Regulation of Ammonium Cellular Levels is An Important Adaptive Trait for the Euhalophytic Behavior of Salicornia europaea
Abstract
1. Introduction
2. Results
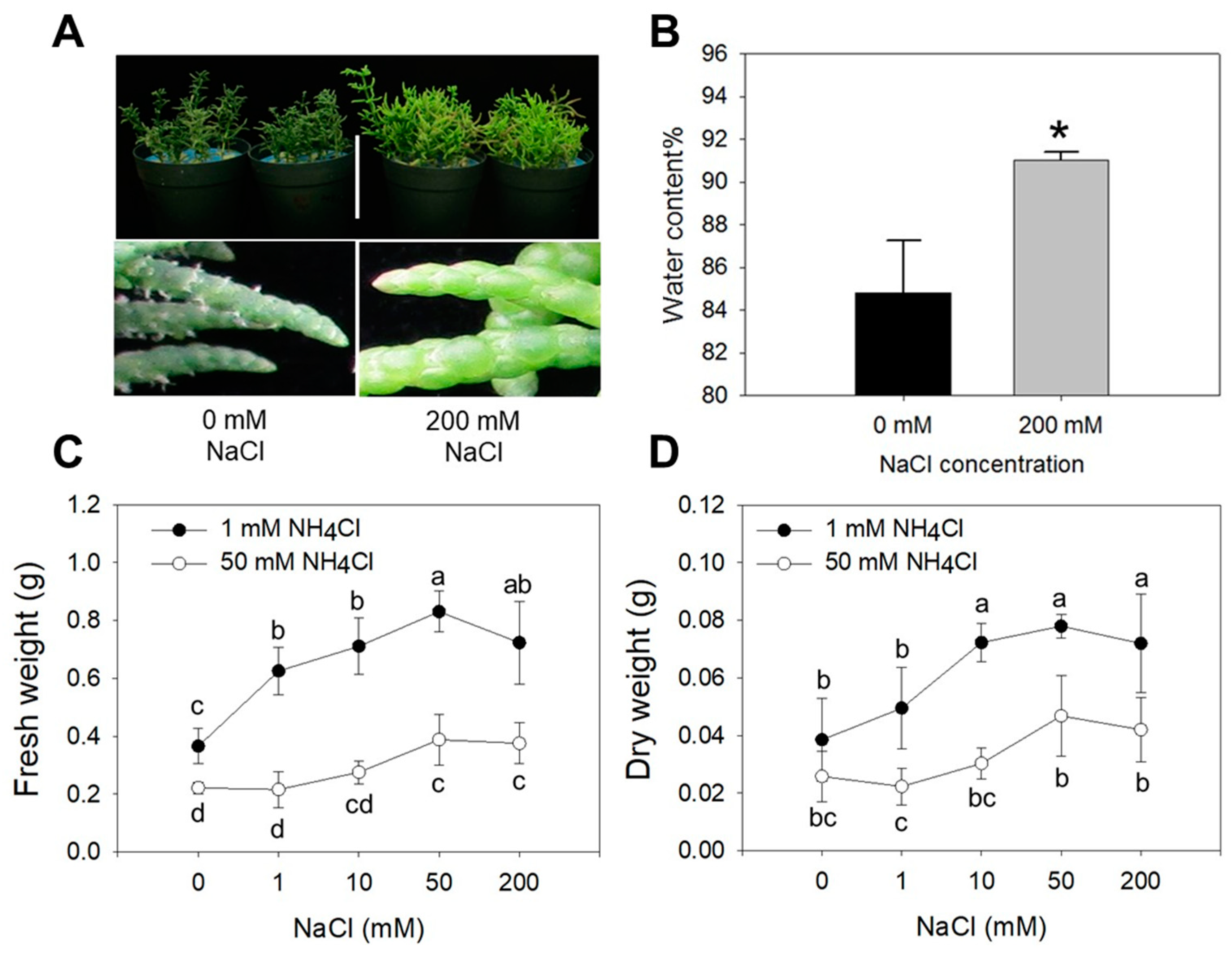
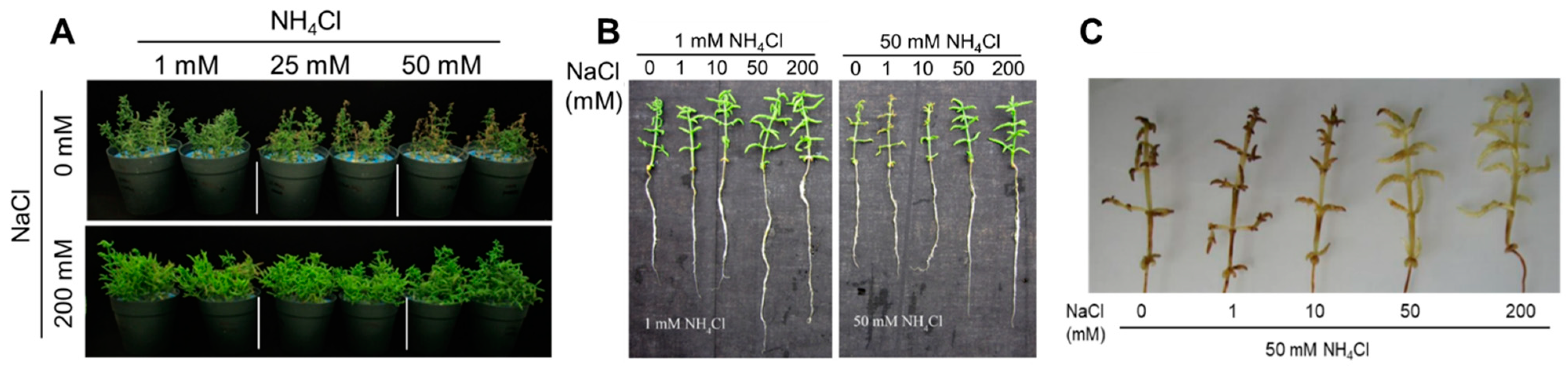
2.1. Effects of NH4Cl/NaCl on Growth and Morpho-Physiological Traits
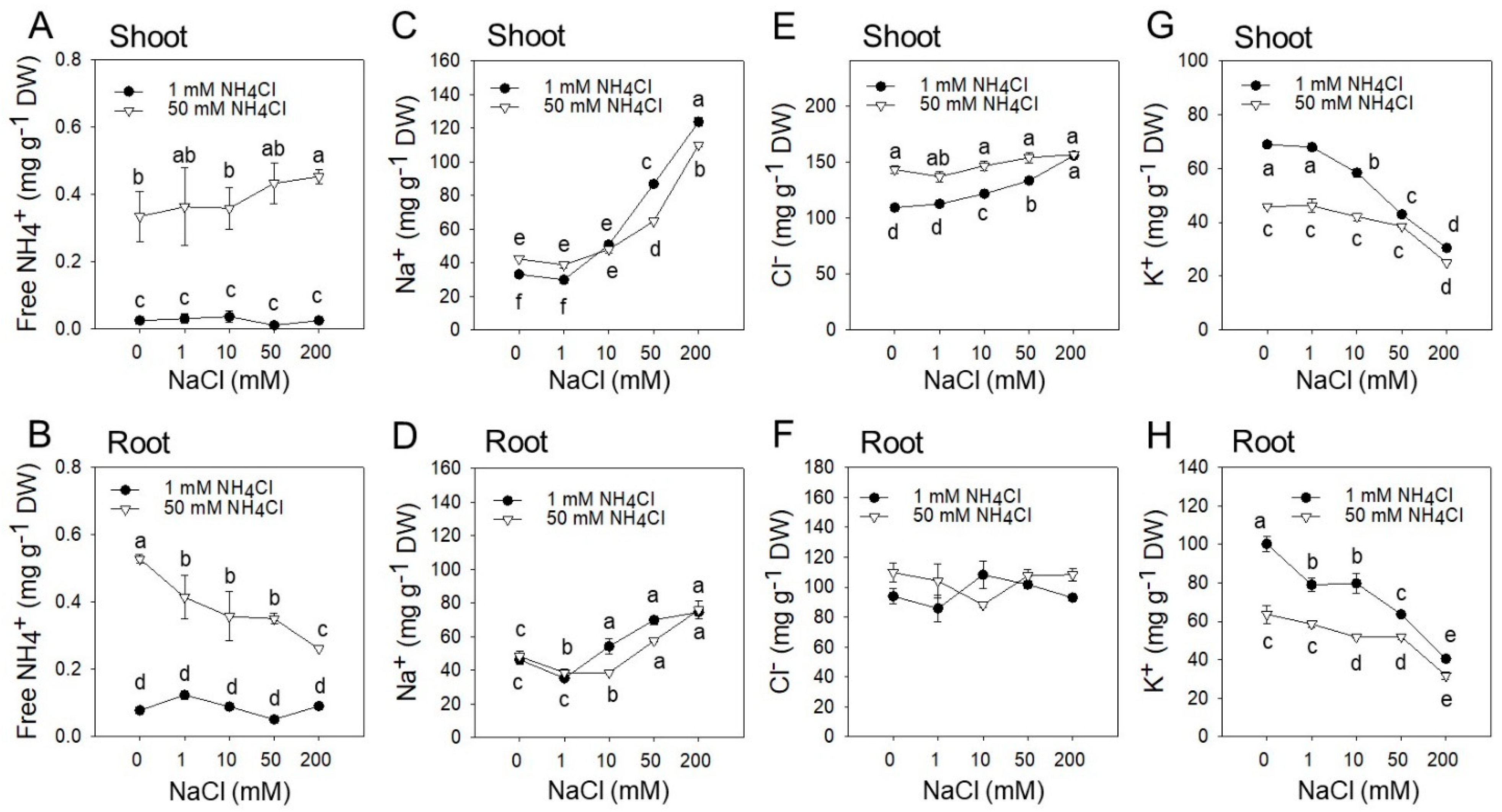
2.2. Shoot and Root Ionic Profile in Response to Increasing NaCl and NH4Cl Concentrations
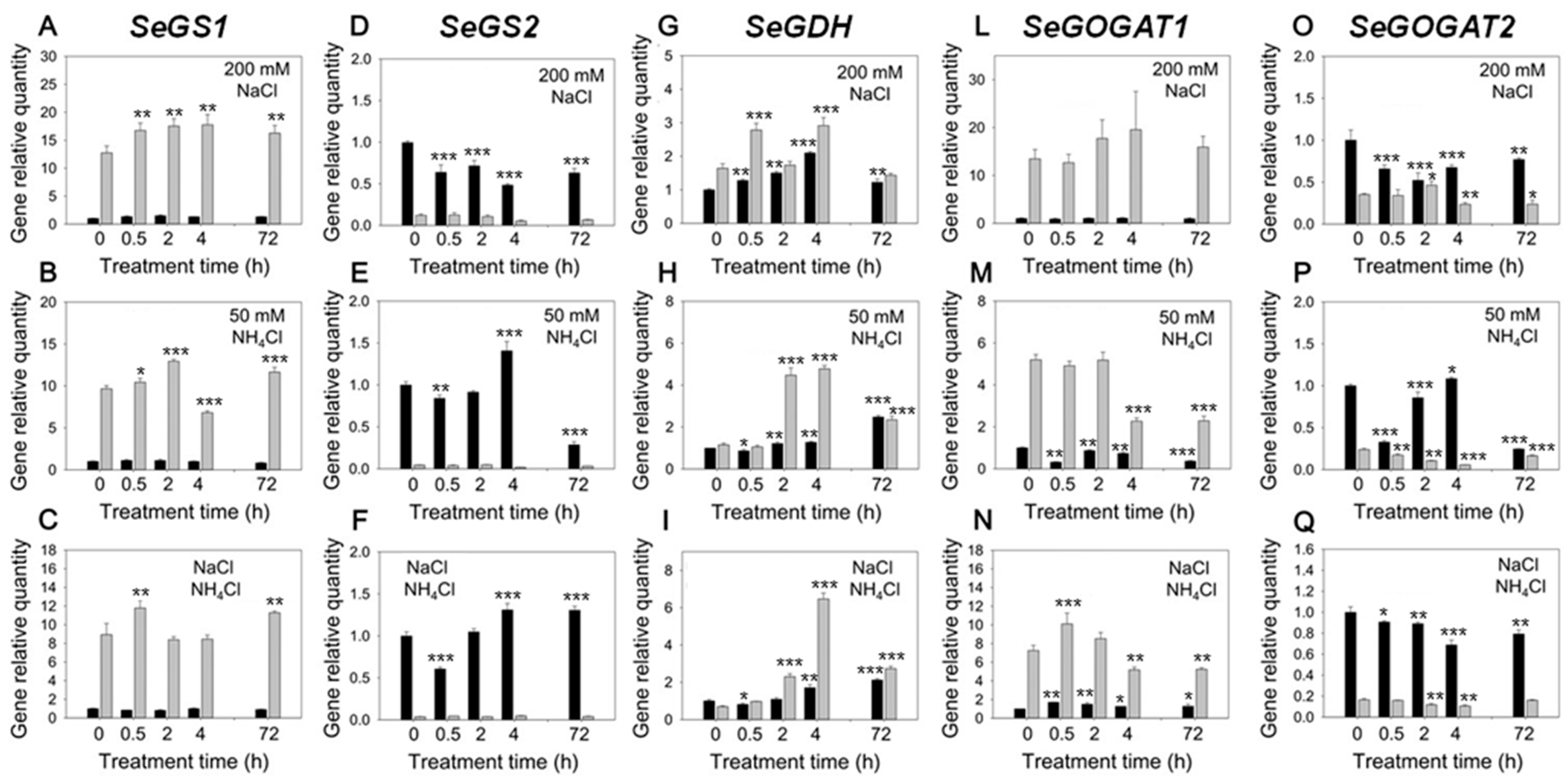
2.3. Expression Pattern of Genes Involved in the Ammonium Assimilation Metabolism in Response to Increasing NaCl and NH4Cl Concentrations
2.4. Effects of NH4+/Na+ on Enzyme Activities Related with Ammonium Assimilation Metabolism
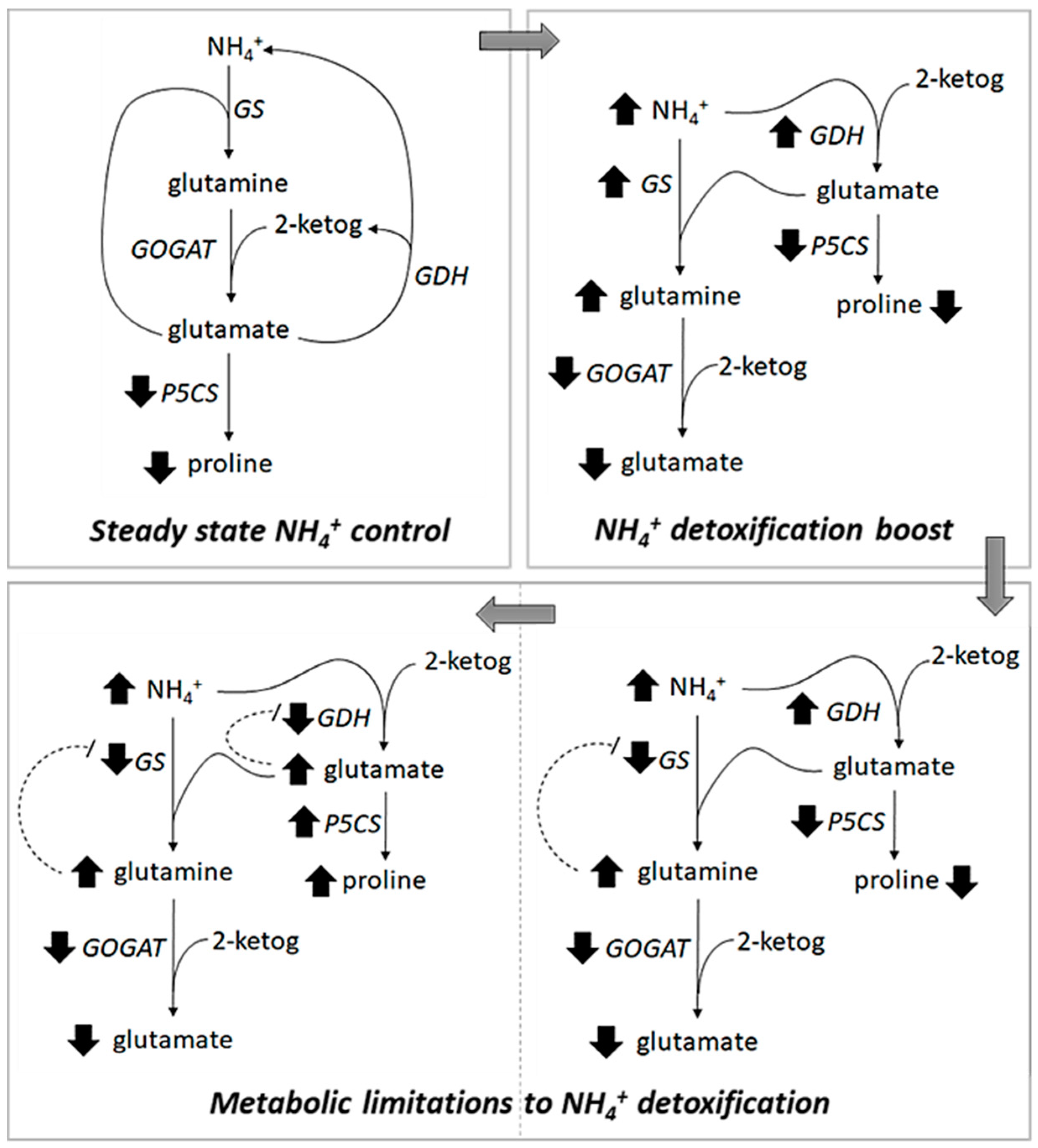
3. Discussion
3.1. Regulation of NH4+ Toxicity is a Key Determinant of Salicornia Growth at High Salinity
3.2. NH4+ Detoxification is Mediated by Salt Induced Activation of the NH4+ Assimilation System
3.3. Role of GOGAT in NH4+ Detoxification and Osmoprotection
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Ion Contents
4.3. Hydrogen Peroxide Detection
4.4. Determination of NH4+ Contents and Ammonium Assimilation Enzyme Activity
4.5. Gene Expression Analysis Under NH4Cl/NaCl Treatment
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yarra, R. The wheat NHX gene family: Potential role in improving salinity stress tolerance of plants. Plant Gene 2019, 18, 100178. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Zhou, Y.; Han, J.; Shi, D. Effects of salt stress on ion balance and nitrogen metabolism in rice. Plant Soil Environ. 2012, 58, 62–67. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 2015, 206, 557–570. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Orsini, F.; D’Urzo, M.P.; Inan, G.; Serra, S.; Oh, D.-H.; Mickelbart, M.V.; Consiglio, F.; Li, X.; Jeong, J.C.; Yun, D.-J.; et al. A comparative study of salt tolerance parameters in 11 wild relatives of Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 3787–3798. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tanna, B. Halophytes: Potential Resources for Salt Stress Tolerance Genes and Promoters. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; An, J.Y.; Lee, H.J.; Son, D.; Sohn, Y.G.; Kim, C.-G.; Lee, J.J. The growth and accumulation of osmotic solutes of the halophyte common glasswort (Salicornia europaea) under salinity conditions. J. Aquat. Plant Manag. 2013, 51, 103–108. [Google Scholar]
- Riehl, T.E.; Ungar, I.A. Growth and ion accumulation in Salicornia europaea under saline field conditions. Oecologia 1982, 54, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Jiang, P.; Chen, X.; Fan, P.; Wang, X.; Li, Y. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012, 51, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; You, J.; Wang, T.; Xiao, X.; Yao, Y.; Zhang, M.; Wang, J.; Tian, C. Global Transcriptome Profiling of Salicornia europaea L. Shoots under NaCl Treatment. PLoS ONE 2013, 8, e65877. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Sagi, M. Halophyte crop production: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Momonoki, Y.S.; Kamimura, H. Studies on the Mechanism of Salt Tolerance in Salicornia europaea L.: I. Changes in pH and osmotic pressure in Salicornia plants during the growth period. Jpn. J. Crop Sci. 1994, 63, 518–523. [Google Scholar] [CrossRef][Green Version]
- Xu, C.; Tang, X.; Shao, H.; Wang, H. Salinity Tolerance Mechanism of Economic Halophytes From Physiological to Molecular Hierarchy for Improving Food Quality. Curr. Genom. 2016, 17, 207–214. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef]
- Wei, Z.; Qing-jie, S.U.N.; Chu-fu, Z.; Yong-ze, Y.; Ji, Z.; Bin-bin, L.U. Effect of Salt Stress on Ammonium Assimilation Enzymes of the Roots of Rice (Oryza sativa) Cultivars Differing in Salinity Resistance. Acta Bot. Sin. 2004, 46, 921–926. [Google Scholar]
- Nguyen, H.T.T.; Shim, I.S.; Kobayashi, K.; Usui, K. Regulation of Ammonium Accumulation during Salt Stress in Rice (Oryza sativa L.) Seedlings. Plant Prod. Sci. 2005, 8, 397–404. [Google Scholar] [CrossRef]
- Nevin, J.M.; Lovatt, C.J. Demonstration of ammonia accumulation and toxicity in avocado leaves during water-deficit stress. Biology 1987, 51–54. [Google Scholar]
- Nguyen, H.T.T.; Shim, I.S.; Kobayashi, K.; Kenji, U. Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2003, 41, 159–164. [Google Scholar]
- Billard, J.P.; Boucaud, J. Effect of NaCl on the activities of glutamate synthase from a halophyte Suaeda maritima and from a glycophyte Phaseolus vulgaris. Phytochemistry 1980, 19, 1939–1942. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic Stress Generates ROS That Signal Expression of Anionic Glutamate Dehydrogenases to Form Glutamate for Proline Synthesis in Tobacco and Grapevine. Plant Cell Online 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Fidalgo, F. Salt stress affects glutamine synthetase activity and mRNA accumulation on potato plants in an organ-dependent manner. Plant Physiol. Biochem. 2009, 47, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Su, L.; Li, Y.; Wang, Y.; Zhang, C.; Zhao, Z. Nitrate and ammonium contribute to the distinct nitrogen metabolism of Populus simonii during moderate salt stress. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Dias, A.S.; De Lima, G.S.; Gheyi, H.R.; Nobre, R.G.; Dos Santos, J.B. Emergence, Growth and Production of Sesame Under Salt Stress and Proportions of Nitrate and Ammonium. Rev. Caatinga 2017, 30, 458–467. [Google Scholar] [CrossRef]
- Fernández-Crespo, E.; Camañes, G.; García-Agustín, P. Ammonium enhances resistance to salinity stress in citrus plants. J. Plant Physiol. 2012, 169, 1183–1191. [Google Scholar] [CrossRef]
- Hessini, K.; Hamed, K.B.; Gandour, M.; Mejri, M.; Abdelly, C.; Cruz, C. Ammonium nutrition in the halophyte Spartina alterniflora under salt stress: Evidence for a priming effect of ammonium? Plant Soil 2013, 370, 163–173. [Google Scholar] [CrossRef]
- Munzarova, E.; Lorenzen, B.; Brix, H.; Vojtiskova, L.; Votrubova, O. Effect of NH4+/NO3− availability on nitrate reductase activity and nitrogen accumulation in wetland helophytes Phragmites australis and Glyceria maxima. Environ. Exp. Bot. 2006, 55, 49–60. [Google Scholar] [CrossRef]
- Camañes, G.; Cerezo, M.; Primo-Millo, E.; Gojon, A.; García-Agustín, P. Ammonium transport and CitAMT1 expression are regulated by N in Citrus plants. Planta 2009, 229, 331–342. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, X.; Li, L.; Maggio, A.; Zhang, D.; Abdelshafy Mohamad, O.A.; Van Oosten, M.; Huang, G.; Sun, Y.; Tian, C.; et al. Large-scale de novo transcriptome analysis reveals specific gene expression and novel simple sequence repeats markers in salinized roots of the euhalophyte Salicornia europaea. Acta Physiol. Plant. 2018, 40. [Google Scholar] [CrossRef]
- Pang, Q.; Chen, S.; Dai, S.; Chen, Y.; Wang, Y.; Yan, X. Comparative proteomics of salt tolerance in arabidopsis thaliana and thellungiella halophila. J. Proteome Res. 2010, 9, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. Protein contribution to plant salinity response and tolerance acquisition. Int. J. Mol. Sci. 2013, 14, 6757–6789. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, P.; Song, H.; Chen, X.; Li, X.; Li, Y. Comparative Proteomic Analysis of Differentially Expressed Proteins in Shoots of Salicornia europaea under Different Salinity. J. Proteome Res. 2009, 8, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi Komaresofla, B.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved growth and salinity tolerance of the halophyte Salicornia sp. by co–inoculation with endophytic and rhizosphere bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Jahn, T.P.; Schjoerring, J.K.; Cuin, T.A.; Pedas, P.; Shabala, S.; Hegelund, J.N.; Hoopen, F.T. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J. Exp. Bot. 2010, 61, 2303–2315. [Google Scholar]
- Orsini, F.; Alnayef, M.; Bona, S.; Maggio, A.; Gianquinto, G. Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ. Exp. Bot. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Almeida, D.M.; Margarida Oliveira, M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Frechilla, S.; Lasa, B.; Ibarretxe, L.; Lamsfus, C.; Aparicio-Tejo, P. Pea responses to saline stress is affected by the source of nitrogen nutrition (ammonium or nitrate). Plant Growth Regul. 2001, 35, 171–179. [Google Scholar] [CrossRef]
- Katschnig, D.; Bliek, T.; Rozema, J.; Schat, H. Constitutive high-level SOS1 expression and absence of HKT1;1 expression in the salt-accumulating halophyte Salicornia dolichostachya. Plant Sci. 2015, 234, 144–154. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review I. Introduction. J. Plant Physiol. 2002, 584, 567–584. [Google Scholar] [CrossRef]
- Coleto, I.; Vega-Mas, I.; Glauser, G.; González-Moro, M.B.; Marino, D.; Ariz, I. New insights on Arabidopsis thaliana root adaption to ammonium nutrition by the use of a quantitative proteomic approach. Int. J. Mol. Sci. 2019, 20, 814. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Liao, Q.; Song, H.; Liu, Q.; Lepo, J.E.; Guan, C.; Zhang, J.; Ismail, A.M.; Zhang, Z. NRT1.1-Related NH4+ Toxicity Is Associated with a Disturbed Balance between NH4+ Uptake and Assimilation. Plant Physiol. 2018, 178, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.; Joy, K.W. Glutamine Synthetase of Pea Leaves. Plant Physiol. 1974, 54, 773–779. [Google Scholar] [CrossRef]
- Bottacin, A.; Cacco, G.; Saccomani, M. Nitrogen absorption and assimilation in NaCl-resistant and NaCl-susceptible millet genotypes (Pennisetum americanum). Can. J. Bot. 1985, 63, 517–520. [Google Scholar] [CrossRef]
- Abouelsaad, I.; Weihrauch, D.; Renault, S. Effects of salt stress on the expression of key genes related to nitrogen assimilation and transport in the roots of the cultivated tomato and its wild salt-tolerant relative. Sci. Hortic. (Amsterdam) 2016, 211, 70–78. [Google Scholar] [CrossRef]
- Cruz, C.; Bio, A.F.M.; Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Martins-Loução, M.A. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta 2006, 223, 1068–1080. [Google Scholar] [CrossRef]
- Fontaine, J.-X.; Tercé-Laforgue, T.; Armengaud, P.; Clément, G.; Renou, J.-P.; Pelletier, S.; Catterou, M.; Azzopardi, M.; Gibon, Y.; Lea, P.J.; et al. Characterization of a NADH-Dependent Glutamate Dehydrogenase Mutant of Arabidopsis Demonstrates the Key Role of this Enzyme in Root Carbon and Nitrogen Metabolism. Plant Cell 2012, 24, 4044–4065. [Google Scholar] [CrossRef]
- Konishi, N.; Ishiyama, K.; Matsuoka, K.; Maru, I.; Hayakawa, T.; Yamaya, T.; Kojima, S. NADH-dependent glutamate synthase plays a crucial role in assimilating ammonium in the Arabidopsis root. Physiol. Plant. 2014, 152, 138–151. [Google Scholar] [CrossRef]
- Kan, C.C.; Chung, T.Y.; Juo, Y.A.; Hsieh, M.H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 1–15. [Google Scholar] [CrossRef]
- Rigano, C.; Di Martino Rigano, V.; Vona, V.; Carfagna, S.; Carillo, P.; Esposito, S. Ammonium assimilation by young plants of Hordeum vulgare in light and darkness: Effects on respiratory oxygen consumption by roots. New Phytol. 1996, 132, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Norenberg, M.D. Glutamine: A Trojan horse in ammonia neurotoxicity. Hepatology 2006, 44, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Albaladejo, I.; Meco, V.; Morales, B.; Sevilla, A.; Bolarin, M.C.; Flores, F.B. The drought-tolerant Solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Sci. Rep. 2018, 8, 2791. [Google Scholar] [CrossRef] [PubMed]
- Ferrario-Mery, S.; Masclaux, C.; Suzuki, A.; Valadier, M.H.; Hirel, B.; Foyer, C.H. Glutamine and α-ketoglutarate are metabolite signals involved in nitrate reductase gene transcription in untransformed and transformed tobacco plants deficient in ferredoxinglutamine-α-ketoglutarate aminotransferase. Planta 2001, 213, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Viégas, R.A.; Albenísio, J. Ammonia assimilation and proline accumulation in young cashew plants during long term exposure to NaCl-salinity. Rev. Bras. Fisiol. Veg. 1999, 11, 153–159. [Google Scholar]
- Wang, Z.Q.; Yuan, Y.Z.; Ou, J.Q.; Lin, Q.H.; Zhang, C.F. Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (Triticum aestivum) seedlings exposed to different salinity. J. Plant Physiol. 2007, 164, 695–701. [Google Scholar] [CrossRef]
- Lee, B.R.; Muneer, S.; Park, S.H.; Zhang, Q.; Kim, T.H. Ammonium-induced proline and sucrose accumulation, and their significance in antioxidative activity and osmotic adjustment. Acta Physiol. Plant. 2013, 35, 2655–2664. [Google Scholar] [CrossRef]
- Kovaleva, N.P.; Ushakova, S.A.; Tikhomirova, N.A.; Kolmakova, A.A.; Gribovskaya, I.V. Effect of photosynthetically active radiation, salinization, and type of nitrogen nutrition on growth of Salicornia europaea plants. Russ. J. Plant Physiol. 2006, 53, 785–792. [Google Scholar]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Egan, T.P.; Ungar, I.A. Mortality of the Salt Marsh Species Salicornia europaea and Atriplex Prostrata (Chenopodiaceae ) in Response to Inundation. Ohio J. Sci. 2000, 100, 24–27. [Google Scholar]
- Cui, Y.W.; Zhang, H.Y.; Ding, J.R.; Peng, Y.Z. The effects of salinity on nitrification using halophilic nitrifiers in a Sequencing Batch Reactor treating hypersaline wastewater. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tissue, D.T.; Nguyen, L.T.T.; Bange, M.P.; Anderson, I.C.; Singh, B.K.; Braunack, M.; Osanai, Y. Impacts of waterlogging on soil nitrification and ammonia-oxidizing communities in farming system. Plant Soil 2018, 426, 299–311. [Google Scholar]
- Tho, B.T.; Lambertini, C.; Eller, F.; Brix, H.; Sorrell, B.K. Ammonium and nitrate are both suitable inorganic nitrogen forms for the highly productive wetland grass Arundo donax, a candidate species for wetland paludiculture. Ecol. Eng. 2017, 105, 379–386. [Google Scholar] [CrossRef]
- Daudi, A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio. Protoc. 2016, 2, 4–7. [Google Scholar] [CrossRef]
- Husted, S.; Hebbern, C.; Mattsson, M.; Schjoerring, J. A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant. 2001, 109, 167–179. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, J.; Wang, J.; Wu, X.; Li, P.; Yao, Y. Validation of suitable reference genes for gene expression analysis in the halophyte Salicornia europaea by real-time quantitative PCR. Front. Plant Sci. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Cirillo, V.; Zhang, D.; Maggio, A.; Wang, L.; Xiao, X.; Yao, Y. Regulation of Ammonium Cellular Levels is An Important Adaptive Trait for the Euhalophytic Behavior of Salicornia europaea. Plants 2020, 9, 257. https://doi.org/10.3390/plants9020257
Ma J, Cirillo V, Zhang D, Maggio A, Wang L, Xiao X, Yao Y. Regulation of Ammonium Cellular Levels is An Important Adaptive Trait for the Euhalophytic Behavior of Salicornia europaea. Plants. 2020; 9(2):257. https://doi.org/10.3390/plants9020257
Chicago/Turabian StyleMa, Jinbiao, Valerio Cirillo, Dayong Zhang, Albino Maggio, Lei Wang, Xinlong Xiao, and Yinan Yao. 2020. "Regulation of Ammonium Cellular Levels is An Important Adaptive Trait for the Euhalophytic Behavior of Salicornia europaea" Plants 9, no. 2: 257. https://doi.org/10.3390/plants9020257
APA StyleMa, J., Cirillo, V., Zhang, D., Maggio, A., Wang, L., Xiao, X., & Yao, Y. (2020). Regulation of Ammonium Cellular Levels is An Important Adaptive Trait for the Euhalophytic Behavior of Salicornia europaea. Plants, 9(2), 257. https://doi.org/10.3390/plants9020257





