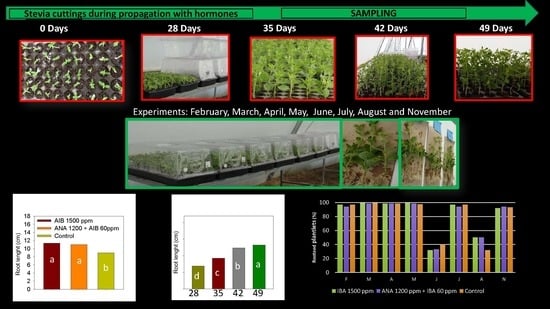
Growth and Development of Stevia Cuttings During Propagation with Hormones in Different Months of the Year
Abstract
1. Introduction
2. Results
2.1. Temperatures during the Development of the Experiment
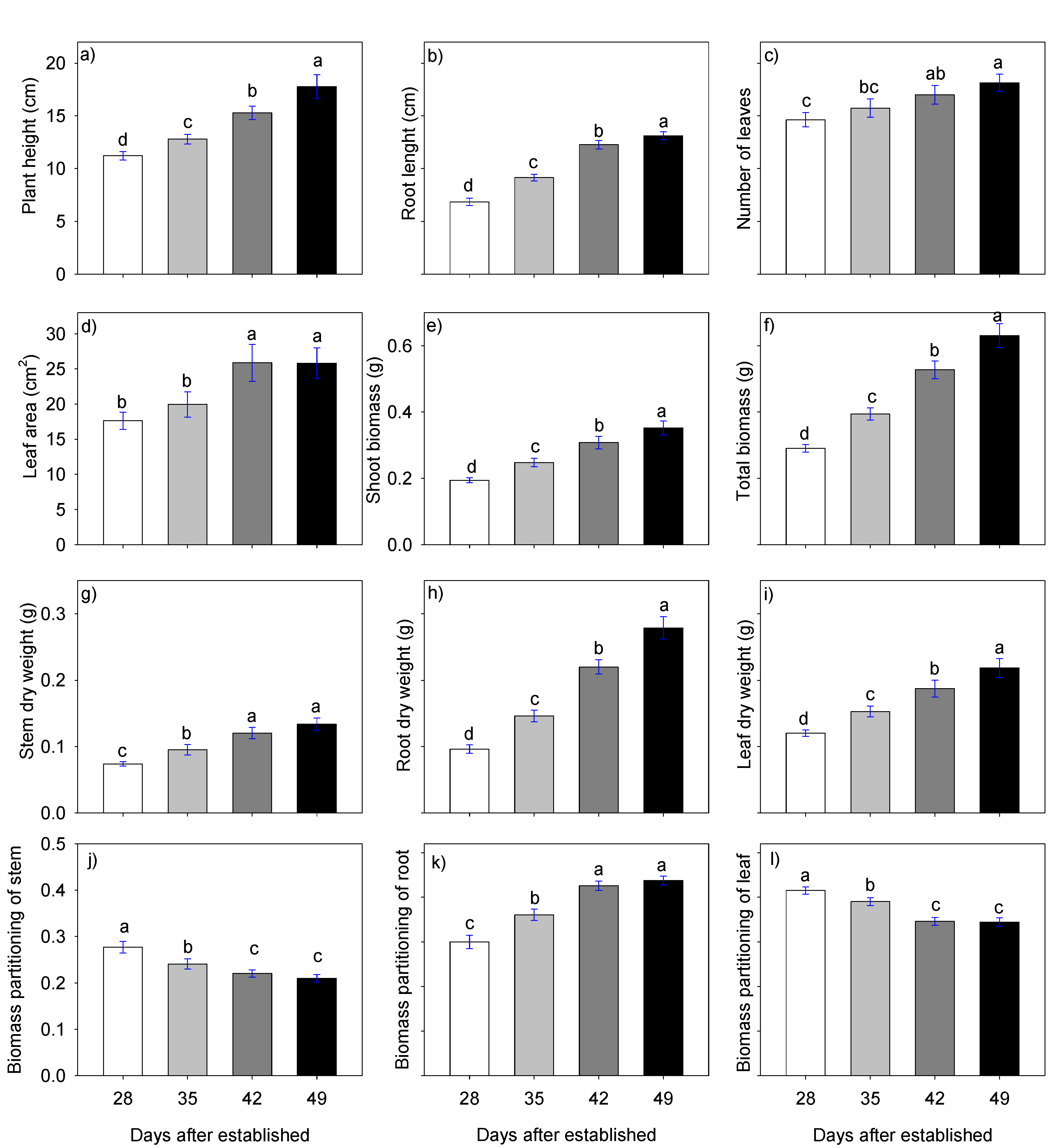
2.2. Stevia Plantlet Growth and Development 28, 35, 42, and 49 Days after Establishment (Dae)
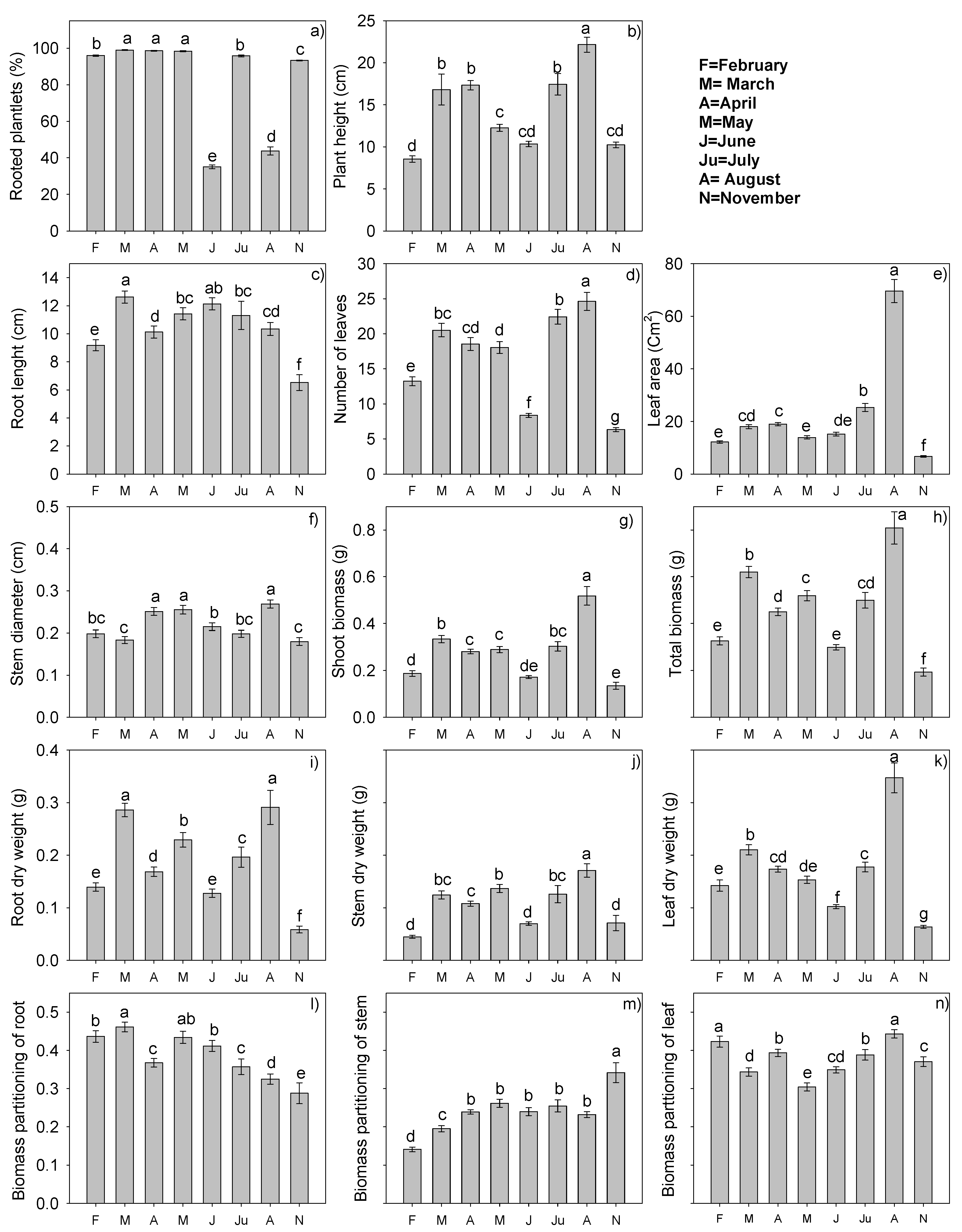
2.3. Growth and Development of Stevia Plantlets in Different Months of the Year
2.4. Effect of hormones on Growth and Development of Stevia Plantlets
3. Discussion
3.1. Temperatures during Development of the Experiments
3.2. Growth and Development of Stevia Plantlets at 28, 35, 42 and 49 Dae
3.3. Growth and Development of Stevia Plants in Different Months of the Year
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aranda-González, G.; Barbosa-Martín, E.; Toraya-Avilés, R.; Segura-Campos, M.; Moguel-Ordoñez, Y. Betancur-Ancona. Evaluación de la inocuidad de Stevia rebaudiana Bertoni cultivada en el sureste de México como edulcorante de alimentos. Nutr. Hosp. 2014, 3, 594–601. [Google Scholar] [CrossRef]
- Ramírez, J.G. Programa Estratégico para el Desarrollo Rural Sustentable de la Región Sur- Sureste de México: Trópico Húmedo Paquete Tecnológico Estevia (Stevia rebaudiana), Establecimiento y Mantenimiento; Centro de Investigación Regional Sureste, Campo experimental Mocochá: Yucatán, México, 2011; pp. 1–14. [Google Scholar]
- Hossain, M.F.; Islam, M.T.; Islam, M.A.; Akhtar, S. Cultivation and uses of stevia (Stevia rebaudiana Bertoni): A review. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12745–12757. [Google Scholar] [CrossRef]
- Singh, A.; Verma, P.P.S. Survival and growth performance of stevia cutting under different growing media. J. Med. Plants 2015, 3, 111–113. [Google Scholar]
- Megeji, N.W.; Kumar, J.K.; Singh, V.; Kaul, V.K.; Ahuja, P.S. Introducing Stevia rebaudiana, a natural zero-calorie sweetener. Curr. Sci. 2005, 88, 801–804. [Google Scholar]
- Gunasena, M.D.K.M.; Senarath, W.T.P.S.K. In vitro plant regeneration of Stevia rebaudiana through indirect organogenesis. Int. J. Bot. Stud. 2019, 4, 199–203. [Google Scholar]
- Joseph, D.; George, J. Remedial Potentials of Sweet Leaf: A Review on Stevia rebaudiana. Int. J. Pharm. Sci. Rev. Res. 2019, 54, 91–95. [Google Scholar]
- Salvador-Reyes, R.; Sotelo-Herrera, M.; Paucar-Menacho, L. Estudio de la Stevia (Stevia rebaudiana Bertoni) como edulcorante natural y su uso en beneficio de la salud. Sci. Agropecu. 2014, 5, 157–163. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Aceves, L.M.; Dublán-García, O.; López-Martínez, L.-X.; Novoa-Luna, K.A.; Islas-Flores, H.; Galar-Martínez, M.; Gómez-Oliván, L.M. Reduction of the Oxidative Stress Status Using Steviol Glycosides in a Fish Model (Cyprinus carpio). Biomed. Res. Int. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia Stevia rebaudiana (Bertoni). Can. J. Plant. Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Angelini, L.G.; Martini, A.; Passera, B.; Tavarini, S. Cultivation of Stevia rebaudiana Bertoni and Associated Challenges. In Reference Series in Phytochemistry; Mérillon, S.J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Oviedo-Pereira, D.; Alvarenga, V.S.; Evangelista, L.S.; Sepúlveda, J.G. Rodríguez-Monroy, M. Micropropagación de Stevia rebaudiana Bertoni, un Cultivo promisorio para México. BioTecnologia 2015, 19, 14–23. [Google Scholar]
- Khalil, S.A.; Zamir, R.; Ahmad, N. Selection of suitable propagation method for consistent plantlets production in Stevia rebaudiana (Bertoni). Saudi J. Biol. Sci. 2014, 21, 566–573. [Google Scholar] [CrossRef] [PubMed]
- López, M.E.; Gil, R.A.E.; López, Z.A. Enraizamiento de esquejes de Stevia rebaudiana Bertoni (Asteraceae) “estevia”, aplicando dosis creciente de ácido indolbutírico. Arnaldoa 2016, 23, 569–576. [Google Scholar] [CrossRef][Green Version]
- Muñoz, M.; Molina, R. Efecto del ácido indolbutírico (AIB) y edad de las estacas en el enraizamiento de Myrceugenia exsucca. Bosque 2016, 37, 637–641. [Google Scholar] [CrossRef][Green Version]
- Babaie, H.; Zarei, H.; Nikdel, K.; Firoozjai, M.N. Effect of different concentrations of IBA and time of taking cutting on rooting, growth and survival of Ficus binnendijkii Amstel Queen cuttings. Not. Sci. Biol. 2014, 6, 163–166. [Google Scholar] [CrossRef]
- Kassahun, B.M.; Mekonnen, S.A. Effect of Cutting Position and Rooting Hormone on Propagation Ability of Stevia (Stevia rebaudiana Bertoni). Afr. J. Plant. Sci Biotechnol. Glob. Sci. Books 2011, 6, 5–8. [Google Scholar]
- Zubenko, V.F.; Rogovskii, S.V.; Chudnovskii, B.D. Effect of the leafiness of cuttings and of day length on the rooting and transplant growth of Stevia rebaudiana. Fiziol. Biokhim. Kul’t Rast 1991, 23, 407–411. [Google Scholar]
- Britos, R.; Park, J. Ka’a he’ẽ Stevia Rebaudiana Bertoni: La Dulce Planta de Paraguay Para el Mundo; alternativa para la diversificación de la finca: Caacupé, Paraguay, 2016; p. 116. [Google Scholar]
- Herrera, C.F.; Gómez, J.R.; González, R.C. El Cultivo de Stevia (Stevia rebaudiana) Bertoni en condiciones Agroambientales de Nayarit, México; Folleto Técnico, 19, INIFAP- Pacifico Centro, Campo Experimental Santiago Ixcuintla: Nayarit, México, 2012; p. 43. [Google Scholar]
- De Lima, F.O.F. Análise Quantitativa do Vrescimento da Estevia; Embrapa Agropecuária Oeste: Dourados-MS, Brazil, 2004; p. 29. [Google Scholar]
- Yücesan, B.; Mohammed, A.; Büyükgöçmen, R.; Altuğ, C.; Kavas, Ö.; Gürel, S.; Gürel, E. In vitro and ex vitro propagation of Stevia rebaudiana Bertoni with high Rebaudioside-A content A commercial scale application. Sci. Hortic. 2016, 203, 20–28. [Google Scholar] [CrossRef]
- Quezada, N.F.E. Propagación por Esquejes de Stevia (Stevia rebaudiana bert) en Tres Sustratos y dos Dosis de Hormona de Enraizamiento Bajo Invernadero en el Cantón Santa Isabel. Bachelor’s Thesis, Facultad de Ciencias Agropecuarias, Escuela de Ingeniería Agronómica, Cuenca, Ecuador, 2002. [Google Scholar]
- Jarma, A.; Rengifo, T.; Aramendiz-Tatis, H. Aspectos fisiológicos de estevia (Stevia rebaudiana Bertoni) en el Caribe colombiano: I. Efecto de la radiación incidente sobre el área foliar y la distribución de biomasa. Agron. Colomb 2005, 23, 206–216. [Google Scholar]
- Sedano, C.G.; Gonzales, H.V.A.; Engelman, E.M.; Villanueva, V.C. Dinámica del crecimiento y eficiencia fisiológica de la planta de calabacita. Rev. Chapingo Ser. Hort. 2005, 11, 91–97. [Google Scholar] [CrossRef]
- Aguilar, G.L.; Escalante, E.J.A.; Fucikovsky, Z.L.; Tijerina, C.H.L.; Engleman, E.M. Área foliar, tasa de asimilación neta, rendimiento y densidad de población en girasol. Terra Lat. 2005, 23, 303–310. [Google Scholar]
- SAS Institute. SAS Versión 9, 13 Para Windows; SAS Institute: Cary, NC, EUA, 2007. [Google Scholar]

| Month | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | Jan. (°C) | Feb. (°C) | Mar. (°C) | Apr. (°C) | May (°C) | June (°C) | July (°C) | Aug. (°C) | Sep. (°C) | Oct. (°C) | Nov. (°C) | Dec. (°C) |
| Low | 5.8 | 7.3 | 8.4 | 5.9 | 8.9 | 10.1 | 15.9 | 17.3 | 15.3 | 13.6 | 6.9 | 6.4 |
| High | 32.0 | 35.5 | 36.8 | 41.4 | 42.7 | 41.6 | 33.6 | 32.9 | 33.7 | 34.9 | 33.3 | 32.0 |
| Average | 16.4 | 19.2 | 20.2 | 22.1 | 24.9 | 22.9 | 22.5 | 22.5 | 21.8 | 21.6 | 18.7 | 16.6 |
| RGR | NAR | LAR | LWR | SLA | |
|---|---|---|---|---|---|
| date | Sampling | ||||
| 35 | 0.036106a | 0.0009481a | 56.747a | 0.082789a | 2028.06a |
| 42 | 0.032940a | 0.0007141a | 46.482b | 0.069684b | 1317.48b |
| 49 | 0.024810a | 0.0006367a | 41.263c | 0.061688c | 935.92c |
| Month | |||||
| February | 0.03020b | 0.0009348a | 41.202de | 0.082318b | 838.2d |
| March | 0.02123b | 0.0008937a | 29.759f | 0.058670d | 450.7e |
| April | 0.01741b | 0.0004562a | 43.201d | 0.075211b | 923.5cd |
| May | 0.01017b | 0.0007527a | 27.350f | 0.045195e | 378.4e |
| June | 0.02334b | 0.0005088a | 48.278c | 0.061167cd | 1160.2c |
| July | 0.03716b | 0.0008458a | 54.599b | 0.075678b | 1513.8b |
| August | 0.07730a | 0.0007989a | 101.752a | 0.105242a | 5374.1a |
| November | 0.03348b | 0.0009396a | 39.173e | 0.067615c | 778.3d |
| mM | Hormone | ||||
| IBA 7.4 | 0.028792a | 0.0007577a | 49.859a | 0.064068b | 1625.73a |
| ANA 6.4 + IBA 0.3 | 0.034672a | 0.0007955a | 47.347b | 0.062876b | 1354.38b |
| Control | 0.030391a | 0.0007457a | 47.287b | 0.087218a | 1301.34b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Saucedo, M.C.; Tapia-Campos, E.; Ramírez-Anaya, J.d.P.; Beltrán, J. Growth and Development of Stevia Cuttings During Propagation with Hormones in Different Months of the Year. Plants 2020, 9, 294. https://doi.org/10.3390/plants9030294
Castañeda-Saucedo MC, Tapia-Campos E, Ramírez-Anaya JdP, Beltrán J. Growth and Development of Stevia Cuttings During Propagation with Hormones in Different Months of the Year. Plants. 2020; 9(3):294. https://doi.org/10.3390/plants9030294
Chicago/Turabian StyleCastañeda-Saucedo, Ma Claudia, Ernesto Tapia-Campos, Jessica del Pilar Ramírez-Anaya, and Jaqueline Beltrán. 2020. "Growth and Development of Stevia Cuttings During Propagation with Hormones in Different Months of the Year" Plants 9, no. 3: 294. https://doi.org/10.3390/plants9030294
APA StyleCastañeda-Saucedo, M. C., Tapia-Campos, E., Ramírez-Anaya, J. d. P., & Beltrán, J. (2020). Growth and Development of Stevia Cuttings During Propagation with Hormones in Different Months of the Year. Plants, 9(3), 294. https://doi.org/10.3390/plants9030294