Why Does the Halophyte Mesembryanthemum crystallinum Better Tolerate Ni Toxicity than Brassica juncea: Implication of Antioxidant Defense Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Growth Analysis
2.3. Estimation of Lipid Peroxidation
2.4. Enzyme Extractions
2.5. Enzyme Assays
2.6. Non-Protein Thiols Determination
2.7. Glutathione Determination
2.8. Free Proline Concentration
2.9. Statistical Analysis
3. Results
3.1. Plant Morphology and Growth
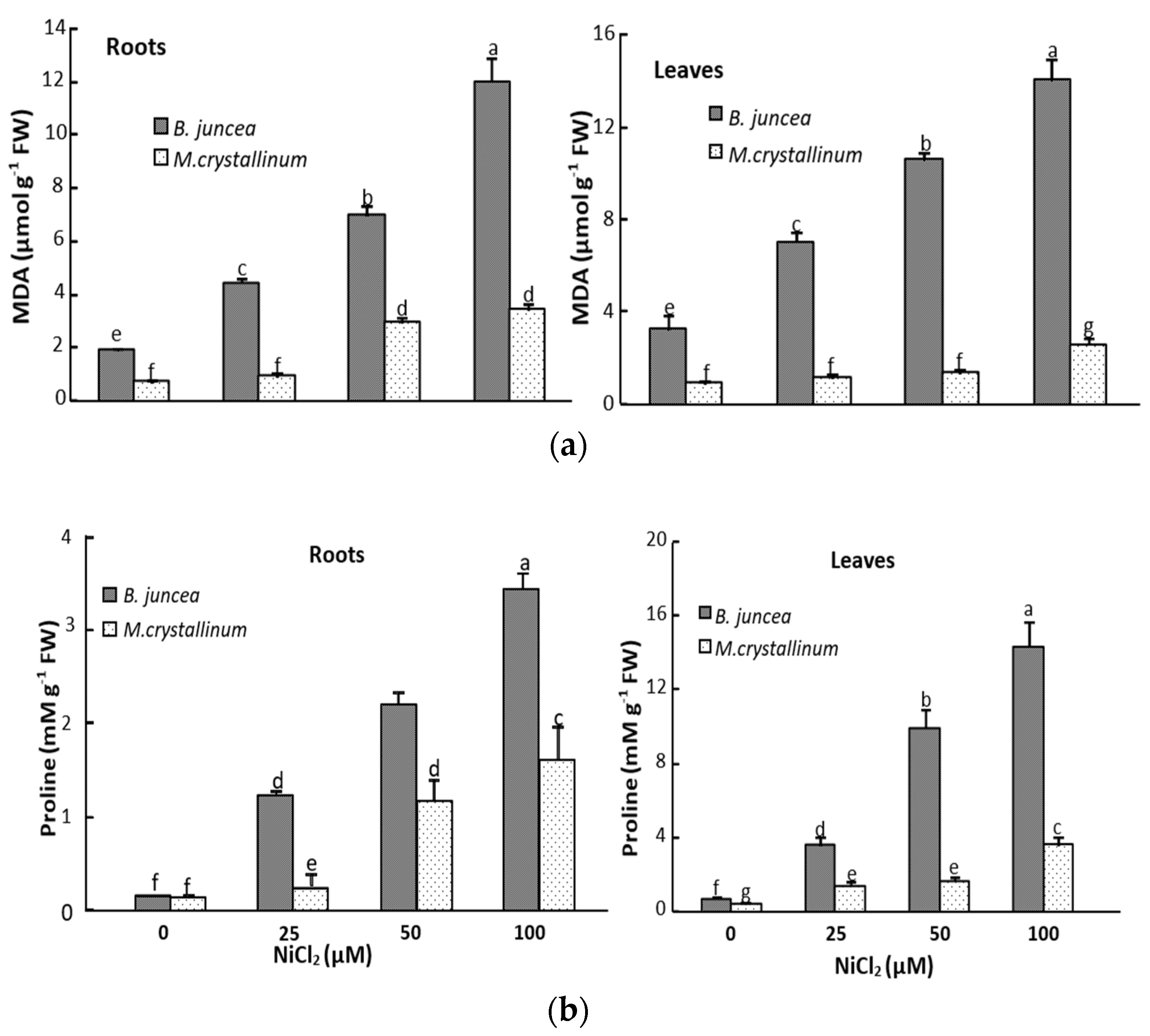
3.2. Ni Effect on Malondialdehyde (MDA) and Proline Tissus Contents
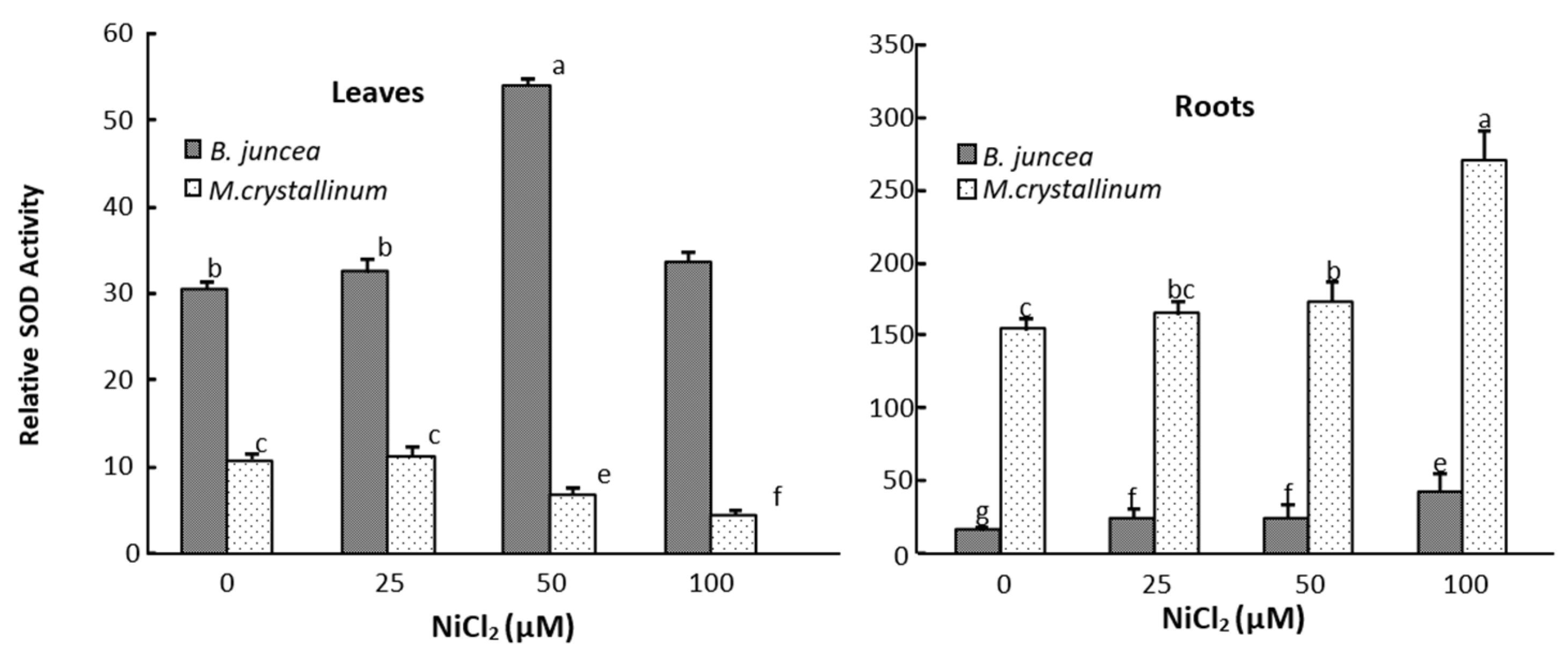
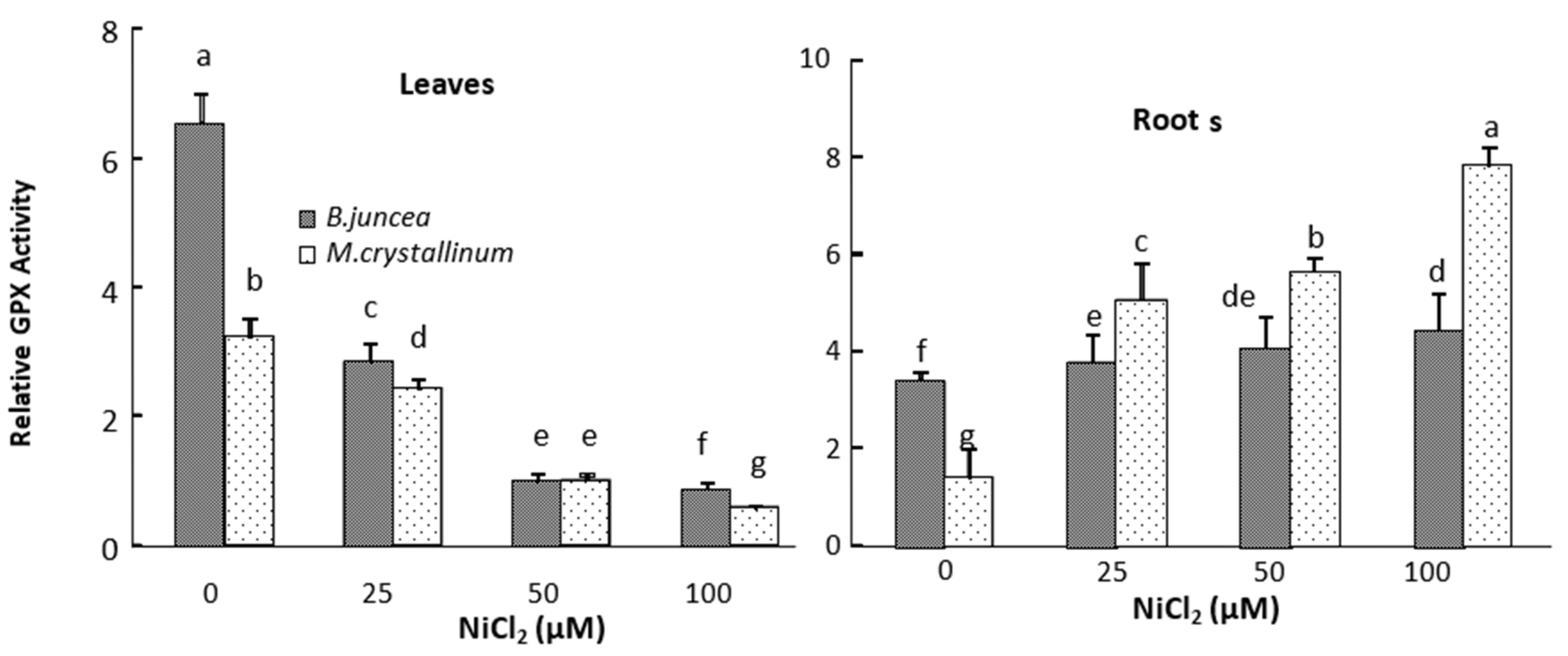
3.3. Leaf and Root Antioxidant Enzyme Activities
3.4. Non-Protein Thiol and Glutathione Contents
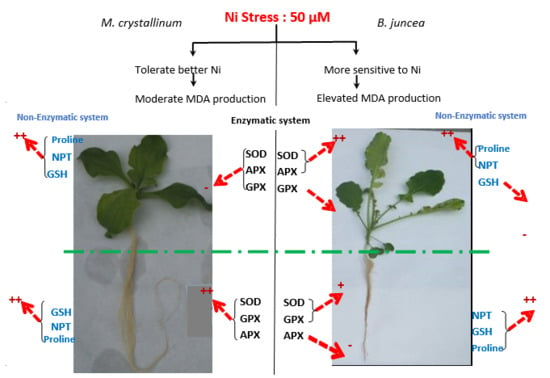
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| DTPA | diethylenetriamine pentaacetic acid |
| DTNB | dithiobis-nitrobenzoic acid |
| DTNB | dithionitrobenzoic acid |
| DTT | dithiothreitol |
| DW | Dry Weight |
| ETDA | Ethylenediaminetetraacetic acid |
| FW; GSH | glutathione, Fresh weight |
| GPX | Guaiacol peroxidase |
| H2O2 | Hydrogen Peroxide |
| OH | hydroxyl radicals |
| MDA | Malondialdehyde |
| NBT | nitroblue tetrazolium |
| NPT | Non-protein thiols |
| GSSG | oxidized glutathione |
| PMSF | phenyl-methyl-sulphonyl-fluoride |
| PS | Photosystem |
| ROS | reactive Oxygen Species |
| RGR | Relative growth rate |
| RH | Relative humidity |
| RDW | root biomass |
| SDW | shoot biomass |
| SSA | sulfosalicylic acid |
| O2− | Superoxide anion |
| SOD | superoxide dismutase |
| TBA | Thiobarbituric acid |
| TDFHA | tridecafluoroheptanoic acid |
| WPDW | whole plant dry weight |
References
- Khan, S.; Hesham, A.E.L.; Qiao, M.; Rehman, S.; He, J.Z. Effects of Cd and Pb on soil microbial community structure and activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Austruy, A.; Pierart, A.; Shahid, M. Kinetic study of phytotoxicity induced by foliar lead uptake for vegetables exposed to fine particles and implications for sustainable urban agriculture. J. Environ. Sci. 2016, 46, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Pierart, A.; Shahid, M.; Séjalon-Delmas, N.; Dumat, C. Antimony bioavailability: Knowledge and research perspectives for sustainable agricultures. J. Hazard. Mater. 2015, 289, 219–234. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Tezotto, T.; Favarin, J.L.; Azevedo, R.A.; Alleoni, L.R.F.; Mazzafera, P. Coffee is highly tolerant to cadmium, nickel and zinc: Plant and soil nutritional status, metal distribution and bean yield. Field Crop. Res. 2012, 125, 25–34. [Google Scholar] [CrossRef]
- Hussain, M.B.; Ali, S.; Azam, A.; Hina, S.; Farooq, A.M.; Ali, B.; Bharwana, A.S.; Gill, M.B. Morphological, physiological and biochemical responses of plants to nickel stress: A review. Afr. J. Agric. Res. 2013, 8, 1596–1602. [Google Scholar]
- Yusuf, M.; Fariduddin, Q.; Hayat, S.; Ahmad, A. Nickel: An overview of uptake, essentiality and toxicity in plants. Bull. Environ. Contam. Toxicol. 2011, 86, 1–17. [Google Scholar] [CrossRef]
- Gratão, P.L.; Pompeu, G.B.; Capaldi, F.R.; Vitorello, V.A.; Lea, P.J.; Azevedo, R.A. Antioxidant response of Nicotiana tabacum cv. Bright Yellow 2 cells to cadmium and nickel stress. Plant Cell Tissue Organ Cult. 2008, 94, 73–83. [Google Scholar] [CrossRef]
- Polacco, J.C.; Mazzafera, P.; Tezotto, T. Opinion–nickel and urease in plants: Still many knowledge gaps. Plant Sci. 2013, 200, 79–90. [Google Scholar] [CrossRef]
- Dourado, M.N.; Franco, M.R.; Peters, L.P.; Martins, P.F.; Souza, L.A.; Piotto, F.A.; Azevedo, R.A. Antioxidant enzymes activities of Burkholderia spp. strains—oxidative responses to Ni toxicity. Environ. Sci. Pollut. Res. 2015, 22, 19922–19932. [Google Scholar] [CrossRef]
- Vogel-Mikus, K.; Drobne, D.; Regvar, M. Zn, Cd and Pb accumulation and arbuscular mycorrhizal colonization of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environ. Pollut. 2005, 133, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Kato, A.; Tsuzuki, C.; Yoshida, J.; Mizuno, T. Induction of nickel accumulation in response to zinc deficiency in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 9420–9430. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, V.S.G.; Majorel-Loulergue, C.; Gonzalez, D.A.; Soubigou-Taconnat, L.; Rigaill, G.J.; Pillon, Y.; Barreau, L.; Thomine, S.; Fogliani, B.; Burtet-Sarramegna, V. Wide cross-species RNA-Seq comparison reveals a highly conserved role for Ferroportins in nickel hyperaccumulation in plants. bioRxiv 2018, 420–729. [Google Scholar] [CrossRef]
- Mohseni, R.; Ghaderian, S.M.; Schat, H. Nickel uptake mechanisms in two Iranian nickel hyperaccumulators, Odontarrhenabracteata and Odontarrhenainflata. Plant Soil 2018, 434, 263–269. [Google Scholar] [CrossRef]
- Takafumi, M.; Koji, U.; Kenji, H.; Shiro, N.; Naoharu, M.; Hitoshi, O. Cloning of three ZIP/Nramp transporter genes from a Ni hyperaccumulator plant Thlaspi japonicum and their Ni2+-transport abilities. Plant Physiol. Biochem. 2005, 43, 793–801. [Google Scholar]
- Seregin, I.V.; Kozhevnikova, A.D. Roles of root and shoot tissues in transport and accu-mulation of cadmium, lead, nickel, and strontium. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar]
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and future prospects. Clean Soil Air Water 2009, 7, 304–313. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Debez, A.; Taamali, M.; Benyoussef, N.; Lucchini, G.; Sacchi, G.A.; Abdelly, C. Comparative Ni tolerance and accumulation potentials between Mesembryanthemumcrystallinum (halophyte) and Brassica juncea: Metal accumulation, nutrient status and photosynthetic activity. J. Plant Physiol. 2014, 171, 1634–1644. [Google Scholar] [CrossRef]
- Gajewska, E.; Wielanek, M.; Bergier, K.; Skłodowska, M. Nickelinduced depression of nitrogen assimilation in wheat roots. Acta Physiol. Plant 2009, 31, 1291–1300. [Google Scholar] [CrossRef]
- Hao, F.; Wang, X.; Chen, J. Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci. 2006, 170, 151–158. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. Oxidative Decay of DNA. J. Biol. Chem. 1997, 32, 19633–19636. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Sklodowska, M. Effect of nickel on ROS content and antioxidative enzymeactivities in wheat leaves. Biometals 2007, 20, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ghnaya, T.; Zaier, H.; Baioui, R.; Sghaier, S.; Lucchini, G.; Sacchi, G.A.; Lutts, S.; Abdelly, C. Implication of organic acids in the long-distance transport and the accumulation of lead in Sesuviumportulacastrum and Brassica juncea. Chemosphere 2013, 90, 1449–1454. [Google Scholar] [CrossRef]
- Mnasri, M.; Ghabriche, R.; Fourati, E.; Zaier, H.; Sabally, K.; Suzelle, B.S.; Lutts, S.; Abdelly, C.; Ghnaya, T. Cd and Ni transport and accumulation in the halophyte Sesuviumportulacastrum: Implication of organic acids in these processes. Front. Plant Sci. 2015, 5, 165–169. [Google Scholar]
- Amari, T.; Lutts, S.; Taamali, M.; Lucchini, G.; Sacchi, G.A.; Abdelly, C.; Ghnaya, T. Implication of citrate, malate and histidine in the accumulation and Transport of nickel in Mesembryanthemumcrystallinum and Brassica juncea. Ecotox. Environ. Saf. 2016, 126, 122–128. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Sharma, S.S.; Schat, H.; Vooijs, R. In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry 1998, 49, 1531–1535. [Google Scholar] [CrossRef]
- Rastgoo, L.; Alemzadeh, A. Biochemical responses of Gouan (Aeluropuslittoralis) to heavy metal stress. Aust. J. Crop. Sci. 2011, 5, 375–383. [Google Scholar]
- Saiyood, S.; Vangnai, A.S.; Inthorn, D.; Thiravetyan, P. Treatment of total dissolve solids from plastic industrial effluent by halophyte plants. Water Air Soil Pollut. 2012, 223, 4865–4873. [Google Scholar] [CrossRef]
- Freeman, J.L.; Persans, M.W.; Nieman, K.; Albrecht, C.; Peer, W.; Pickering, I.J.; Salt, D.E. Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 2004, 16, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Tukiendorf, A. Glutathione in adaptation of Arabidopsis thaliana to cadmium stress. Biol. Plant 2011, 55, 125–132. [Google Scholar] [CrossRef]
- Yin, H.; Chen, Q.; Yi, M. Effets of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. J. Plant Growth Regul. 2008, 54, 45–54. [Google Scholar] [CrossRef]
- Bulbovas, P.; Souza, S.R.; Esposito, J.B.N.; Moraes, R.M.; Alves, E.S.; Domingos, M.; Azevedo, R.A. Assessment of the ozone tolerance of two soybean cultivars (Glycine max cv. Sambaíba and Tracajá) cultivated in Amazonian areas. Environ. Sci. Pollut. Res. 2014, 21, 10514–10524. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Dubey, R.S. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul. 2009, 59, 37–49. [Google Scholar] [CrossRef]
- Ghnaya, T.; Nouairi, I.; Slama, I.; Messedi, D.; Grignon, C.; Abdelly, C.; Ghorbel, M.H. Cadmium effects on growth and mineral nutrition of two halophytes: SesuviumportulacastrumGovindjee and Mesembryanthemumcrystallinum. J. Plant Physiol. 2005, 162, 1133–1140. [Google Scholar] [CrossRef]
- Reboreda, R.; Caçador, I. Halophyte vegetation influences in salt marsh retention capacity for heavy metals. Environ. Pollut. 2007, 146, 147–154. [Google Scholar] [CrossRef]
- Zaier, H.; Ghnaya, T.; Lakhdar, A.; Baioui, R.; Ghabriche, R.; Mnasri, M.; Sghaier, S.; Lutts, S.; Abdelly, C. Comparative study of Pb-phytoextraction potential in Sesuviumportulacastrum and Brassica juncea: Tolerance and accumulation. J. Hazard. Mater. 2010, 183, 609–615. [Google Scholar] [CrossRef]
- Mazharia, M.; Homaeed, M. Annual halophyte Chenopidiumbotrys can phytoextract cadmium from contaminated soils. J. Basic Appl. Sci. Res. 2012, 2, 1415–1422. [Google Scholar]
- Taamalli, M.; Ghabriche, R.; Amari, T.; Mnasri, M.; Zolla, L.; Lutts, S.; Abdely, C.; Ghnaya, T. Comparative study of Cd tolerance and accumulation potential between Cakilemaritima L. (halophyte) and Brassica juncea L. Ecol. Eng. 2014, 71, 623–627. [Google Scholar] [CrossRef]
- Arnon, D.I.; Hoagland, D.R. Crop production in artificial solutions and in soils withspecial reference to factors affecting yields and absorption of inorganic nutrients. Soil Sci. 1940, 50, 463–484. [Google Scholar]
- Aldrich, M.V.; Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; Parsons, J.G. Uptake and reduction of Cr (VI) to Cr (III) by mesquite (Prospis spp.): Chromate-plant interaction in hydroponics and solid media studied using XAS. Environ. Sci. Technol. 2003, 37, 1859–1864. [Google Scholar] [CrossRef]
- Hunt, R. Basic Growth Analysis: Plant Growth Analysis for Beginners; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hodges, M.D.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantization of micrograms quantities of protein utilizing the principle of protein-dye binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Protective enzymes against activated oxygen species in wheat (Triticum aestivum L.) seedlings: Responses to cold acclimation. J. Plant Physiol. 1999, 155, 762–768. [Google Scholar] [CrossRef]
- Luck, H. Methods of Enzymatic Aanalysis; Verlag Chemie Academic Press: New York, NY, USA, 1965; pp. 885–888. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Fielding, J.L.; Hall, J.L. Abiochemical and cytochemical study of peroxidase activity in root of Pisum sativum. J. Exp. Bot. 1978, 29, 979–986. [Google Scholar]
- Rijstenbil, J.W.; Haritonidis, S.; Malea, P.; Seferlis, M.; Wijnholds, J.A. Thiol pools and glutathione redox ratios as possible indicators of copper toxicity in the green macroalgae Enteromorpha spp. from the Scheldt estuary (SW Netherlands, Belgium) and Thermaikos Gulf (Greece, N Aegean Sea). Hydrobiologia 1998, 385, 171–181. [Google Scholar] [CrossRef]
- Sgherri, C.L.M.; Navari-Izzo, F. Sunflower seedlings subjected to increasing water deficit stress: Oxidative stress and defence mechanisms. Physiol. Plant 1995, 93, 25–30. [Google Scholar] [CrossRef]
- Armstrong, M.; Jonscher, K.; Reisdorph, N.A. Analysis of 25 underivatized amino acids in human plasma using ion-pairing reversed-phase liquid chromatography/time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2717–2726. [Google Scholar] [CrossRef]
- Bai, C.; Reilly, C.C.; Wood, B.W. Nickel deficiency disrupts metabolism of ureides, aminoacids, and organic acids of young pecan foliage. Plant Physiol. 2006, 140, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Fourati, E.; Wali, M.; Vogel-Mikus, K.; Abdelly, C.; Ghnaya, T. Nickel tolerance, accumulation and subcellular distribution in the halophytes Sesuviumportulacastrum and Cakilemaritima. Plant Physiol. Biochem. 2016, 108, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Boominathan, R.; Doran, P.M. Ni induced oxidative stress in roots of the Ni hyperac-cumulator, Alyssum bertolonii. New Phytol. 2002, 156, 205–215. [Google Scholar] [CrossRef]
- Ferraz, P.; Fidalgo, F.; Almeida, A.; Teixeira, J. Phytostabilization of nickel by the zinc and cadmium hyperaccumulator Solanum nigrum L. Are metallothioneins involved? Plant Physiol. Biochem. 2012, 57, 254–260. [Google Scholar] [CrossRef]
- Shahid, M.; Ferrand, E.; Schreck, E.; Dumat, C. Behavior and impact of zirconium in the soil-plant system: Plant and phytotoxicity. Rev. Environ. Contam. Toxicol. 2013, 221, 107–127. [Google Scholar]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Differential biochemical responses of wheat shoots and roots to nickel stress: Antioxidative reactions and proline accumulation. Plant Growth Regul. 2008, 54, 179–188. [Google Scholar] [CrossRef]
- Dubey, D.; Pandey, A. Effect of nickel (Ni) on chlorophyll, lipid peroxidation and antioxidant enzymes activities in black gram (Vigna mungo) leaves. Int. J. Sci. Nat. 2011, 2, 395–401. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford Science Publications: Oxford, UK, 1999. [Google Scholar]
- Maksymiec, W. Signaling responses in plants to heavy metal stress. Acta. Physiol. Plant. 2007, 29, 177–187. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Corpas, F.J.; Rodriguez-Serrano, M.; Gomez, M.; del Río, L.A.; Sandalio, L.M. Differential expression and regulation of antioxidative enzymes by Cd in pea plants. J. Plant Physiol. 2007, 164, 1346–1357. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Li, Y.Y.; Pang, C.H.; Lu, C.M.; Wang, B.S. NaCl enhances thylakoid-bound activity in the leaves of C3 halophyte Suaeda salsa L. Plant Sci. 2005, 168, 423–430. [Google Scholar]
- Gomes-Juniora, R.A.; Moldes, C.A.; Delite, F.S.; Gratão, P.L.; Mazzafer, P.; Leac, P.J.; Azevedoa, R.A. Nickel elicits a fast antioxidant response in Coffea arabica cells. Plant Physiol. Biochem. 2006, 44, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Sharma, D.; Kumar, V. Nickel-induced oxidative stress and role of antioxidant defence in Barley roots and leaves. Int. J. Environ. Biol. 2012, 2, 121–128. [Google Scholar]
- Papadopoulos, A.; Prochaska, C.; Papadopoulos, F.; Gantidis, N.; Metaxa, E. Determination and evaluation of cadmium, copper, nickel, and zinc in agricultural soils of western Macedonia, Greece. Environ. Manag. 2007, 40, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Ivanov, V.B. Physiological Aspects of Cadmium and Leads Toxic Effects on the Higher Plants. Russ. J. Plant Physiol. 2001, 48, 606–630. [Google Scholar] [CrossRef]
- Ros, R.; Morales, A.; Segura, J.; Picazo, I. In Vivo and In Vitro Effects of Nickel and Cadmium on the Plasmalemma ATPase from Rice (Oryza sativa L.) Shoots and Roots. Plant Sci. 1992, 83, 1–6. [Google Scholar] [CrossRef]
- Gajewska, E.; Sklodowska, M.; Slaba, M.; Mazur, J. Effect of nickel on antioxidative enzyme activities, proline and chlorophyll content in wheat shoots. Biol. Plant 2006, 50, 653–659. [Google Scholar] [CrossRef]
- Jocsak, I.; Vegvari, G.; Rabnecz, G.; Droppa, M. Investigation of nickel stress induction in terms of metal accumulation and antioxidative enzyme activity in barley seedlings. Acta Biol. Szeged. 2008, 52, 167–171. [Google Scholar]
- Radotic, K.; Ducic, T.; Mutavdzic, D. Changes in peroxidase activity and isozymes in spruce needles after exposure to different concentrations of cadmium. Environ. Exp. Bot. 2000, 44, 105–113. [Google Scholar] [CrossRef]
- Gonnelli, C.; Galardi, F.; Gabbrielli, R. Nickel and copper tolerance and toxicity in three Tuscan populations of Sileneparadoxa. Physiol. Plant 2001, 113, 507–514. [Google Scholar] [CrossRef]
- Gajewska, E.; Sklodowska, M. Antioxidative responses and proline level in leaves and roots of pea plants subjected to nickel stress. Acta. Physiol. Plant. 2005, 27, 329–339. [Google Scholar] [CrossRef]
- Cardoso, P.F.; Gratao, P.L.; Gomes-Junior, A.L.; Medici, L.O.; Azevedo, R.A. Response of Crotalaria juncea to nickel exposure. Braz. J. Plant Physiol. 2005, 17, 267–272. [Google Scholar] [CrossRef]
- Bouazizi, H.; Jouili, H.; Geitmann, A.; ElFerjani, E. Cupric stress induces oxidative damage marked by accumulation of H2O2 and changes to chloroplast ultrastructure in primary leaves of beans (Phaseolus vulgaris L.). Acta Biol. Hung. 2010, 61, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signalling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Montagu, M.V.; Inzé, D.; Camp, W.V. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef] [PubMed]
- Rabenstein, D.L.; Guevremont, R.; Evans, C.A. Glutathione and Its Metal-Complexes. In Metal Ions in Biological Systems; Singel, H., Ed.; Marcel Dekker: New York, NY, USA, 1985; pp. 104–141. [Google Scholar]
- Cánovas, D.; Vooijs, R.; Schat, H.; De Lorenzo, V. The role of thiol species in the hypertolerance of Aspergillus sp. P37 to arsenic. J. Biol. Chem. 2004, 279, 51234–51240. [Google Scholar] [CrossRef]
- Nocito, F.F.; Lancilli, C.; Crema, B.; Fourcroy, P.; Davidian, J.C.; Sacchi, G.A. Heavy metal stress and sulfate uptake in maize roots. Plant Physiol. 2006, 141, 1138–1148. [Google Scholar] [CrossRef]
- Estrella-Gomez, N.; Mendoza-Cózatl, D.; Moreno-Sanchez, R.; Gonzalez-Mendoza, D.; Zapata-Perez, O.; Martinez-Hernandez, A.; Santamaria, J.M. The Pb-hyperaccumulator aquatic fern Salvinia minima Baker, responds to Pb2+ by increasing phytochelatins via changes in SmPCS expression and in phytochelatin synthase activity. Aquat. Toxicol. 2009, 91, 320–328. [Google Scholar] [CrossRef]
- Spuches, A.M.; Kruszyna, H.G.; Rich, A.M.; Wilcox, D.E. Thermodynamics of the As (III)-thiol interaction: Arsenite and monomethylarsenite complexes with glutathione, dihydrolipoic acid, and other thiol ligands. Inorg. Chem. 2005, 44, 2964–2972. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kafel, A.; Kandziora-Ciupa, M.; Gospodarek, J.; Zawisza-Raszka, A. Accumulation of heavy metals and antioxidant responses in Viciafaba plants grown on monometallic contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Boaretto, L.F.; Carvalho, G.; Borgo, L.; Creste, S.; Landell, M.G.A.; Mazzafera, P.; Azevedo, R.A. Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol. Biochem. 2014, 74, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.B.; Korpelainen, H.; Li, C.Y. Physiological and biochemical responses to high Mn concentrations in two contrasting Populuscathayana populations. Chemosphere 2007, 68, 686–694. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2009, 15, 89–97. [Google Scholar] [CrossRef]
- Okuma, E.; Murakami, Y.; Shimoishi, Y.; Tada, M.; Murata, Y. Effects of exogenous application of proline and betaine on the growth of tobacco cultured cells under saline conditions. Soil Sci. Plant Nut. 2004, 50, 1301–1305. [Google Scholar] [CrossRef]
- Kaul, S.; Sharma, S.; Mehta, I. Free radical scavenging potential of L-proline: Evidence from in vitro assays. Amino Acids 2008, 34, 315–320. [Google Scholar] [CrossRef]
- Kolodyazhnaya, Y.S.; Titov, S.E.; Kochetov, A.V.; Trifonova, E.A.; Romanova, A.V.; Komarova, M.L.; Shumny, V.K. Tobacco transformants expressing antisense sequence of proline dehydrogenase gene possess tolerance to heavy metals. Russ. J. Genet. 2007, 43, 825–828. [Google Scholar] [CrossRef]
- Tamas, L.; Dudikova, J.; Durcekova, K.; Haluskova, L.; Huttova, J.; Mistrik, I.; Olle, M. Alterations of the gene expression, lipid peroxidation, proline and thiol content along the barley root exposed to cadmium. J. Plant Physiol. 2008, 165, 1193–1203. [Google Scholar] [CrossRef]
- Dinakar, N.; Nagajyothi, P.C.; Suresh, S.; Udaykiran, Y.; Damodharam, T. Phytotoxicity of cadmium on protein, proline and antioxidant enzyme activities in growing Arachis hypogaea L. seedlings. J. Environ. Sci. 2008, 20, 199–206. [Google Scholar] [CrossRef]
- Shevyakova, N.I.; Netronina, I.A.; Aronova, E.E.; Kuznetsov, V.V. Compartmentation of cadmium and iron in Mesembryanthemumcrystallinum plants during the adaptation to cadmium stress. Russ. J. Plant Physiol. 2003, 50, 678–685. [Google Scholar] [CrossRef]
- Ozturk, L.; Demir, Y. In vivo and in vitro protective role of proline. Plant Growth Regul. 2002, 38, 259–261. [Google Scholar] [CrossRef]

| NiCl2 | RDW (g) | SDW (g) | WPDW (g) | RGR (d−1) |
|---|---|---|---|---|
| M. crystallinum | ||||
| 0 | 0.70 ± 0.06a | 2.17 ± 0.11a | 2.77 ± 0.12a | 0.081 ± 0.003a |
| 25 | 0.47 ± 0.02b | 1.62 ± 0.04b | 2.09 ± 0.03b | 0.075 ± 0.004b |
| 50 | 0.41 ± 0.03c | 1.41 ± 0.07c | 1.82 ± 0.10c | 0.064 ± 0.004c |
| 100 | 0.30 ± 0.02d | 0.89 ± 0.04d | 1.19 ± 0.08d | 0.049 ± 0.002d |
| B. juncea | ||||
| 0 | 1.19 ± 0.10a | 5.22 ± 0.32a | 6.41 ± 0.41a | 0.121 ± 0.004a |
| 25 | 0.89 ± 0.09b | 3.94 ± 0.28b | 4.82 ± 0.37b | 0.086 ± 0.004b |
| 50 | 0.61 ± 0.04c | 2.13 ± 0.14c | 2.76 ± 0.18c | 0.054 ± 0.002c |
| 100 | 0.50 ± 0.05d | 1.60 ± 0.21d | 2.1 ± 0.26d | 0.042 ± 0.003d |
| NiCl2 (µM) | Brassica juncea | Mesembryanthemum crystallinum | ||
|---|---|---|---|---|
| Root | Shoot | Root | Shoot | |
| NPT (nmol/g FW) | ||||
| 0 µM | 305.29 ± 29.13c | 19.7 ± 0.053b | 301.90 ± 26.18b | 96.29 ± 7.35c |
| 25 µM | 301.47 ± 28.12c | 18.76 ± 1.34b | 309.90 ± 6.13b | 97.99 ± 6.72c |
| 50 µM | 457.81 ± 40.74ab | 14.32 ± 1.12c | 547.60 ± 24.52a | 156.45 ± 12.61b |
| 100 µM | 579.82 ± 53.67a | 82.74 ± 5.21a | 522.28 ± 31.19a | 601.30 ± 55.11a |
| GSH (µmol/g FW) | ||||
| 0 µM | 0.135 ± 0.017d | 0.147 ± 0.006a | 0.106 ± 0.004d | 0.221 ± 0.005b |
| 25 µM | 0.123 ± 0.013cd | 0.076 ± 0.008b | 0.136 ± 0.001c | 0.213 ± 0.041c |
| 50 µM | 0.205 ± 0.012b | 0.064 ± 0.005c | 0.172 ± 0.015b | 0.318 ± 0.034a |
| 100 µM | 0.265 ± 0.010a | 0.068 ± 0.004c | 0.318 ± 0.018a | 0.307 ± 0.030a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amari, T.; Souid, A.; Ghabriche, R.; Porrini, M.; Lutts, S.; Sacchi, G.A.; Abdelly, C.; Ghnaya, T. Why Does the Halophyte Mesembryanthemum crystallinum Better Tolerate Ni Toxicity than Brassica juncea: Implication of Antioxidant Defense Systems. Plants 2020, 9, 312. https://doi.org/10.3390/plants9030312
Amari T, Souid A, Ghabriche R, Porrini M, Lutts S, Sacchi GA, Abdelly C, Ghnaya T. Why Does the Halophyte Mesembryanthemum crystallinum Better Tolerate Ni Toxicity than Brassica juncea: Implication of Antioxidant Defense Systems. Plants. 2020; 9(3):312. https://doi.org/10.3390/plants9030312
Chicago/Turabian StyleAmari, Taoufik, Aymen Souid, Rim Ghabriche, Mauro Porrini, Stanley Lutts, Gian Attilio Sacchi, Chedly Abdelly, and Tahar Ghnaya. 2020. "Why Does the Halophyte Mesembryanthemum crystallinum Better Tolerate Ni Toxicity than Brassica juncea: Implication of Antioxidant Defense Systems" Plants 9, no. 3: 312. https://doi.org/10.3390/plants9030312
APA StyleAmari, T., Souid, A., Ghabriche, R., Porrini, M., Lutts, S., Sacchi, G. A., Abdelly, C., & Ghnaya, T. (2020). Why Does the Halophyte Mesembryanthemum crystallinum Better Tolerate Ni Toxicity than Brassica juncea: Implication of Antioxidant Defense Systems. Plants, 9(3), 312. https://doi.org/10.3390/plants9030312