australian plant species important for post-mining restoration. CryoLetters 2013, 34, 508–519. [Google Scholar]
- Lin, L.; Yuan, B.; Wang, D.; Li, W. Cryopreservation of adventitious shoot tips of Paraisometrum mileense by droplet vitrification. CryoLetters 2014, 35, 22–28. [Google Scholar]
- Marco-Medina, A.; Casas, J.L.; González-Benito, M.E. Comparison of vitrification and encapsulation-dehydration for cryopreservation of Thymus moroderi shoot tips. CryoLetters 2010, 31, 301–309. [Google Scholar]
- Marco-Medina, A.; Casas, J.L.; Swennen, R.; Panis, B. Cryopreservation of Thymus moroderi by droplet vitrification. CryoLetters 2010, 31, 14–23. [Google Scholar]
- Coelho, N.; González-Benito, M.E.; Romano, A. Cryopreservation of shoot tips from the rare endemic species Tuberaria major. Acta Physiol. Plant. 2014, 36, 3333–3336. [Google Scholar] [CrossRef]
- Turner, S.; Krauss, S.L.; Bunn, E.; Senaratna, T.; Dixon, K.W.; Tan, B.; Touchell, D.H. Genetic fidelity and viability of Anigozanthos viridis following tissue culture, cold storage and cryopreservation. Plant Sci. 2001, 161, 1099–1106. [Google Scholar] [CrossRef]
- Turner, S.; Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K.; Tan, B. Stereochemical arrangement of hydroxyl groups in sugar and polyalcohol molecules as an important factor in effective cryopreservation. Plant Sci. 2001, 160, 489–497. [Google Scholar] [CrossRef]
- Danova, K.; Nikolova-Damianova, B.; Denev, R.; Markovska, Y. Impact of pre-culture on short- and long-term in vitro recovery of the biosynthetic potential and enzymatic and non-enzymatic antioxidant defense of Hypericum rumeliacum Boiss. after cryostorage. Plant Growth Regul. 2012, 68, 447–457. [Google Scholar] [CrossRef]
- Turner, S.; Tan, B.; Senaratna, T.; Bunn, E.; Dixon, K.; Touchell, D. Cryopreservation of the Australian species Macropidia fuliginosa (Haemodoraceae) by vitrification. CryoLetters 2000, 21, 379–388. [Google Scholar] [PubMed]
- Choi, C.-H.; Popova, E.; Lee, H.; Park, S.-U.; Ku, J.; Kang, J.-H.; Kim, H.-H. Cryopreservation of Endangered Wild Species, Aster altaicus var. uchiyamae Kitam, Using Droplet-Vitrification Procedure. CryoLetters 2019, 40, 113–122. [Google Scholar] [PubMed]
- Barraco, G.; Sylvestre, I.; Iapichino, G.; Engelmann, F. Investigating the cryopreservation of nodal explants of Lithodora rosmarinifolia (Ten.) Johnst., a rare, endemic Mediterranean species. Plant Biotechnol. Rep. 2013, 7, 141–146. [Google Scholar] [CrossRef]
- Menon, A.; Funnekotter, B.; Kaczmarczyk, A.; Bunn, E.; Turner, S.; Mancera, R.L. Cryopreservation of Lomandra sonderi (Asparagaceae) shoot tips using droplet-vitrification. CryoLetters 2012, 33, 259–270. [Google Scholar]
- Ozudogru, E.A.; Kaya, E. Cryopreservation of Thymus cariensis and T. vulgaris shoot tips: Comparison of three vitrification-based methods. CryoLetters 2012, 33, 363–375. [Google Scholar]
- Coelho, N.; González-Benito, M.E.; Martín, C.; Romano, A. Cryopreservation of Thymus lotocephalus shoot tips and assessment of genetic stability. CryoLetters 2014, 35, 119–128. [Google Scholar]
- González-Benito, M.E.; Nunez-Moreno, Y.; Martín, C. A protocol to cryopreserve nodal explants of Antirrhinum microphyllum by encapsulation-dehydration. CryoLetters 1998, 19, 225–230. [Google Scholar]
- González-Benito, M.E.; Pérez, C. Cryopreservation of nodal explants of an endangered plant species (Centaurium rigualii Esteve) using the encapsulation-dehydration method. Biodivers. Conserv. 1997, 6, 583–590. [Google Scholar] [CrossRef]
- Funnekotter, B.; Bunn, E.; Mancera, R.L. Cryo-mesh: A simple alternative cryopreservation protocol. CryoLetters 2017, 38, 155–159. [Google Scholar] [PubMed]
- Funnekotter, B.; Whiteley, S.; Turner, S.; Bunn, E.; Mancera, R. Evaluation of the New Vacuum Infiltration Vitrification (VIV) Cryopreservation Technique for Native Australian Plant Shoot Tips. CryoLetters 2015, 36, 104–113. [Google Scholar] [PubMed]
- Filipin, E.; Zitta, C.; Schmidt, E.; Randi, A. Pleopeltis lepidopteris Langsd. & Fisch. (Polypodiaceae), an endemic fern from Brazilian “restingas”: Viability of spores under different storage conditions. Braz. J. Bot. 2017, 40, 59–65. [Google Scholar] [CrossRef]
- Reed, B.M.; Uchendu, E. Controlled Rate Cooling. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; Section I; Chapter 5; p. 77. [Google Scholar]
- Wang, Q.; Li, P.; Batuman, O.; Gafny, R.; Mawassi, M. Effect of benzyladenine on recovery of cryopreserved shot tips of grapevine and citrus cultured in vitro. CryoLetters 2003, 24, 293–302. [Google Scholar] [PubMed]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Hirai, D.; Niino, T. Development of PVS-Based Vitrification and Encapsulation-Vitrification Protocols. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; Section I; Chapter 3; p. 33. [Google Scholar]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar]
- Normah, M.N.; Sulonga, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 87, 1–14. [Google Scholar] [CrossRef]
- Engelmann, F.; Gonzalez-Arnao, M.T.; Wu, Y.; Escobar, R. The Development of Encapsulation Dehydration. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; Section I; Chapter 4; p. 59. [Google Scholar]
- Reed, B.M. Plant cryopreservation: A continuing requirement for food and ecosystem security. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 285–288. [Google Scholar] [CrossRef]
- Kartha, K.K.; Leung, N.L.; Mroginski, L.A. In vitro growth responses and plant regeneration from cryopreserved meristems of Cassava (Manihot esculenta Crantz). Zeitschrift Pflanzenphysiol. 1982, 107, 133–140. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Yamamoto, S.; Rafique, T.; Priyantha, W.S.; Fukui, K.; Matsumoto, T.; Niino, T. Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters 2011, 32, 256–265. [Google Scholar] [PubMed]
- Niino, T.; Thwin, W.; Watanabe, K.; Nohara, N.; Rafique, T.; Yamamoto, S.; Fukui, K.; Arizaga, M.V.; Castillo-Martínez, C.; Matsumoto, T.; et al. Cryopreservation of mat rush lateral buds by air dehydration using aluminum cryo-plate. Plant Biotechnol. 2014, 31, 281–287. [Google Scholar] [CrossRef][Green Version]
- Nadarajan, J.; Pritchard, H.W. Biophysical characteristics of successful oilseed embryo cryoprotection and cryopreservation using vacuum infiltration vitrification: An innovation in plant cell preservation. PLoS ONE 2014, 9, e96169. [Google Scholar] [CrossRef] [PubMed]
- Funnekotter, B.; Mancera, R.L.; Bunn, E. Advances in understanding the fundamental aspects required for successful cryopreservation of Australian flora. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 289–298. [Google Scholar] [CrossRef]
- Teixeira, A.S.; González-Benito, M.E.; Molina-García, A.D. Determination of glassy state by cryo-SEM and DSC in cryopreservation of mint shoot tips by encapsulation– dehydration. Plant Cell Tissue Organ Cult. 2014, 119, 269–280. [Google Scholar] [CrossRef]
- Funnekotter, B.; Sortey, A.; Bunn, E.; Turner, S.; Mancera, R. Influence of abiotic stress preconditioning on antioxidant enzymes in shoot tips of Lomandra sonderi (Asparagaceae) prior to cryostorage. Aust. J. Bot. 2016, 64, 260–268. [Google Scholar] [CrossRef]
- Marco-Medina, A.; Casas, J.L. RAPD and phytochemical analysis of Thymus moroderi plantlets after cryopreservation. CryoLetters 2013, 34, 119–127. [Google Scholar]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef]
- Pence, V.C. The Application of Biotechnology for the Conservation of Endangered Plants. In Plant Conservation Biotechnology; Benson, E.E., Ed.; Taylor & Francis: London, UK, 1999; Part 2; Chapter 15; p. 227. [Google Scholar]
- Coelho, N.; Martín, C.; González-Benito, M.E.; Romano, A. Estimation of genetic diversity in seedlings of Plantago algarbiensis, an endangered species endemic to the south of Portugal in risk of global extinction. Braz. J. Bot. 2016, 40, 257–264. [Google Scholar] [CrossRef]
- Niazian, M. Application of genetics and biotechnology for improving medicinal plants. Planta 2019, 249, 953–973. [Google Scholar] [CrossRef]
- Sipes, S.; Wolf, P. Clonal Structure and Patterns of Allozyme Diversity in the Rare Endemic Cycladenia humilis var. Jonesii (Apocynaceae). Am. J. Bot. 1997, 84, 401–409. [Google Scholar] [CrossRef]
- Torres, E.; Iriondo, J.M.; Pérez, C. Genetic structure of an endangered plant, Antirrhinum microphyllum (Scrophulariaceae): Allozyme and RAPD analysis. Am. J. Bot. 2003, 90, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kholina, A.; Koren, O.; Zhuravlev, Y. Genetic structure and differentiation of populations of the tetraploid species Oxytropis chankaensis (Fabaceae). Russ. J. Genet. 2009, 45, 70–80. [Google Scholar] [CrossRef]
- Šajna, N.; Kavar, T.; Šustar-Vozlič, J.; Kaligarič, M. Population Genetics of the Narrow Endemic Hladnikia Pastinacifolia RCHB. (Apiaceae) Indicates Survival in Situ During the Pleistocene. Acta Biol. Cracov. Bot. 2012, 54, 84–96. [Google Scholar] [CrossRef]
- Trindade, H.; Sena, I.; Gonçalves, S.; Romano, A. Genetic diversity of wild populations of Tuberaria major (Cistaceae), an endangered species endemic to the Algarve region (Portugal), using ISSR markers. Biochem. Syst. Ecol. 2012, 45, 49–56. [Google Scholar] [CrossRef]
- Tang, A.-J.; Zhou, R.; Ge, X.-J.; Wu, W. Development of 11 Microsatellite Loci for an Endangered Herb, Paraisometrum mileense (Gesneriaceae), in Southwest China. Appl. Plant Sci. 2013, 1, apps.1200133. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Matos, M.; Correia, S.; Martins, N.; Gonçalves, S.; Romano, A.; Pinto-Carnide, O. Genetic diversity of two endemic and endangered Plantago species. Biochem. Syst. Ecol. 2013, 51, 37–44. [Google Scholar] [CrossRef]
- Shivaprakash, K.N.; Ramesha, B.T.; Uma Shaanker, R.; Dayanandan, S.; Ravikanth, G. Genetic Structure, Diversity and Long Term Viability of a Medicinal Plant, Nothapodytes nimmoniana Graham. (Icacinaceae), in Protected and Non-Protected Areas in the Western Ghats Biodiversity Hotspot. PLoS ONE 2014, 9, e112769. [Google Scholar] [CrossRef]
- Gonçalves-Oliveira, R.; Wöhrmann, T.; Iseppon, A.; Krapp, F.; Alves, M.; Wanderley, M.; Weising, K. Population genetic structure of the rock outcrop species Encholirium spectabile (Bromeliaceae): The role of pollination vs. seed dispersal and evolutionary implications. Am. J. Bot. 2017, 104, 868–878. [Google Scholar] [CrossRef]
| Species | Region | Explant | Reference |
|---|---|---|---|
| Aechmea ramosa Mart. ex Schult. f. | Atlantic Forest (Brazil) | Leaf | [26] |
| Asparagus macrorrhizus (Pedrol, Regalado & López-Encina) | “Mar Menor” lagoon, Murcia (Spain) | Rhizome buds | [27] |
| Brachystelma glabrum Hook.f. | Eastern Ghats (India) | Shoot tips and nodal explants | [28] |
| Calophyllum apetalum Willd. | Western Ghats of southern India | Shoot tips and nodal explants | [29] |
| Ceropegia noorjahaniae Ans. | Western Ghats (India) | Shoot segments (node explant) | [30] |
| Coelogyne nervosa A. Rich. | Western Ghats (South India) | Seedlings | [31] |
| Cryptanthus sinuosus (L.B. Smith) | Rio de Janeiro State (Brazil) | Stem–root axes with intact shoot apices and approximately 6–10 nodes | [32] |
| Cycladenia humilis Benth. var. jonesii (Eastw.) S.L. Welsh &N.D. Atwood | Canyonlands region of Utah and Arizona (Western U.S.A.) | Seedlings and nodal segments | [33] |
| Dianthus giganteus D’Urv subsp. banaticus (Heuff.) Tutin | Southwest Carpathians (Romenia) | Nodal explants | [34] |
| Dianthus pinifolius Sibth. et Sm. | South-eastern Balkans | Seedlings | [35] |
| Eryngium viviparum Gay | Southwest Europe (France, Spain and Portugal) | Seedlings | [24] |
| Henckelia incana (Vahl) Spreng. | Peninsular hills of India | Leaves | [36] |
| Hladnikia pastinacifolia Rchb | Plateau of Trnovski Gozd, Alps (Slovenia) | Seedlings and shoots | [37] |
| Hypericum richeri ssp. transsilvanicum (Čelak) Ciocârlan | Southeast Carpathians (Romania) | Seedlings (nodes, leaves, internodal stems and and root segments) | [38] |
| Juniperus navicularis Gand | Southwest Portugal | Shoot tips and nodal segments | [39] |
| Juniperus thurifera L. | Aure‘s Mountains (Northeastern Algeria) | Shoots | [40] |
| Magnolia dealbata Zucc. | Mexico (south–central) | Zygotic embryos | [41] |
| Laelia anceps Lindl | Mexico | Seedlings | [42] |
| Leucocroton havanensis Borhidi | Cuba | Seedlings | [43] |
| Picconia azorica (Tutin) Knobl. | Azores (Portugal) | Nodal segments | [44] |
| Plantago algarbiensis Samp. | Algarve Region (Portugal) | Seedlings | [45] |
| Plantago almogravensis Franco | Portuguese Southwest coast | Seedlings | [45] |
| Quercus euboica Pap. | Northeastern Euboia island (middle eastern Greece) | Seedlings | [46] |
| Quercus lusitanica Lam. | Iberian Peninsula and North Africa | Shoots | [47] |
| Reseda pentagyna Abdallah & A.G. Miller | Saudi Arabia | Axillary and apical buds | [48] |
| Salvia valentina Vahl and Salvia blancoana Webb & Heldr subsp. mariolensis Figuerola | Valencia Community (Spain) | Apical and nodal segments | [49] |
| Thymus lotocephalus G. López & R. Morales | Algarve Region (Portugal) | Seedlings | [50] |
| Tuberaria major (Willk.) P. Silva and Rozeira | Algarve Region (Portugal) | Seedlings | [51] |
| Zelkova sicula Di Pasquale, Garfì and Quézel | South-eastern Sicily (Italy) | Shoot segments | [52] |
| Species | Germination Rate (%) | Reference | IUCN |
|---|---|---|---|
| Encholirium spectabile Martius ex Schultes f. | 97 | [67] | LC |
| Ermania parryoides Cham. Ex Botsch. | 70 | [66] | - |
| Ferula iliensis Krasn. ex Korov | 90 | [68] | - |
| Hedysarum austrokurilense (N.S. Pavlova) N.S. Pavlova | 85 | [66] | - |
| Hedysarum sachalinense B. Fedtch. (Fabaceae) | 75 | [66] | - |
| Melocactus conoideus Buining and Brederoo | 10 | [69] | CR |
| Micranthocereus flaviflorus subsp. densiflorus (Buining and Brederoo) P. J. Braun and Esteves | 59 | [69] | NT |
| Micranthocereus polyanthus subsp. alvinii M. Machado and Hofacker | 23 | [69] | EN |
| Myosotis sachalinensis M. Pop. | 30 | [66] | - |
| Oxytropis chankaensis Jurtz. | 75 | [66] | - |
| Oxytropis kamtschatica Hult. | 90 | [66] | - |
| Oxytropis retusa Matsum. | 40 | [66] | - |
| Pitcairnia encholirioides L.B.Sm. | 85 | [65] | - |
| Plantago algarbiensis Samp. | 100 | [70] | EN |
| Saxifraga purpurascens Kom. | 20 | [66] | - |
| Stellaria eschscholziana Fenzl | 35 | [66] | - |
| Tanacetum ulutavicum Tzvel. | 72 | [71] | - |
| Thymus lotocephalus G. López & R. Morales | 90 | [63] | NT |
| Tuberaria major (Willk.) P. Silva and Rozeira | 65 | [64] | EN |
| Vaccinium vulcanorum Kom. | 90 | [66] | - |
| Vicia subrotunda (Maxim.) Czefr. | 50 | [66] | - |
| Method(s) | Species | Region | Explant | Rate (%) | Reference | IUCN |
|---|---|---|---|---|---|---|
| Vit | Anigozanthos viridis ssp terraspectans Hopper | Western Australia | Shoot tips | 89 (S) | [83,84] | - |
| Hypericum rumeliacum Boiss. | Balkan Peninsula | Shoot tips | 2 (S) | [85] | - | |
| Luisia macrantha Blatt. & McCann | Western Ghats (India) | Pollinia | 56 (G) | [75] | - | |
| Macropidia fuliginosa (Hook.) Druce | Western Australia | Shoot tips Somatic embryos | 84 (S) 91 (S) | [86] | - | |
| Thymus moroderi Pau ex Martínez | South-eastern Spain | Shoot tips | 70 (S) | [81] | - | |
| Tuberaria major (Willk.) P. Silva and Rozeira | Algarve Region (Portugal) | Shoo tips | 60 (R) | [82] | EN | |
| D-V | Aster altaicus var. uchiyamae Kitam | Korea | Shoot tips | 65 (R) | [87] | - |
| Cycladenia humilis Benth. var. jonesii (Eastw.) S.L. Welsh & N.D. Atwood | Canyonlands region of Utah and Arizona (USA) | Shoot tips | 54 (R) | [33] | - | |
| Dianthus giganteus D’Urv subsp. banaticus (Heuff.) Tutin | Southwest Carpathians (Romenia) | Shoot tips | 43 (R) | [34] | - | |
| Hypericum richeri ssp. transsilvanicum (Čelak) Ciocârlan | Southeast Carpathians, Transilvania (Romania) | Axillary buds | 68 (S) | [38] | - | |
| Lithodora rosmarinifolia (Ten.) I. M. Johnst. | Sicily (Italy) | Nodal segments | 33 (R) | [88] | - | |
| Lomandra sonderi (F.Muell.) Ewart | Western Australia | Shoot tips | 32 (S) | [89] | - | |
| Loxocarya cinerea R. Br. | Western Australia | Callus | 90 (S) | [78] | - | |
| Paraisometrum mileense W. T. Wang | Yunnan, China | Shoot tips | 86 (R) | [79] | - | |
| Plantago algarbiensis Samp. | Algarve Region (Portugal) | Nodal segments | 60 (R) | [70] | EN | |
| Thymus cariensis Hub-Mor. & Jalas | Turkey | Shoot tips | 25 (R) | [90] | - | |
| Thymus lotocephalus G. López & R. Morales | Algarve Region (Portugal) | Shoot tips | 67 (R) | [91] | NT | |
| Thymus moroderi Pau ex Martínez | South-eastern Spain | Shoot tips | 79 (S) | [80] | - | |
| E-D | Antirrhinum microphyllum Rothm. | Spain | Nodal segments | 70 (S) | [92] | - |
| Hladnikia pastinacifolia Rchb | Plateau of Trnovski Gozd, Alps (Slovenia) | Shoot tips | 53 (R) | [62] | DD | |
| Centaurium rigualii Esteve | Iberian Peninsula | Nodal segments | 70 (S) | [93] | - | |
| Plantago algarbiensis Samp. | Algarve Region (Portugal) | Nodal segments | 63 (R) | [70] | EN | |
| Pteris adscensionis Swartz | Ascension Island | Gametophytes | 48 (S) | [21] | CR | |
| Thymus moroderi Pau ex Martínez | South-eastern Spain | Shoot tips | 50 (S) | [81] | - | |
| Thymus lotocephalus G. López & R. Morales | Algarve Region (Portugal) | Shoot tips | 44 (R) | [91] | NT | |
| Tuberaria major (Willk.) P. Silva and Rozeira | Algarve Region (Portugal) | Shoot tips | 67 (R) | [82] | EN | |
| E-V | Hladnikia pastinacifolia Rchb | Plateau of Trnovski Gozd, Alps (Slovenia) | Shoot tips | 64 (R) | [62] | DD |
| Cryo-mesh | Anigozanthos viridis Endl. | Western Australia | Shoot tips | 83 (R) | [94] | - |
| VIV | Anigozanthos viridis ssp. terraspectans Hopper | Western Australia | Shoo tips | 31 (R) | [95] | - |
| Lomandra sonderi (F.Muell.) Ewart | Western Australia | Shoo tips | 42 (R) | [95] | - | |
| Loxocarya cinerea R. Br. | Western Australia | Shoo tips | 10 (R) | [95] | - | |
| Desiccation | Nothapodytes nimmoniana (Graham) Melbbery | Western Ghats (India) | Embryonic axes | 60 (G) | [74] | - |
| Direct LN | Pleopeltis lepidopteris Langsd. & Fisch. | South Brazil | Spores | 89 (G) | [96] | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. https://doi.org/10.3390/plants9030345
Coelho N, Gonçalves S, Romano A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants. 2020; 9(3):345. https://doi.org/10.3390/plants9030345
Chicago/Turabian StyleCoelho, Natacha, Sandra Gonçalves, and Anabela Romano. 2020. "Endemic Plant Species Conservation: Biotechnological Approaches" Plants 9, no. 3: 345. https://doi.org/10.3390/plants9030345
APA StyleCoelho, N., Gonçalves, S., & Romano, A. (2020). Endemic Plant Species Conservation: Biotechnological Approaches. Plants, 9(3), 345. https://doi.org/10.3390/plants9030345





