Abstract
To characterize cultivar variation in resistance gene (R-gene)-mediated calcium signaling and hormonal regulation in effector-triggered immunity (ETI) and disease susceptibility, Xanthomonas campestris pv. campestris (Xcc) was inoculated in two Brassica napus cultivars (cvs. Capitol and Mosa). At 14 days post inoculation (DPI) with Xcc, there was a necrotic lesion in cv. Mosa along with the significant accumulation of H2O2 and malondialdehyde (MDA), whereas no visual symptom was observed in cv. Capitol. The cultivar variations in the R-gene expressions were found in response to Xcc. ZAR1 is a coiled-coil-nucleotide binding site-leucine-rich repeat (CC-NB-LRR)-type R-gene that is significantly induced in cv. Capitol, whereas toll/interleukin-1 receptor-nucleotide binding site-leucine-rich repeat (TIR-NB-LRR)-type R-gene, TAO1, is significantly upregulated in cv. Mosa Xcc-inoculated plants. The defense-related gene’s non-race-specific disease resistance 1 (NDR1) and mitogen-activated protein kinase 6 (MAPK6) were enhanced, whereas calcium-dependent protein kinase (CDPK5) and calcium-sensing protein 60g (CBP60g) were depressed in cv. Capitol Xcc inoculated plants, and opposite results were found in cv. Mosa. The calcium-sensing receptor (CAS), calmodulin (CaM), expression was induced in both the cultivars. However, the CAS induction rate was much higher in cv. Mosa than in cv. Capitol in response to Xcc. The phytohormone salicylic acid (SA) and jasmonic acid (JA) levels were significantly higher in cv. Capitol along with the enhanced SA receptors (NPR3 and NPR4) and JA synthesis and signaling-related gene expression (LOX2, PDF1.2), whereas the JA level was significantly lower in cv. Mosa Xcc inoculated plants. The SA synthesis and signaling-related genes (ICS1, NPR1) and SA were present at higher levels in cv. Mosa; additionally, the SA level present was much higher in the susceptible cultivar (cv. Mosa) than in the resistant cultivar (cv. Capitol) in response to Xcc. These results indicate that ZAR1 mediated the coordinated action of SA and JA synthesis and signaling to confirm ETI, whereas TAO1 enhanced the synthesis of SA through CAS and CBP60g to antagonize JA synthesis and signaling to cause disease susceptibility in the Brassica napus–Xcc pathosystem.
1. Introduction
Black rot disease of oilseed rape (Brassica napus) caused by Xanthomonas campestris pv. campestris (Xcc) is a major threat that reduces the quality and productivity of Brassicaceae crops. Yellow v-shaped necrotic lesions and darkened leaf veins are the most common symptoms [1,2].
Effector-triggered immunity (ETI) is initiated by resistance genes (R-genes) [3,4]. Coiled-coil-nucleotide binding site-leucine-rich repeat (CC-NB-LRR) and toll/interleukin-1 receptor-nucleotide binding site-leucine-rich repeat (TIR-NB-LRR) are two major types of R-genes that regulate the activation of the plant immune response, especially phytohormone signaling to counteract pathogenic infection [5]. Reactive oxygen species (ROS) and calcium signaling play important roles in pattern-triggered immunity (PTI) or ETI [4]. H2O2 is the primary detectable oxide that is capable of inducing cytosolic Ca2+ and calcium-dependent protein kinases (CDPKs) during pathogenic attack [4,6,7]. Phytohormone salicyclic acid (SA), a critical messenger, is induced for immune response during plant–pathogen interaction modulated by different Ca2+ signaling [8,9,10].
The phytohormones SA and jasmonic acid (JA) are the central regulators of plant immune responses. SA induces the defense mechanism against biotrophic pathogens, whereas JA induces the defense mechanism against necrotrophic pathogens. SA and JA signaling are mutually antagonistic, and the antagonism depends on SA signaling gene nonexpressor of pathogenesis-related gene 1 (NPR1) [11]. The fine-tuning of this hormonal signaling is key to activating effective defense responses to hemibiotrophic pathogens such as Xcc [7], which has both biotrophic and necrotrophic phases of infection. Liu et al. (2016) [12] reported that the nonexpressor of pathogenesis-related genes 3 and 4 (NPR3 and NPR4) act as SA receptors, which induce JA synthesis and signaling towards ETI against the hemibiotrophic bacterial pathogen Pseudomonas syringae pv. tomato. The necrotrophic or hemibiotrophic pathogens in the necrotrophic phase of the infection may use SA signaling to induce disease progression by antagonizing JA signaling [13].
We hypothesized that the genotypic difference in the expression of the R-gene might play a significant role in activating the different hormonal signalings through the calcium signaling in resistant and susceptible B. napus–Xcc interactions. To test this hypothesis, the expressions of two different R-genes ZAR1 (CC-NB-LRR) and TAO1 (TIR-NB-LRR) [14] (different calcium signaling genes) were interpreted along with endogenous alteration of JA and SA levels as well as their synthesis and signaling genes in two contrasting B. napus cultivars in responses to Xcc.
2. Results
2.1. Development of Symptoms and Oxidative Stress
In the response to Xcc, there were no visual symptoms observed in cv. Capitol (Figure 1A), whereas a visible v-shaped necrotic lesion was clearly observed in cv. Mosa (Figure 1B). In the cultivar Mosa, H2O2 and lipid peroxidation levels in Xcc-inoculated plants were significantly higher (18.8% and 30.8%, respectively) than those of non-Xcc-inoculated plants (Figure 1C,D).

Figure 1.
Visible disease symptom development (A,B), H2O2 concentration (C), and lipid peroxidation level (D) in the non-Xcc-inoculated (white bar) and Xcc-inoculated leaves (black bar) of two Brassica napus cultivars. Vertical bars indicate mean ± SE (n=3). Significant difference levels between non-inoculated and Xcc-inoculated plants are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
2.2. Expression of the Resistance Genes and Related Genes
In cv. Capitol, Xcc inoculation enhanced the expression of CC-NB-LRR-type R-gene ZAR1 (Figure 2A). However, in cv. Mosa, TIR-NB-LRR-type R-gene TAO1 was significantly enhanced by Xcc inoculation, whereas it was downregulated in cv. Capitol (Figure 2B). NDR1 gene (Figure 2C) was significantly induced (102.75-fold) in cv. Capitol, whereas it was not changed in cv. Mosa. Xcc inoculation induced MAPK6 (Figure 2D) significantly (47.5-fold) in cv. Capitol, and it was downregulated in cv. Mosa.

Figure 2.
Relative gene expression of Hop (Hrp-dependent outer protein) Z-activated resistance 1 (ZAR1, A) and target of AvrB operation 1 (TAO1, B), non-race-specific disease resistance 1 (NDR1, C), mitogen-activated protein kinase 6 (MAPK6, D) in the non-Xcc-inoculated (white bar) and Xcc-inoculated leaves (black bar) of two Brassica napus cultivars. Vertical bars mean ± SE (n=3). Significant levels (between non-inoculated and Xcc-inoculated plants) are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
2.3. Expression of the Calcium Signaling-Related Genes
The calcium sensors Ca2+ATPase, CDPK5, and CBP60g were downregulated in cv. Capitol, whereas these were significantly upregulated in cv. Mosa (5.24-fold, 34.71%, 1.00-fold, respectively) by Xcc inoculation (Figure 3A,B,C). However, Xcc induced the expression of CAS and Calmodulin (CaM) significantly in both cultivars (Figure 3D,E).

Figure 3.
Relative expression of calcium signaling genes. (A) Ca2+ATPase, (B) calcium-dependent protein kinase (CDPK5), (C) calmodulin-binding protein 60g (CBP60g), (D) calcium-sensing receptor (CAS), (E) calmodulin (CaM) in the non-Xcc-inoculated (white bar) and Xcc-inoculated leaves (black bar) of two Brassica napus cultivars. Vertical bars mean ± SE (n=3). Significant levels (between non-inoculated and Xcc-inoculated plants) are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
2.4. Expression of Hormonal Synthesis-Related Genes and Endogenous Content of SA and JA
JA synthesis-related gene lipoxygenase 2 (LOX2) was significantly (p < 0.001) enhanced by Xcc inoculation in cv. Capitol, whereas it was depressed (-40.16%) in cv. Mosa when compared to the respective non-Xcc-inoculated plants (Figure 4A). Xcc inoculation significantly (p < 0.05) depressed two SA synthesis-related genes, EDS1 and ICS1, in cv. Capitol. However, a significant enhancement of ICS1 expression was observed only in cv. Mosa (Figure 4B,C).

Figure 4.
Relative expression of hormonal synthesis-related genes (A–C) and the endogenous level of jasmonic acid (JA, D), salicylic acid (SA, E), and SA/JA ratio (F) in the non-Xcc-inoculated (white bar) and Xcc-inoculated leaves (black bar) of two Brassica napus cultivars. Vertical bars mean ± SE (n=3). Significant differences between non-inoculated and Xcc-inoculated plants are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
Xcc inoculation increased (p < 0.05) the endogenous level of JA in cv. Capitol, while it significantly decreased (p < 0.001) in cv. Mosa (Figure 4D). Xcc inoculation significantly increased the SA content in both cultivars. The increase of SA was much higher in cv. Mosa (2.9-fold) than in cv. Capitol (1.2-fold) (Figure 4E). Xcc inoculation-induced alteration in the endogenous SA and JA levels was much more distinct in cv. Mosa (5.3-fold of SA/JA ratio), whereas the ratio was not significantly changed in cv. Capitol (Figure 4F).
2.5. Expression of Hormonal Signaling-Related Genes
In cv. Capitol, Xcc inoculation highly enhanced the expression of JA signaling-related gene plant defensin 1.2 (PDF1.2) (Figure 5A), whereas enhanced expression of the SA signaling gene NPR1 was observed in cv. Mosa (Figure 5B). SA receptor genes (NPR3 and NPR4) were significantly expressed in cv. Capitol (Figure 5C,D).
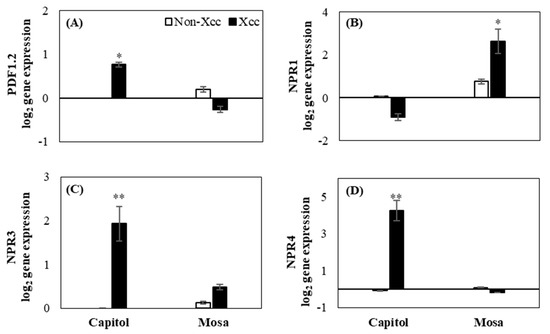
Figure 5.
Relative expression of hormonal signaling genes. (A) Plant defensing factor 1.2 (PDF1.2), (B) non-expresser of pathogenesis-related (PR) genes 1 (NPR1), (C) NPR3, and (D) NPR4 in the non-Xcc-inoculated (white bar) and Xcc-inoculated leaves (black bar) of two Brassica napus cultivars. Vertical bars mean ± SE (n=3). Significant levels (between non-inoculated and Xcc-inoculated plants) are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
3. Discussion
During plant–pathogen interactions, H2O2 is the primary detectable oxide that can initiate the destruction of the challenged plant cell through lipid peroxidation or programmed cell death [6,7]. In the present study, cultivar variation in symptoms and oxidative stress development was observed (Figure 1A,B), as shown by a yellow v-shaped necrotic lesion along with enhanced H2O2 (Figure 1C) and lipid peroxidation (Figure 1D) in cv. Mosa, whereas no visible symptoms were observed in cv. Capitol in response to Xcc inoculation. ROS (especially H2O2 production) relies on calcium signaling in response to pathogens. The primary detectable oxide H2O2 is capable of inducing cytosolic Ca2+ during pathogenic attack. Interestingly, the full activation of respiratory burst oxidase homolog protein D (RBOHD) for ROS production requires phosphorylation by Ca2+-induced calcium-dependent protein kinases (CDPKs) [4,6,7], as we found that different calcium signaling genes were upregulated in cv. Mosa Xcc-inoculated plants along with H2O2 and MDA (Figure 1C,D and Figure 3).
In our previous study, it was found that cultivar variation in hormonal status regulates the development of Xcc symptoms in different B. napus cultivars [7,13,15]. In addition, it has been reported that the plant immune system might play a significant role in fine-tuning hormonal signaling to activate effective immune responses against pathogen infection [11,16].
Plants possess a multilayered immune system to protect them from pathogenic infection [17,18,19]. Pattern recognition receptors (PRRs) initiate the recognition of pathogen-associated molecular patterns (PAMPs) to induce pattern-triggered immunity [17]. ETI is a long-lasting defense response and has been known to be activated by the interactions between the pathogen effector and plant R-genes. During the plant–pathogen interaction, different calcium signaling genes are induced towards plant defense [4,10,20]. In the present study, we hypothesized that the R-genes are involved in regulating hormonal signaling through calcium signaling in the resistant or susceptible interaction in the B. napus–Xcc pathosystem. We thus analyzed the expressions of two types of R-genes, ZAR1 and TAO1, in two B. napus cultivars, contrasting disease susceptibility against Xcc infection. The R-genes are usually nucleotide-binding/leucine-rich-repeat (NLR) receptors [21], and two sub-families of these R-genes could be distinguished as CC-NB-LRR and TIR-NB-LRR, which regulate plant defense through calcium and phytohormonal signaling [5].
ZAR1 is one of the CC-NB-LRR R-genes that regulates ETI in response to Xcc through the binding complex to recognize the bacterial effector in Arabidopsis [22]. It has also been reported that CC-NB-LRR-type R-genes regulate the coordinated actions of SA and JA responses to induce ETI against hemibiotrophic pathogens [12]. For ETI induction, NDR1 and MAPKs are important genes that are activated by the induction of R-genes in biotic stress, and in CC-NB-LRR type R-genes, MAPKs are responsible for regulating SA signaling for inducing ETI [5,23]. In this study, we found that NDR1 and MAPK6 (Figure 2C,D) were significantly upregulated along with ZAR1 (Figure 2A). This indicates that ZAR1 might upregulate NDR1 and MAPK6 after Xcc inoculation in cv. Capitol. However, TAO1 belongs to another group of R-genes (TIR-NB-LRR) responsible for disease resistance by inducing SA signaling [14,15]. Ca2+ATPase is an important sensor in calcium fluctuation in several types of stresses [24], and in this study, it was regulated in cv. Mosa with Xcc inoculation (Figure 3A). Another calcium signaling gene, CDPK5, is an important calcium sensor [6,7,25] in biotic stress that is also induced in cv. Mosa (Figure 3B) after Xcc inoculation. This indicates that TAO1 (Figure 2B), after Xcc inoculation, induced the expression of Ca2+ATPase and CDPK5 (Figure 3A,B) along with symptom development in cv. Mosa. Calcium-sensing receptors and signaling genes are involved in phytohormonal synthesis for the plant immunity system [8,9]. Positive interactions between CaM and CBP60g regulate SA synthesis and signaling through the ICS1 in disease resistance [8,9]. Another report indicated that unknown calcium signaling-induced CAS is involved in SA biosynthesis through ICS1 for mediating ETI of PTI [9,26]. In the current study, CAS and CaM (Figure 3D,E) gene expressions were significantly induced in both cultivars along with SA (Figure 4E), whereas CBP60g (Figure 3C) was highly expressed in cv. Mosa. The results indicate that calcium signaling genes Ca2+ATPase, CDPK5, and CaM (Figure 3A,B,E) might be induced by the induction of TAO1 with interaction with H2O2 in cv. Mosa (Figure 1C and Figure 2B). These calcium signaling genes might have induced CBP60g and CAS for SA synthesis and signaling after Xcc inoculation in cv. Mosa (Figure 3C,D and Figure 4E), whereas ZAR1 (Figure 2A) might induce the CAS and CaM (Figure 3D,E) that mediated SA synthesis and signaling in cv. Capitol. However, the question of how CAS and CaM mediate SA synthesis and signaling still needs to be answered.
Phytohormones SA and JA are the usual central regulators of the plant defense system against biotrophic and necrotrophic pathogens, respectively [7,16,27]. In the present study, the expression of R-genes in response to Xcc infection was associated with the endogenous hormonal status in the resistant or susceptible B. napus–Xcc interaction, as shown by a distinct accumulation of JA in cv. Capitol (Figure 4D), while SA in cv. Mosa (Figure 4E) resulted in a much higher SA/JA ratio in cv. Mosa (Figure 4F). Previously, we found with six different B. napus that a higher SA/JA ratio was symptomatic of Xcc disease [7] and that the JA-mediated phenolic metabolite accumulation, especially in the cell wall-bound form, was an important feature of disease resistance [16]. The present data confirmed that JA induction with an antagonistic depression of SA (thus maintaining proper SA/JA ratio) would be part of the resistance mechanism against the hemibiotrophic pathogen Xcc in B. napus.
Furthermore, in the resistant cultivar Capitol, the enhanced expression of ZAR1 (Figure 2A) was concomitant with the enhanced expression of JA synthesis-related LOX2 (Figure 4A) and JA signaling PDF1.2 (Figure 5A) genes, accompanied by higher accumulations of JA (Figure 4D). Interestingly, enhanced expression of ZAR1 in cv. Capitol was accompanied by enhanced expression of SA receptor genes NPR3 and NPR4 (Figure 5C,D). It has been reported that the RPS2, a CC-NB-LRR-type R-gene, induced NPR3 and NPR4 and activated JA synthesis via the degradation of JAZ1 proteins in response to hemibiotrophic pathogen infection [13]. In addition, higher accumulation of SA depressed the expression of NPR3 and NPR4 [28]. In the susceptible cultivar cv. Mosa, the enhanced expression of TIR-NB-LRR-type R-gene TAO1 (Figure 2B) coincided with the enhanced expression of SA synthesis-related genes ICS1 (Figure 4C) along with CBP60g and CAS (Figure 3C,D). Higher disease susceptibility in cv. Mosa (Figure 1B) was characterized by the enhanced expression of TAO1-mediated SA-dependent gene NPR1 (Figure 5B), which was accompanied by a higher SA/JA ratio (Figure 4F), in accordance with previous reports [5,14,29].
This report, as far as we know, is the first to provide evidence that CC-NB-LRR R-gene ZAR1 is involved in mediating CAS and CaM to initiate SA synthesis and signaling-regulated disease-resistant interaction by JA synthesis and signaling (Figure 6A), whereas the TIR-NB-LRR-type R-gene TAO1-mediated SA accumulation by CAS and CBP60g with an antagonistic depression of JA and is part of disease susceptibility (Figure 6B) in the B. napus–Xcc pathosystem.
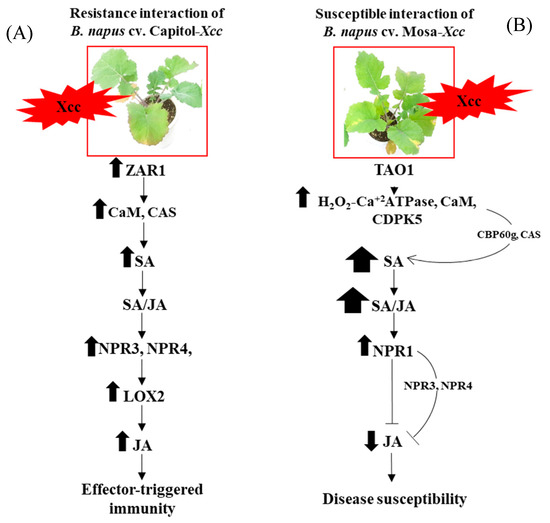
Figure 6.
Outline of R-gene-mediated hormonal signaling in effector-triggered immunity (ETI) in Brassica napus cv. Capitol (A) and disease susceptibility in Brassica napus cv. Mosa (B) in response to Xcc inoculation. The impact of treatment on the measurement was expressed by the thickness of each arrow.
4. Materials and Methods
4.1. Plant Material and Bacterial Inoculation
The seedlings of two B. napus cultivars (cvs. Capitol and Mosa) were grown in plastic pots. After six leaf stages, the plants were divided into two groups of each cultivar. One group was the control group, and another group was the experimental group with pathogen inoculation. The pathogenic bacterial (Xanthomonas campestris pv. campestris) strain was collected from the Korean Agricultural Culture Collection and was cultured in Yeast dextrose calcium carbonate (YDC) in an agar plate at 30 °C for 48 h. The bacterial concentration was then adjusted with 108 CFU/mL, and the plants’ leaves were inoculated with the bacteria by clipping the leaf edges near the veins using mouth–tooth forceps. The experiment was conducted with a completely randomized design with three biological replications. After 14 days of inoculation, six leaves of each plant were sampled from both pathogen-inoculated or control (non-inoculated) plants in the same leaf rank order. Six combined leaves were considered as a biological replicate of each treatment. The collected samples were immediately frozen in liquid nitrogen and stored in a deep freezer (−80 °C) for further analysis.
4.2. Determination of Hydrogen Peroxide (H2O2) and Lipid Peroxidation
Hydrogen peroxide levels were measured according to the method described by Venisse et al., 2019 [30]. The concentration of malondialdehyde (MDA) was measured to determine the lipid peroxidation level, using the method described by Lee et al., 2007 [31].
4.3. Phytohormone Analysis
Quantitative analysis of jasmonic acid and salicylic acid in the leaf tissue was performed according to Pan et al., 2010 [32]. JA and SA extracts from 50 mg of well-ground leaves were injected into a reverse-phase C18 Gemini high-performance liquid chromatography (HPLC) column for HPLC-electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) analysis. Agilent 1100 HPLC (Agilent Technologies), Waters C18 column (150 × 2.1 mm, 5 μm), and API3000 MSMRM (Applied Biosystems) were used for the analysis.
4.4. Isolation of Total RNA and Quantitative Real-Time PCR
Total RNA was isolated from 200 mg of leaf tissue using an RNAiso Plus (TAKARA BIO INC., Nojihigashi 7-4-38, Kusatsu, Shiga 525-0058, Japan). The GoScript Reverse Transcription System (TAKARA BIO INC., Nojihigashi 7-4-38, Kusatsu, Shiga 525-0058, Japan) was used to synthesize complementary DNA (cDNA) from the RNA. A light cycle real-time PCR detection system was used to quantify the gene expression level. The PCR reactions were initiated at 95 °C for 5 min, and then, 45 cycles of 95 °C for 30 s, 54–59 °C for 30 s (depending on target primers), and 72 °C for 30 s were initiated, with the final extension at 72 °C for 5 min. The gene-specific primers used for the qRT-PCR are given in Table S1. The qRT-PCR reactions were performed in duplicate for each of the three independent samples.
4.5. Statistical Analysis
A completely randomized design was used with three replicates for each treatment. Duncan’s multiple range test was performed to compare the means of separate replicates. Statistical significance was determined at p < 0.05. SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA) was used to perform the statistical analysis of all the measurements.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/3/303/s1, Table S1: Specific primers used for qRT-PCR.
Author Contributions
M.A.M., T.-H.K., designed the experiment and interpreted data. M.A.M. wrote the manuscript under the guidance of T.-H.K., M.A.M., M.T.I., B.-R.L., V.H.L., and D.-W.B. carried out the chemical analysis. M.T.I. participated in the critical reading of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation (NRF-2019R1A2C1089340).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jensen, B.D.; Massomo, S.M.S.; Swai, I.S.; Hockenhull, J.; Andersen, S.B. Field evaluation for resistance to the black rot pathogen Xanthomonas campestris pv. campestris in cabbage (Brassica oleracea). Eur. J. Plant Pathol. 2005, 113, 297–308. [Google Scholar] [CrossRef]
- Ignatov, A.; Kuginuki, Y.; Hida, K. Distribution and inheritance of race-specific resistance to Xanthomonas campestris pv. campestris in Brassica rapa and B. napus. J. Russ. Phytopathol. Soc. 2000, 1, 83–87. [Google Scholar]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Nayak, S. Functional characterization and signal transduction ability of nucleotide-binding site-leucine-rich repeat resistance genes in plants. Genet. Mol. Res. 2011, 10, 2637–2652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.X.; Yu, M.; Zhang, N.; Zhou, Z.Q.; Xu, Q.T.; Mei, F.Z.; Qu, L.H. Reactive oxygen species regulate programmed cell death progress of endosperm in winter wheat (Triticum aestivum L.) under waterlogging. Protoplasma 2015, 253, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Lee, B.R.; Park, S.H.; La, V.H.; Bae, D.W.; Kim, T.H. Cultivar variation in hormonal balance is a significant determinant of disease susceptibility to Xanthomonas campestris pv. campestris in Brassica napus. Front. Plant Sci. 2017, 8, 2121. [Google Scholar] [CrossRef]
- Wang, L.; Tsuda, K.; Sato, M.; Cohen, J.D.; Katagiri, F.; Glazebrook, J. Arabidopsis CaM Binding Protein CBP60g Contributes to MAMP-Induced SA Accumulation and Is Involved in Disease Resistance against Pseudomonas syringae. PLoS Pathog. 2009, 5, e1000301. [Google Scholar] [CrossRef]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.N.Y.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1157. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Does, D.V.D.; Zamioudis, C.; Reyes, A.L.; Wees, S.C.M.V. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sonbol, F.M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signaling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.A.E.; Oirdi, M.E.; Lamothe, R.G.; Bouarab, K. Necrotrophic pathogens use the salicylic acid signaling pathway to promote disease development in tomato. Mol. Plant Microbe Interact. 2012, 25, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Eitas, T.K.; Nimchuk, Z.L.; Dangl, J.L. Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. USA 2008, 105, 6475–6480. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Lee, B.T.; La, V.H.; Lee, H.; Jung, W.J.; Bae, D.W.; Kim, T.H. p-Coumaric acid induces jasmonic acid-mediated phenolic accumulation and resistance to black rot disease in Brassica napus. Physiol. Mol. Plant. Pathol. 2019, 106, 270–275. [Google Scholar] [CrossRef]
- Islam, M.T. Hormonal regulations in soluble and cell-wall bound phenolic accumulation in two cultivars of Brassica napus contrasting susceptibility to Xanthomonas campestris pv. campestris. Plant Sci. J. 2019, 285, 132–140. [Google Scholar] [CrossRef]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Bohinc, T.; Ban, S.G.; Ban, D.; Trdan, S. Glucosinolates in plant protection strategies: A review. Arch. Biol. Sci. 2012, 64, 821–828. [Google Scholar] [CrossRef]
- Tortosa, M.; Cartea, M.E.; Velasco, P.; Soengas, P.; Rodriguez, V.M. Calcium-signaling proteins mediate the plant transcriptomic response during a well-established Xanthomonas campestris pv. campestris infection. Hortic. Res. 2019, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Vernaldi, S.; Maekawa, T. Evolution and conservation of plant NLR functions. Front. Immunol. 2013, 4, 297. [Google Scholar] [CrossRef]
- Wang, G.; Roux, B.; Feng, F.; He, C.; Noel, L.D.; Zhou, J.M. The Decoy substrate of a pathogen effector and a pseudokinase Specify pathogen-induced modified-self recognition and immunity in plants. Cell Host Microbe 2015, 18, 285–295. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Du, L.; Wang, H.; Yang, T. Recent Advances in Calcium/Calmodulin-Mediated Signaling with an Emphasis on Plant-Microbe Interactions. Plant Physiol. 2013, 163, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Huda, K.M.D.; Banu, M.S.A.; Tuteja, R.; Tuteja, N. Global calcium transducer P-type Ca2+ATPases open new avenues for agriculture by regulating stress signaling. J. Exp. Bot. 2013, 64, 3099–3109. [Google Scholar] [CrossRef]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.P. Calcium Signalling in Plant Biotic Interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef] [PubMed]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, M.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.R.; Lin, Y.; Joe, A.; Guo, M.; Korneli, C.; Yang, H.; Wang, P.; Yu, M.; Cerny, R.L.; Staiger, D.; et al. Structure function analysis of an ADP ribosyltransferase type III effector and its RNA-binding target in plant immunity. J. Biol. Chem. 2011, 286, 43272–43281. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception and contribution to plant immunity. Curr. Opin. Plant. Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Adlung, N.; Prochaska, H.; Thieme, S.; Banik, A.; Blüher, D.; John, P.; Nagel, O.; Schulze, S.; Gantner, J.; Delker, C.; et al. Non-host resistance induced by the Xanthomonas Effector XopQ is wide spread within the genus nicotiana and functionally depends on EDS1. Front. Plant Sci. 2016, 7, 1796. [Google Scholar] [CrossRef]
- Venisse, J.S.; Gullner, G.; Brisset, M.N. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol. 2019, 125, 2164–2172. [Google Scholar] [CrossRef]
- Lee, B.L.; Kim, K.Y.; Jung, W.J.; Avice, J.C.; Ourry, A.; Kim, T.H. Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J. Exp. Bot. 2007, 58, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).