Long-Chain acyl-CoA Synthetase LACS2 Contributes to Submergence Tolerance by Modulating Cuticle Permeability in Arabidopsis
Abstract
1. Introduction
2. Results
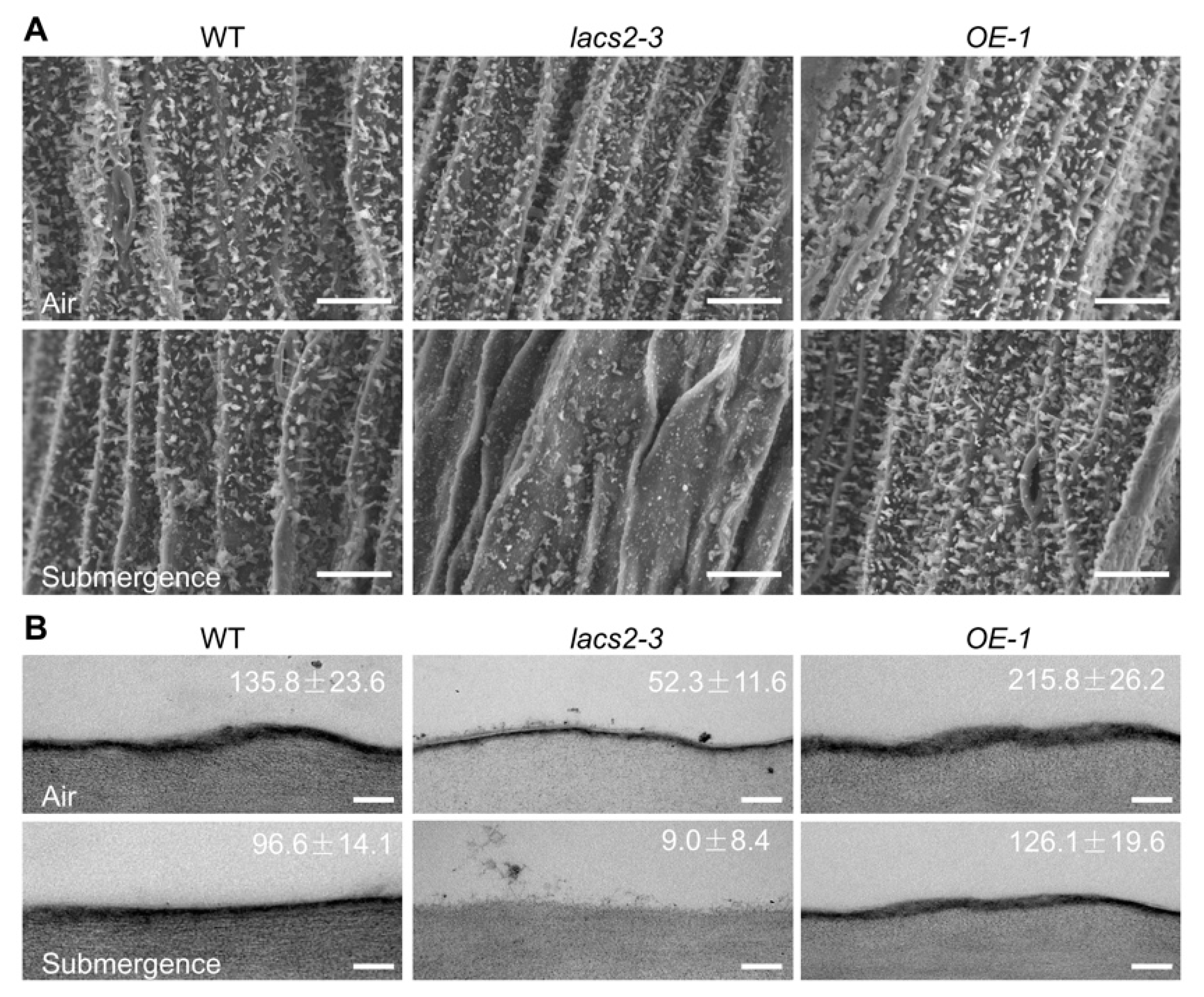
2.1. LACS2 Is Involved in Maintenance of Submergence-Induced Alterations of Epicuticular Wax and Cutin
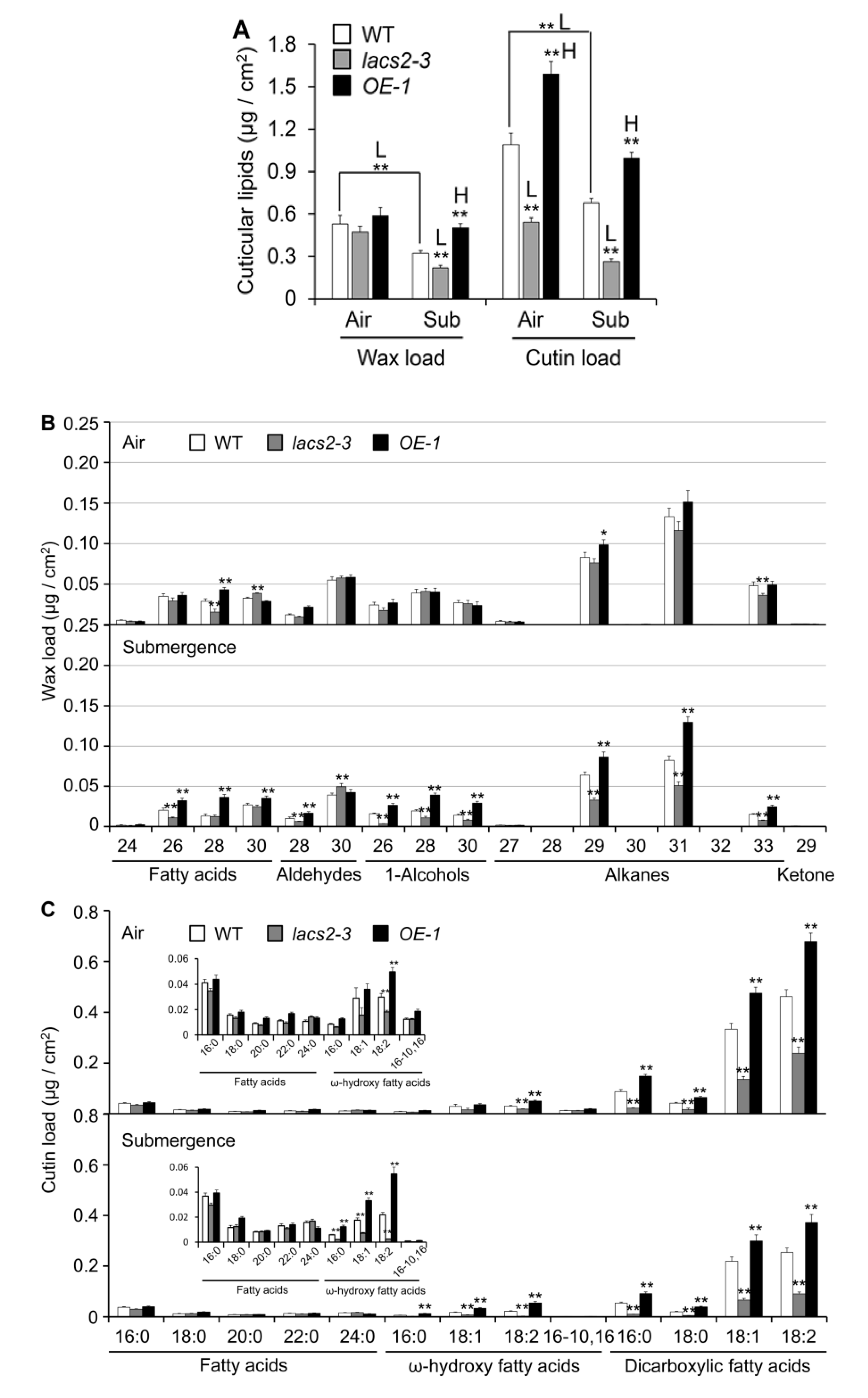
2.2. Cuticular Wax and Cutin Polyester Monomer Profiles of Wild type (WT), lacs2-3, and LACS2-OEs Leaves upon Submergence Exposure
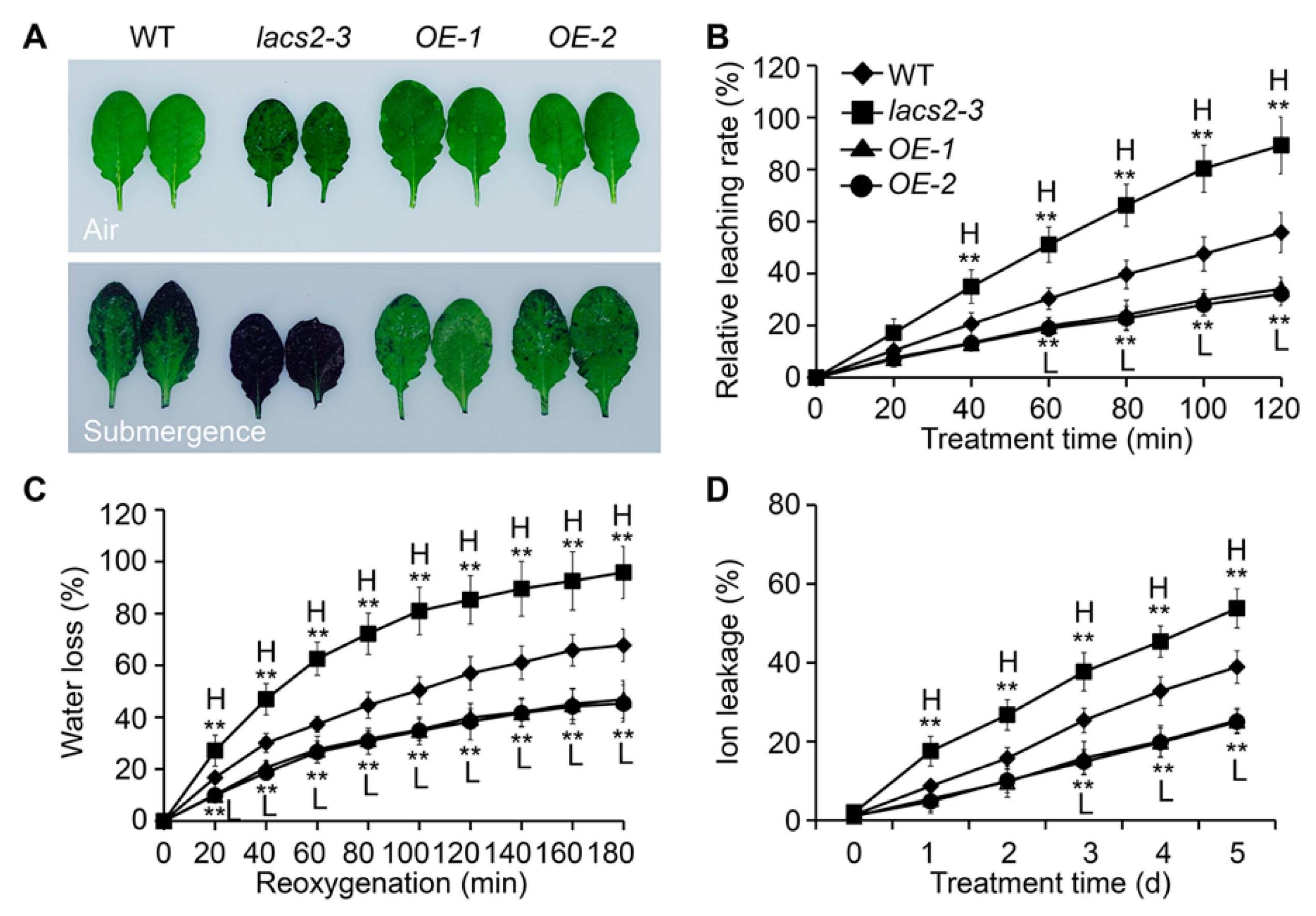
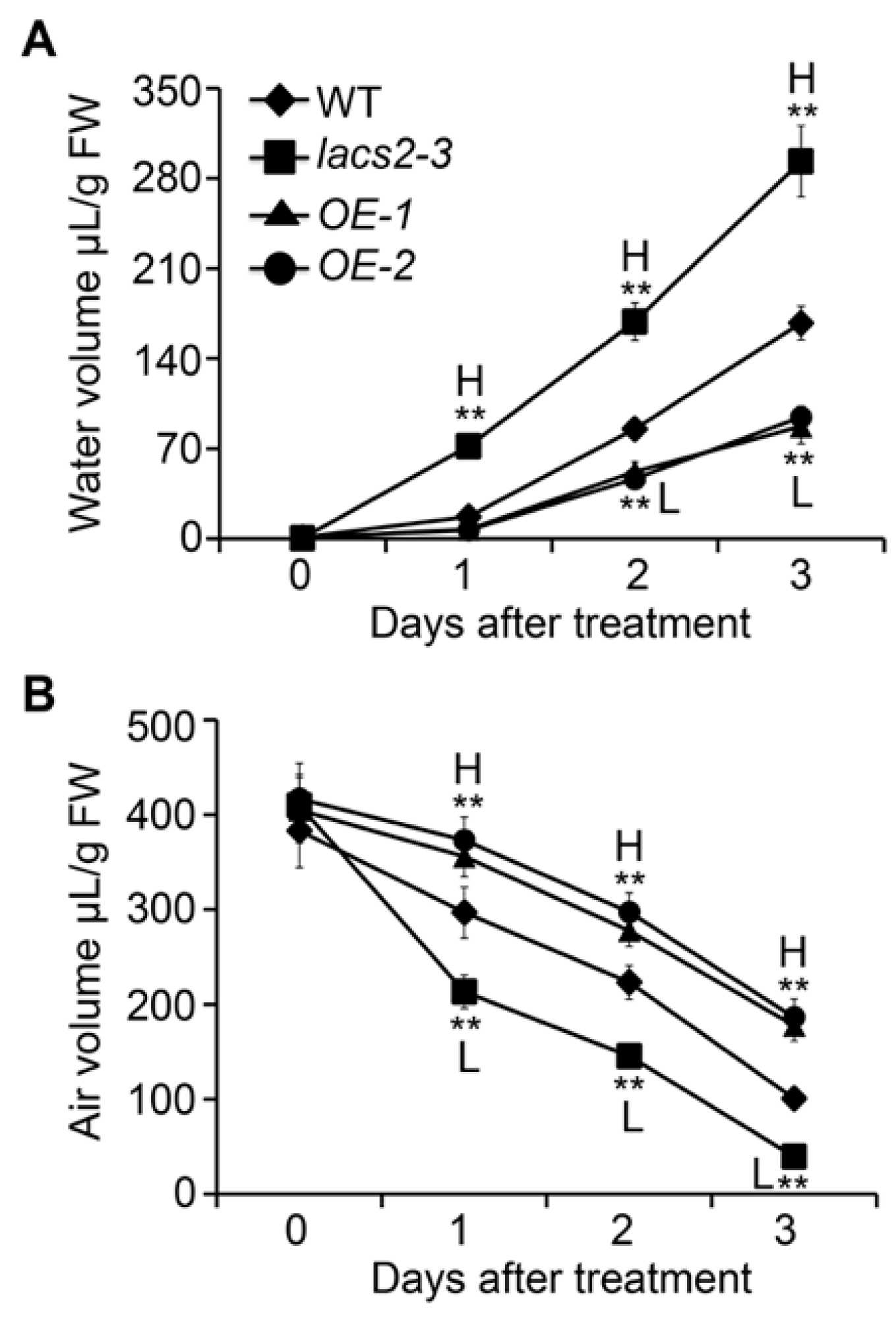
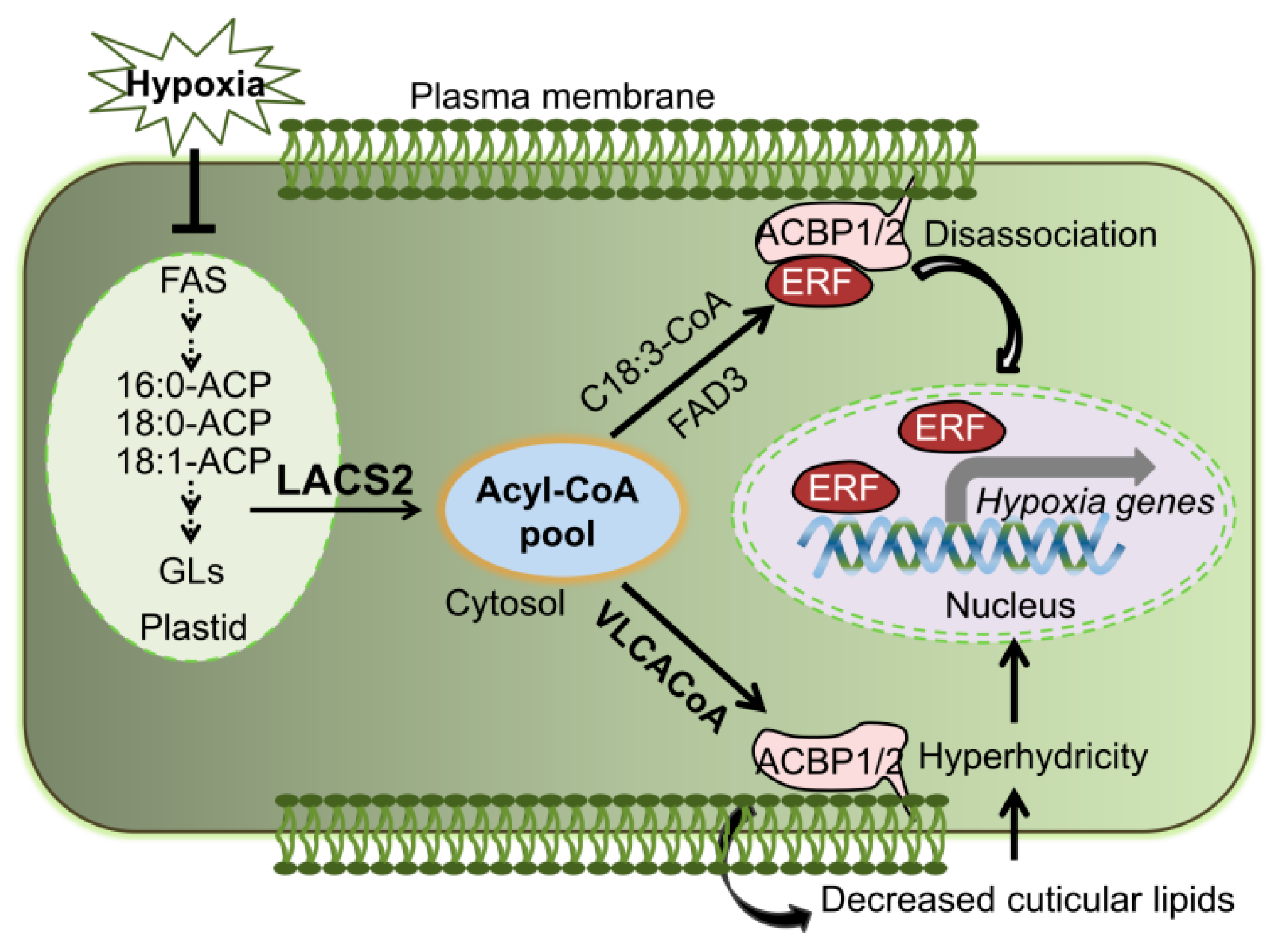
2.3. Plant Surface Permeability Is Associated with Hyperhydricity and Hypoxia Signaling during Submergence
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Culture Conditions, and Treatments
4.2. Scanning Electron Microscopy and Transmission Electron Microscopy
4.3. Leaf Hydrophobicity Analyses
4.4. Wax and Cutin Profiling
4.5. Analysis of Cuticle Permeability
4.6. Measurement of Electrolyte Leakage and Water Loss
4.7. Measurement of Apoplastic Water and Air Volumes and Determination of Malate Dehydrogenase Activity
Author Contributions
Funding
Conflicts of Interest
References
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress, acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Lee, S.C.; Isa, N.M.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J.; et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.C.J.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes, an overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Shabala, S. Hypoxia Sensing in Plants, On a Quest for Ion Channels as Putative Oxygen Sensors. Plant Cell Physiol. 2017, 58, 1126–1142. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Fulda, M.; Paul, M.V.; Anders, M.; Plum, F.; Weits, D.A.; Kosmacz, M.; Larson, T.R.; Graham, I.A.; Beemster, G.T.S.; et al. Low-oxygen response is triggered by an ATP-dependent shift in oleoyl-CoA in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 12101–12110. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, W.J.; Xie, L.J.; Qi, H.; Yang, Y.C.; Huang, L.P.; Lai, Y.X.; Tan, Y.F.; Zhou, D.M.; Yu, L.J.; et al. Polyunsaturated linolenoyl-CoA modulates ERF-VII-mediated hypoxia signaling in Arabidopsis. J. Integr. Plant Biol. 2019. [Google Scholar] [CrossRef]
- Shockey, J.M.; Fulda, M.S.; Browse, J.A. Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol. 2002, 129, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Fulda, M.; Shockey, J.; Weber, M.; Wolter, F.P.; Heinz, E. Two longchain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid b-oxidation. Plant J. 2002, 32, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, J.; Shockey, J.; Browse, J. The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 2004, 16, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Bessire, M.; Chassot, C.; Jacquat, A.C.; Humphry, M.; Borel, S.; Petétot, J.M.; Métraux, J.P.; Nawrath, C. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 2007, 26, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.; Song, T.; Kosma, D.K.; Parsons, E.P.; Rowland, O.; Jenks, M.A. Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J. 2009, 59, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Molina, I.; Shockey, J.; Browse, J. Organ fusion and defective cuticle function in a lacs1 lacs2 double mutant of Arabidopsis. Planta 2010, 231, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Katavic, V.; Li, F.; Haughn, G.W.; Kunst, L. Insertional mutant analysis reveals that long-chain acyl-CoA synthetase 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis. Plant J. 2010, 64, 1048–1058. [Google Scholar] [CrossRef]
- Jessen, D.; Olbrich, A.; Knüfer, J.; Krüger, A.; Hoppert, M.; Polle, A.; Fulda, M. Combined activity of LACS1 and LACS4 is required for proper pollen coat formation in Arabidopsis. Plant J. 2011, 68, 715–726. [Google Scholar] [CrossRef]
- Tang, D.; Simonich, M.T.; Innes, R.W. Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol. 2007, 144, 1093–1103. [Google Scholar] [CrossRef]
- Xie, L.J.; Chen, Q.F.; Chen, M.X.; Yu, L.J.; Huang, L.; Chen, L.; Wang, F.Z.; Xia, F.N.; Zhu, T.R.; Wu, J.X.; et al. Unsaturation of very-long-chain ceramides protects plant from hypoxia-induced damages by modulating ethylene signaling in Arabidopsis. PLoS Genet. 2015, 11, e1005143. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Nagai, K.; Huan, P.D.; Shimazaki, K.; Qu, H.; Mori, Y.; Toda, Y.; Kuroha, T.; Hayashi, N.; Aiga, S.; et al. Rice leaf hydrophobicity and gas films are conferred by a wax synthesis gene (LGF1) and contribute to flood tolerance. New Phytol. 2018, 218, 1558–1569. [Google Scholar] [CrossRef]
- Van den Dries, N.; Giannì, S.; Czerednik, A.; Krens, F.A.; de Klerk, G.J. Flooding of the apoplast is a key factor in the development of hyperhydricity. J. Exp. Bot. 2013, 64, 5221–5230. [Google Scholar] [CrossRef]
- Pollard, M.; Beisson, F.; Li, Y.H.; Ohlrogge, J.B. Building lipid barriers, Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, L. Plant cuticles shine, advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Beisson, F.; Li-Beisson, Y.; Pollard, M. Solving the puzzles of cutin and suberin polymer biosynthesis. Curr. Opin. Plant Biol. 2012, 15, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes, advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yu, K.; Gao, Q.M.; Wilson, E.V.; Navarre, D.; Kachroo, P.; Kachroo, A. Acyl-CoA Binding Proteins are required for cuticle formation and plant responses to microbes. Front. Plant Sci. 2012, 3, 224. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, S.; Kim, J.; Lung, S.C.; Chen, L.; Tanner, J.A.; Suh, M.C.; Chye, M.L. Arabidopsis membrane-associated acyl-CoA-binding protein ACBP1 is involved in stem cuticle formation. J. Exp. Bot. 2014, 65, 5473–5483. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.H.; Wang, K.; Huang, G.; Zhu, Y.X. Genome-scale analysis of the cotton KCS gene family revealed a binary mode of action for gibberellin A regulated fiber growth. J. Integr. Plant Biol. 2016, 58, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, D.; Suh, M.C. Cuticle ultrastructure, cuticular lipid composition, and gene expression in hypoxia-stressed Arabidopsis stems and leaves. Plant Cell Rep. 2017, 36, 815–827. [Google Scholar] [CrossRef]
- Boyer, J.S.; Wong, S.C.; Farquhar, G.D. CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiol. 1997, 114, 185–191. [Google Scholar] [CrossRef]
- Frost-Christensen, H.; Jørgensen, L.B.; Floto, F. Species specificity of resistance to oxygen diffusion in thin cuticular membranes from amphibious plants. Plant Cell Environ. 2003, 26, 561–569. [Google Scholar] [CrossRef]
- Mommer, L.; Pons, T.L.; Wolters-Arts, M.; Venema, J.H.; Visser, E.J. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiol. 2005, 139, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Kannangara, R.; Branigan, C.; Liu, Y.; Penfield, T.; Rao, V.; Mouille, G.; Höfte, H.; Pauly, M.; Riechmann, J.L.; Broun, P. The Transcription Factor WIN1/SHN1 Regulates Cutin Biosynthesis in Arabidopsis thaliana. Plant Cell 2007, 19, 1278–1294. [Google Scholar] [CrossRef]
- Xie, L.J.; Yu, L.J.; Chen, Q.F.; Wang, F.Z.; Huang, L.; Xia, F.N.; Zhu, T.R.; Wu, J.X.; Yin, J.; Liao, B.; et al. Arabidopsis acyl-CoA-binding protein ACBP3 participates in plant response to hypoxia by modulating very-long-chain fatty acid metabolism. Plant J. 2015, 81, 53–67. [Google Scholar] [CrossRef]
- Peng, X.; Wang, M.; Li, Y.; Yan, W.; Chang, Z.; Chen, Z.; Xu, C.; Yang, C.; Deng., X.W.; Wu, J.; et al. Lectin receptor kinase OsLecRK-S.7 is required for pollen development and male fertility. J. Integr. Plant Biol. 2019. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces, an inspiration for biomimetic materials. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1487–1509. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Go, Y.S.; Bae, H.J.; Park, J.H.; Cho, S.H.; Cho, H.J.; Lee, D.S.; Park, O.K.; Hwang, I.; Suh, M.C. Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein gene altered cuticular lipid composition, increased plastoglobules, and enhanced susceptibility to infection by the fungal pathogen Alternaria brassicicola. Plant Physiol. 2009, 150, 42–54. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, H.; Machida, C.; Watanabe, M.; Machida, Y. A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J. 2004, 37, 139–146. [Google Scholar] [CrossRef]
- Chen, L.; Liao, B.; Qi, H.; Xie, L.J.; Huang, L.; Tan, W.J.; Zhai, N.; Yuan, L.B.; Zhou, Y.; Yu, L.J.; et al. Autophagy contributes to regulation of the hypoxia response during submergence in Arabidopsis thaliana. Autophagy 2015, 11, 2233–2246. [Google Scholar] [CrossRef]
- Lee, S.H.; Sakuraba, Y.; Lee, T.; Kim, K.W.; An, G.; Lee, H.Y.; Paek, N.C. Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J. Integr. Plant Biol. 2015, 57, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.E.; Bonner, B.A. An examination of centrifugation as a method of extracting an extracellular solution from peas, and its use for the study of indoleacetic acid-induced growth. Plant Physiol. 1980, 66, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Raskin, I. A method for measuring leaf volume, density, thickness, and internal gas volume. HortScience 1983, 18, 698–699. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.-J.; Tan, W.-J.; Yang, Y.-C.; Tan, Y.-F.; Zhou, Y.; Zhou, D.-M.; Xiao, S.; Chen, Q.-F. Long-Chain acyl-CoA Synthetase LACS2 Contributes to Submergence Tolerance by Modulating Cuticle Permeability in Arabidopsis. Plants 2020, 9, 262. https://doi.org/10.3390/plants9020262
Xie L-J, Tan W-J, Yang Y-C, Tan Y-F, Zhou Y, Zhou D-M, Xiao S, Chen Q-F. Long-Chain acyl-CoA Synthetase LACS2 Contributes to Submergence Tolerance by Modulating Cuticle Permeability in Arabidopsis. Plants. 2020; 9(2):262. https://doi.org/10.3390/plants9020262
Chicago/Turabian StyleXie, Li-Juan, Wei-Juan Tan, Yi-Cong Yang, Yi-Fang Tan, Ying Zhou, De-Mian Zhou, Shi Xiao, and Qin-Fang Chen. 2020. "Long-Chain acyl-CoA Synthetase LACS2 Contributes to Submergence Tolerance by Modulating Cuticle Permeability in Arabidopsis" Plants 9, no. 2: 262. https://doi.org/10.3390/plants9020262
APA StyleXie, L.-J., Tan, W.-J., Yang, Y.-C., Tan, Y.-F., Zhou, Y., Zhou, D.-M., Xiao, S., & Chen, Q.-F. (2020). Long-Chain acyl-CoA Synthetase LACS2 Contributes to Submergence Tolerance by Modulating Cuticle Permeability in Arabidopsis. Plants, 9(2), 262. https://doi.org/10.3390/plants9020262



