AUXIN RESPONSE FACTOR 1 Acts as a Positive Regulator in the Response of Poplar to Trichoderma asperellum Inoculation in Overexpressing Plants
Abstract
1. Introduction
2. Results
2.1. PdPapARF1 Expression Is Responsive to T. asperellum Inoculation
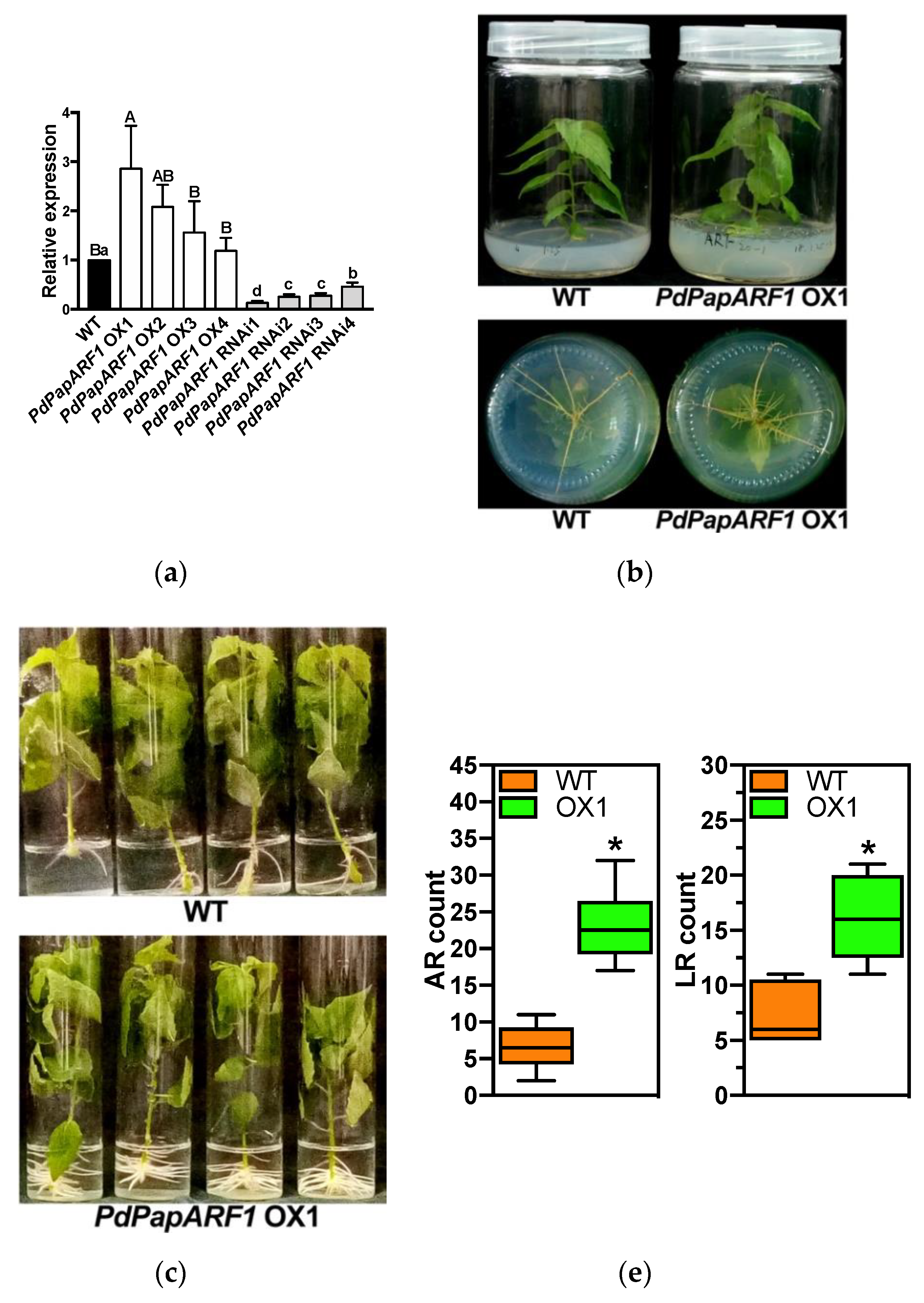
2.2. Production of Transgenic Poplar with Modified Expression of PdPapARF1
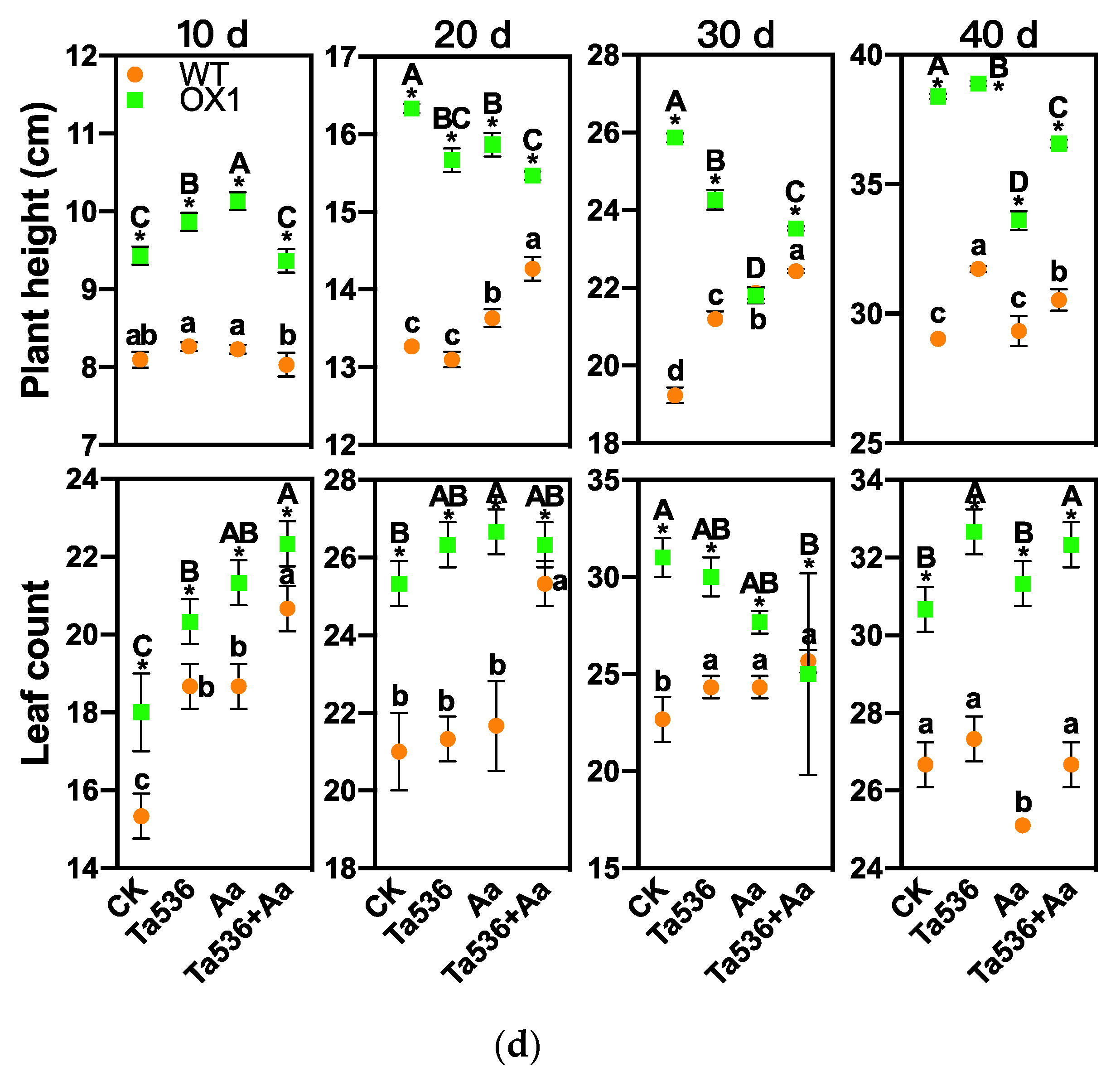
2.3. PdPapARF1 Overexpression Promoted Growth and Adventitious Root Formation
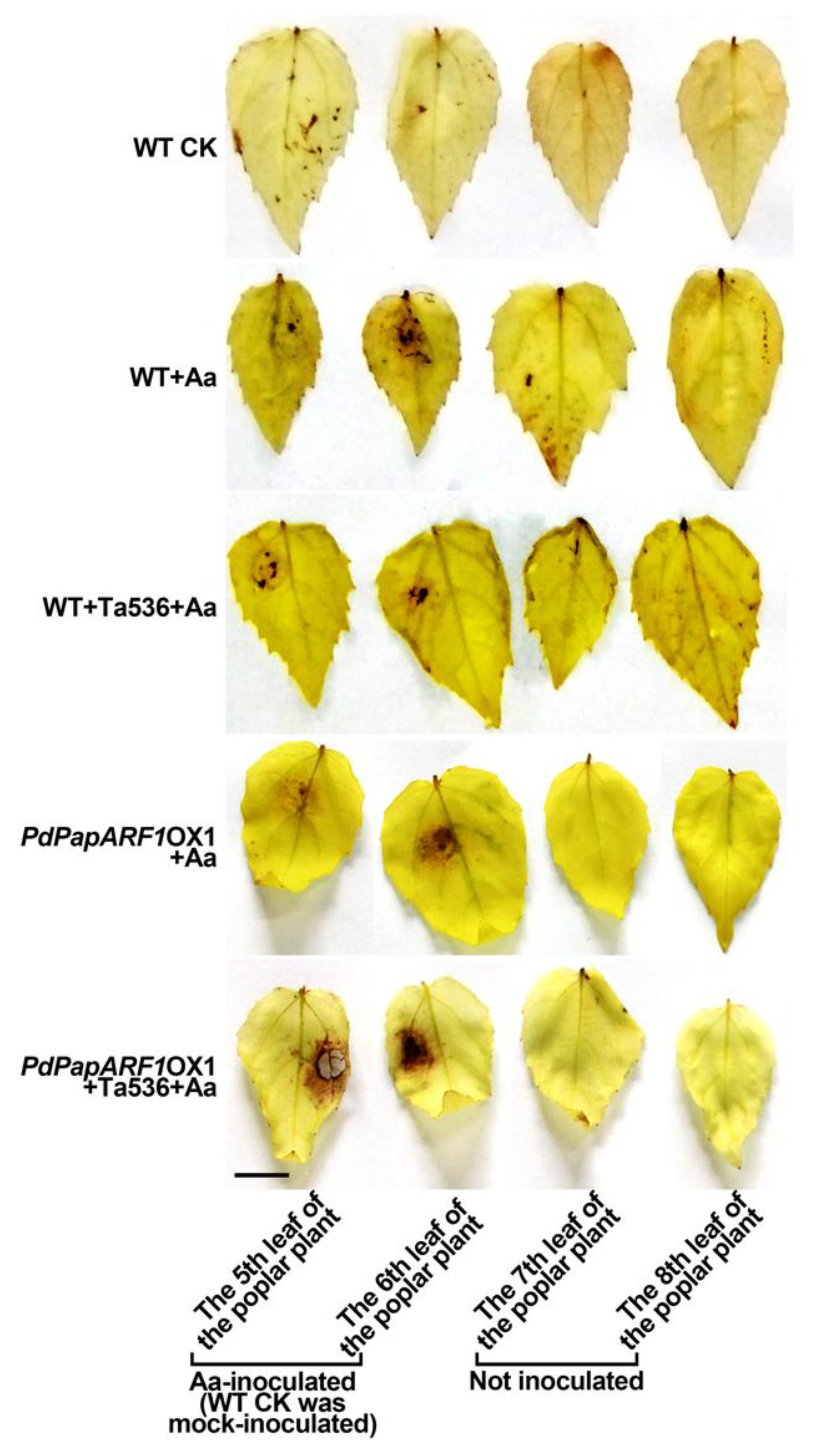
2.4. PdPapARF1 Overexpression Altered the Response of Poplar Leaf to A. alternata Infection
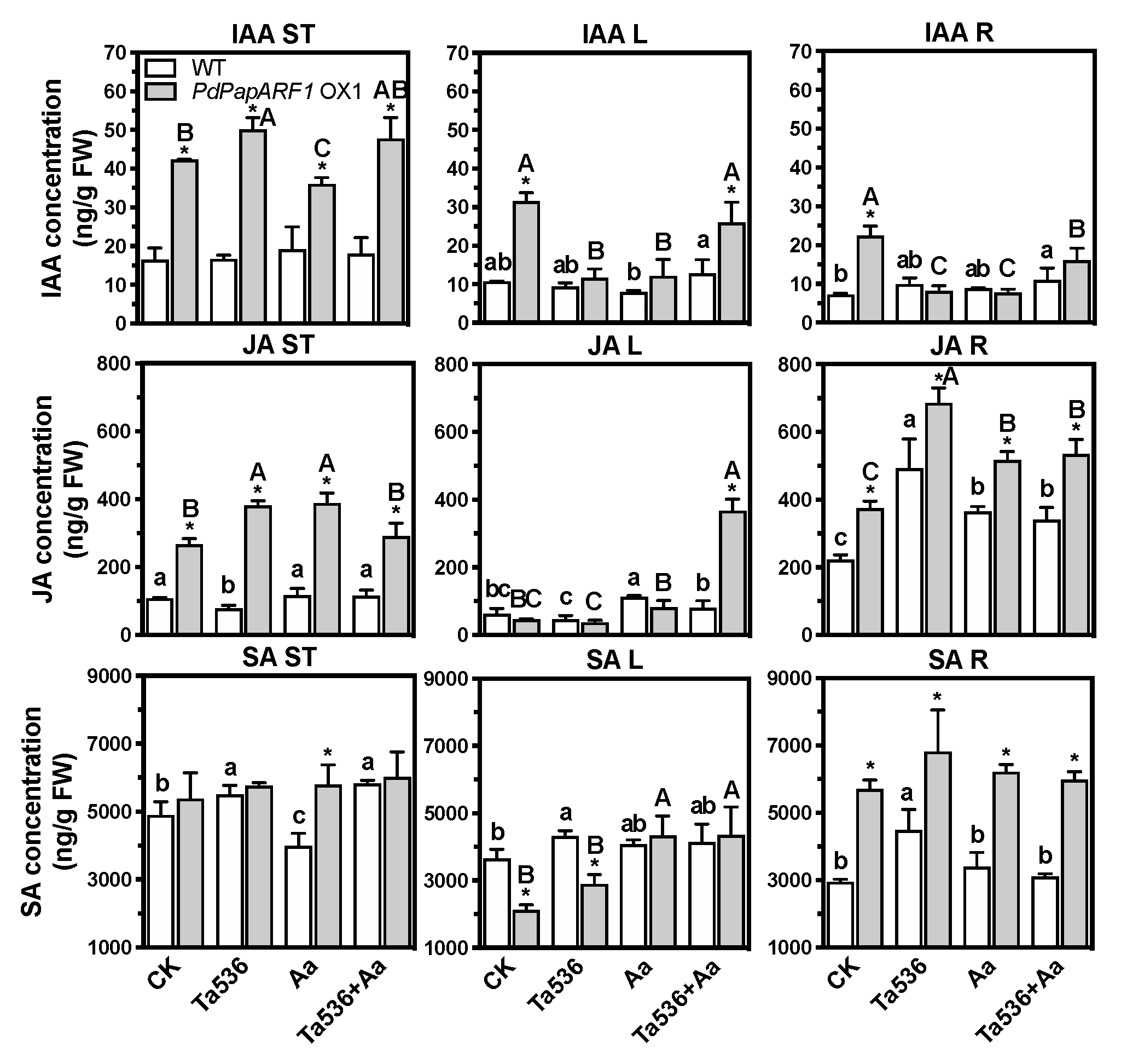
2.5. PdPapARF1 Overexpression Regulated Hormone Levels in Planta
2.6. PdPapARF1 Overexpression Influenced the IAA, JA and SA Signaling Cascades in Planta
3. Discussion
4. Materials and Methods
4.1. Plant, Fungal Material, and Growth
4.2. Cloning of PdPapARF1 Sequences and Production of Transgenic Poplars
4.3. Inoculation of Plants
4.4. DAB Staining Assays, Determination of Hormone concentrations, qRT-PCR, and Statistic Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Topolovec-Pintarić, S. Trichoderma: Invisible Partner for Visible Impact on Agriculture. In Trichoderma-The Most Widely Used Fungicide; IntechOpen: London, UK, 2019. [Google Scholar]
- Chaverri, P.; Gazis, R.O.; Samuels, G.J. Trichoderma amazonicum, a new endophytic species on Hevea brasiliensis and H. guianensis from the Amazon basin. Mycologia 2011, 103, 139–151. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.; Lu, G.-D. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef]
- Harman, G.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2019, 1–18. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Pozo, M.J.; Cammue, B.P.; Vos, C.M. Belowground defence strategies in plants: The plant–Trichoderma dialogue. In Belowground Defence Strategies in Plants; Springer: Berlin, Germany, 2016; pp. 301–327. [Google Scholar]
- Patel, J.; Teli, B.; Bajpai, R.; Meher, J.; Rashid, M.; Mukherjee, A.; Yadav, S.K. Trichoderma-mediated biocontrol and growth promotion in plants: An endophytic approach. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–239. [Google Scholar]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Zemlyanskaya, E.V.; Omelyanchuk, N.A.; Ubogoeva, E.V.; Mironova, V.V. Deciphering auxin-ethylene crosstalk at a systems level. Int. J. Mol. Sci. 2018, 19, 4060. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Shukla, N.; Kabadwal, B.; Tewari, A.; Kumar, J. Review on Plant-Trichoderma-Pathogen Interaction. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2382–2397. [Google Scholar] [CrossRef]
- Engelberth, J.; Koch, T.; Schüler, G.; Bachmann, N.; Rechtenbach, J.; Boland, W. Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol. 2001, 125, 369–377. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Ichikawa, H.; Naznin, H.A.; Kogure, A.; Hyakumachi, M. Systemic resistance induced in Arabidopsis thaliana by Trichoderma asperellum SKT-1, a microbial pesticide of seedborne diseases of rice. Pest Manag. Sci. 2012, 68, 60–66. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Harper, C.P. The roles of auxin during interactions between bacterial plant pathogens and their hosts. J. Exp. Bot. 2017, 69, 245–254. [Google Scholar] [CrossRef]
- Li, S.-B.; Xie, Z.-Z.; Hu, C.-G.; Zhang, J.-Z. A review of auxin response factors (ARFs) in plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Jiang, C.-Y.; Zhai, T.-T.; Chang, Y.; Yao, Z.-H.; Zhang, R.-S. Effects of Trichoderma asperellum combined application on growth and photosynthesis characteristics of Populus davidiana × P. alba var. pyramidlis. Bull. Bot. Res. 2018, 38, 64–74. [Google Scholar] [CrossRef]
- Jiang, C.-Y.; Zhu, G.-D.; Yao, Z.-H.; Yang, X.-T.; Liu, Z.-H.; Zhang, R.-S. Effects of three Trichoderma asperellum strains on the growth and Photosynthetic characteristics of tissue-cultured Populus davidiana × P. alba var. Pyramidalis seedlings. Pratacult. Sci. 2016, 33, 1189–1199. [Google Scholar]
- Apostol, I.; Heinstein, P.F.; Low, P.S. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: Role in defense and signal transduction. Plant Physiol. 1989, 90, 109–116. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Lü, Q.; Yan, D.; Liu, Z.; Zhang, X. Comparative Proteomic Analysis of Plant–Pathogen Interactions in Resistant and Susceptible Poplar Ecotypes Infected with Botryosphaeria dothidea. Phytopathology 2019, 109, 2009–2021. [Google Scholar] [CrossRef]
- Gutierrez, L.; Bussell, J.D.; Păcurar, D.I.; Schwambach, J.; Păcurar, M.; Bellini, C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Păcurar, M.; Demailly, H.; Geiss, G. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef]
- Lakehal, A.; Chaabouni, S.; Cavel, E.; Le Hir, R.; Ranjan, A.; Raneshan, Z.; Novák, O.; Păcurar, D.I.; Perrone, I.; Jobert, F. A Molecular Framework for the Control of Adventitious Rooting by TIR1/AFB2-Aux/IAA-Dependent Auxin Signaling in Arabidopsis. Mol. Plant 2019, 12, 1499–1514. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Evans, M.L.; Ishikawa, H.; Estelle, M.A. Responses of Arabidopsis roots to auxin studied with high temporal resolution: Comparison of wild type and auxin-response mutants. Planta 1994, 194, 215–222. [Google Scholar] [CrossRef]
- Tsukanova, K.; Meyer, J.; Bibikova, T. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The nexus between growth and defence signalling: Auxin and cytokinin modulate plant immune response pathways. J. Exp. Bot. 2015, 66, 4885–4896. [Google Scholar] [CrossRef]
- Asai, S.; Shirasu, K. Plant cells under siege: Plant immune system versus pathogen effectors. Curr. Opin.Plant Biol. 2015, 28, 1–8. [Google Scholar] [CrossRef]
- Caarls, L.; Pieterse, C.M.; Van Wees, S. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Deng, W.; Luo, K.; Li, Z.; Yang, Y. A novel method for induction of plant regeneration via somatic embryogenesis. Plant Sci. 2009, 177, 43–48. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Zhai, T.; Wang, Y.; Baloch, A.M.; Baloch, A.W.; Liu, Z.; Jiang, C.; Zhang, R. Trichoderma aspereullm ACCC30536 inoculation differently regulates the time-course expression of five indole-3-acetic acid amido synthetase genes and the levels of IAA, SA and JA in Populus davidiana × P. alba var. Pyramidalis. Pak. J. Bot. 2019, 51, 689–697. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-F.; Hou, X.-Y.; Deng, J.-J.; Yao, Z.-H.; Lyu, M.-M.; Zhang, R.-S. AUXIN RESPONSE FACTOR 1 Acts as a Positive Regulator in the Response of Poplar to Trichoderma asperellum Inoculation in Overexpressing Plants. Plants 2020, 9, 272. https://doi.org/10.3390/plants9020272
Wang Y-F, Hou X-Y, Deng J-J, Yao Z-H, Lyu M-M, Zhang R-S. AUXIN RESPONSE FACTOR 1 Acts as a Positive Regulator in the Response of Poplar to Trichoderma asperellum Inoculation in Overexpressing Plants. Plants. 2020; 9(2):272. https://doi.org/10.3390/plants9020272
Chicago/Turabian StyleWang, Yue-Feng, Xue-Yue Hou, Jun-Jie Deng, Zhi-Hong Yao, Man-Man Lyu, and Rong-Shu Zhang. 2020. "AUXIN RESPONSE FACTOR 1 Acts as a Positive Regulator in the Response of Poplar to Trichoderma asperellum Inoculation in Overexpressing Plants" Plants 9, no. 2: 272. https://doi.org/10.3390/plants9020272
APA StyleWang, Y.-F., Hou, X.-Y., Deng, J.-J., Yao, Z.-H., Lyu, M.-M., & Zhang, R.-S. (2020). AUXIN RESPONSE FACTOR 1 Acts as a Positive Regulator in the Response of Poplar to Trichoderma asperellum Inoculation in Overexpressing Plants. Plants, 9(2), 272. https://doi.org/10.3390/plants9020272






