The Interaction between Leaf Allelopathy and Symbiosis with Rhizobium of Ulex europaeus on Hawaii Island
Abstract
1. Introduction
2. Methods and Materials
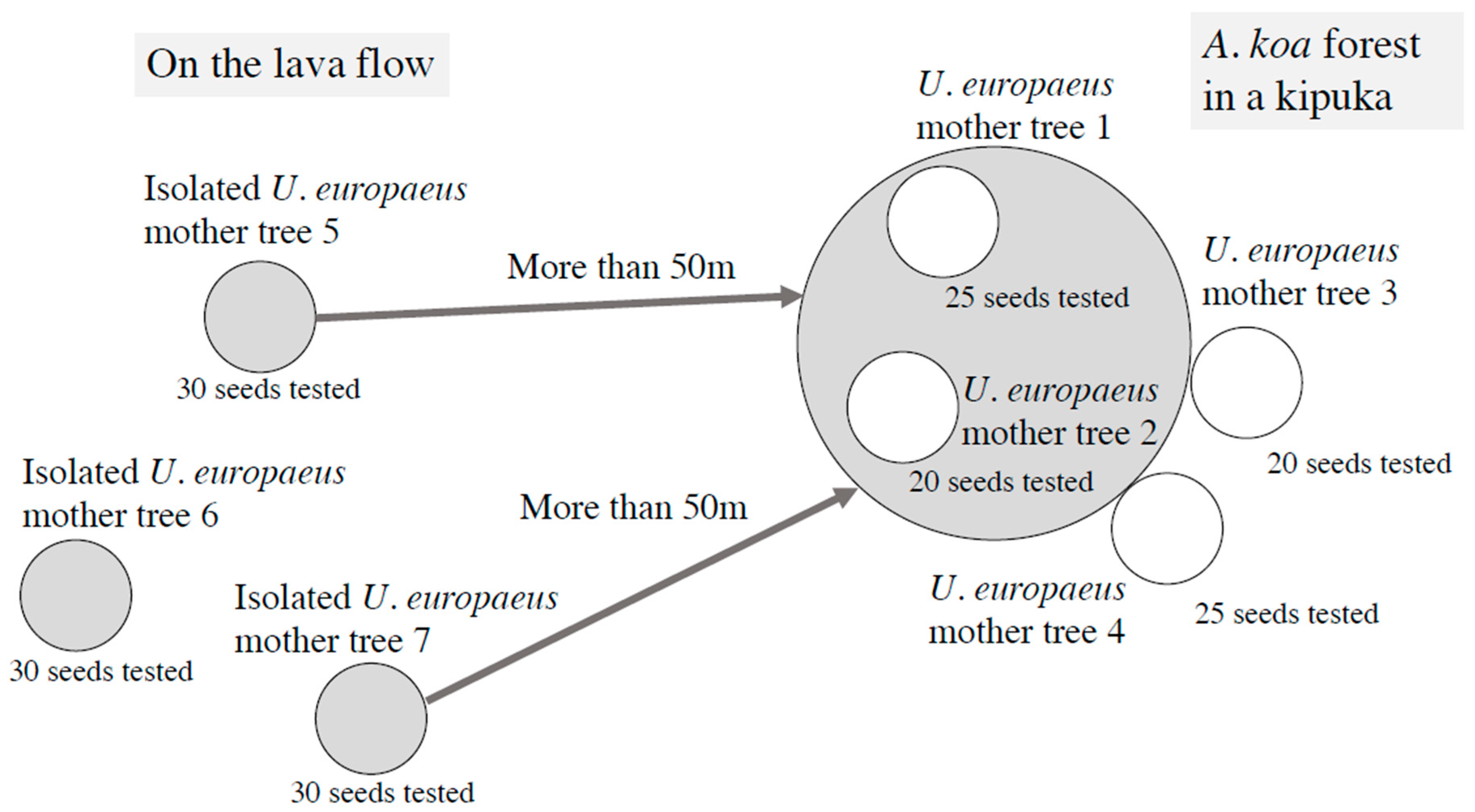
2.1. Plant Samples
2.2. Experiments
2.3. Statistical Analyses
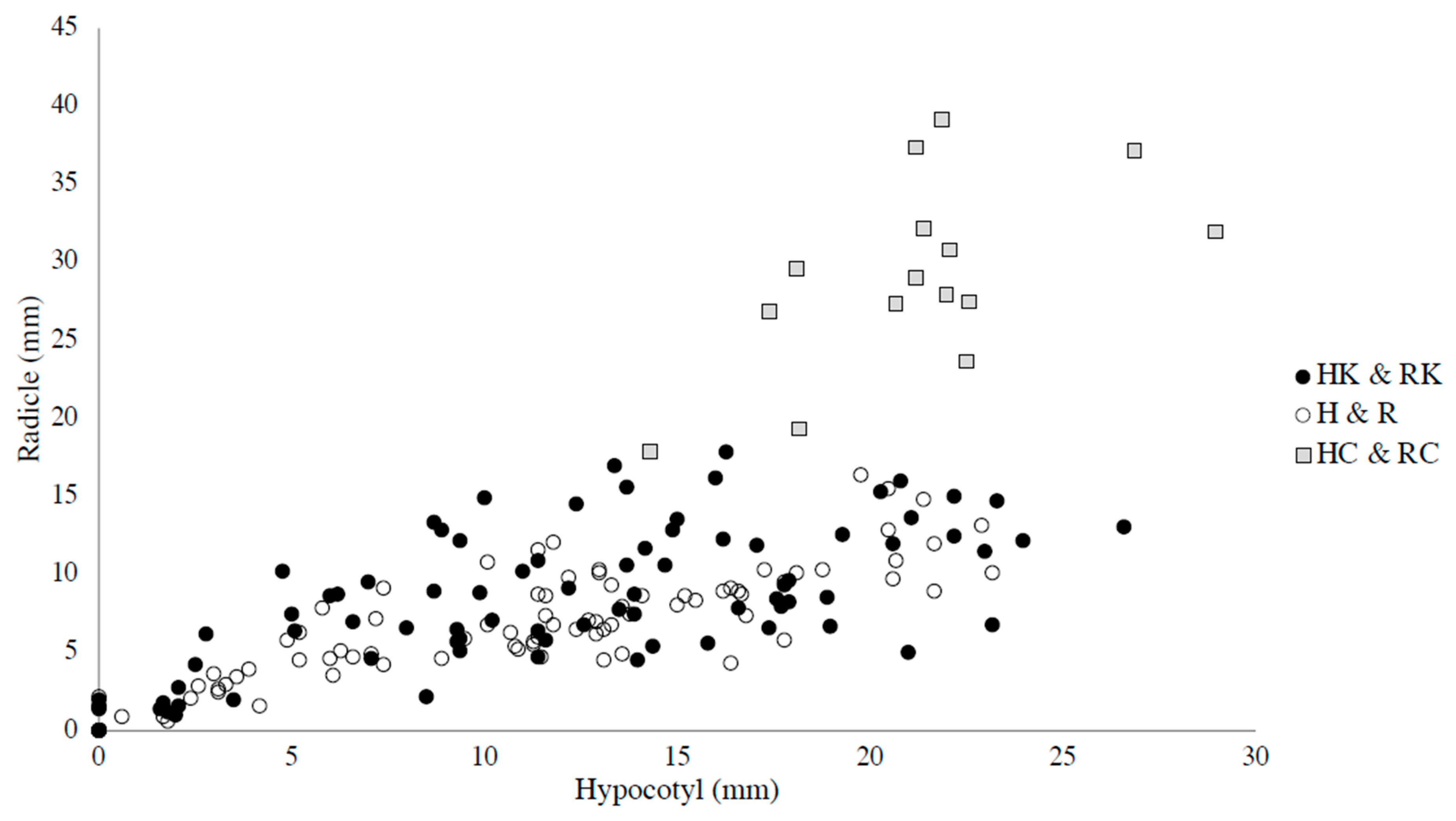
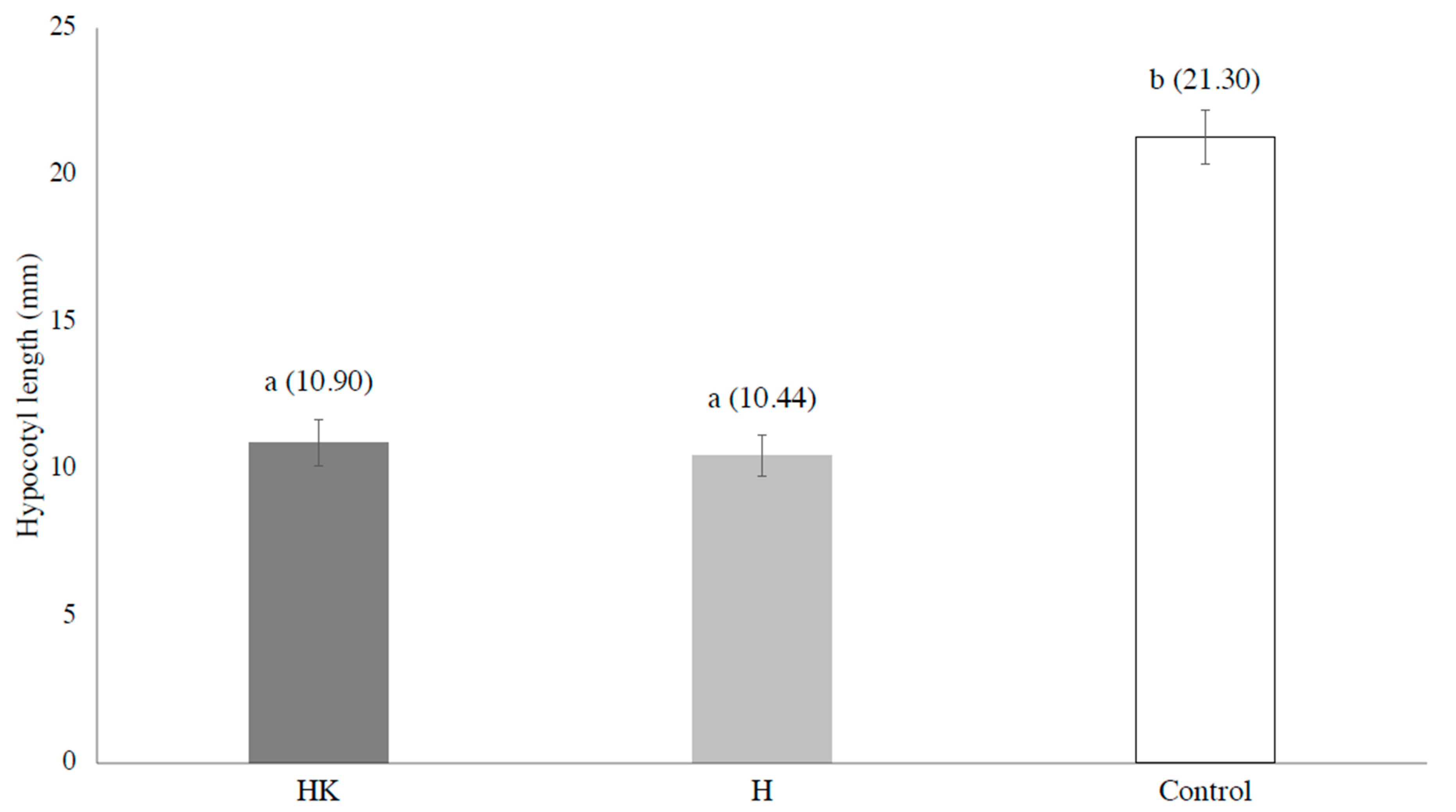
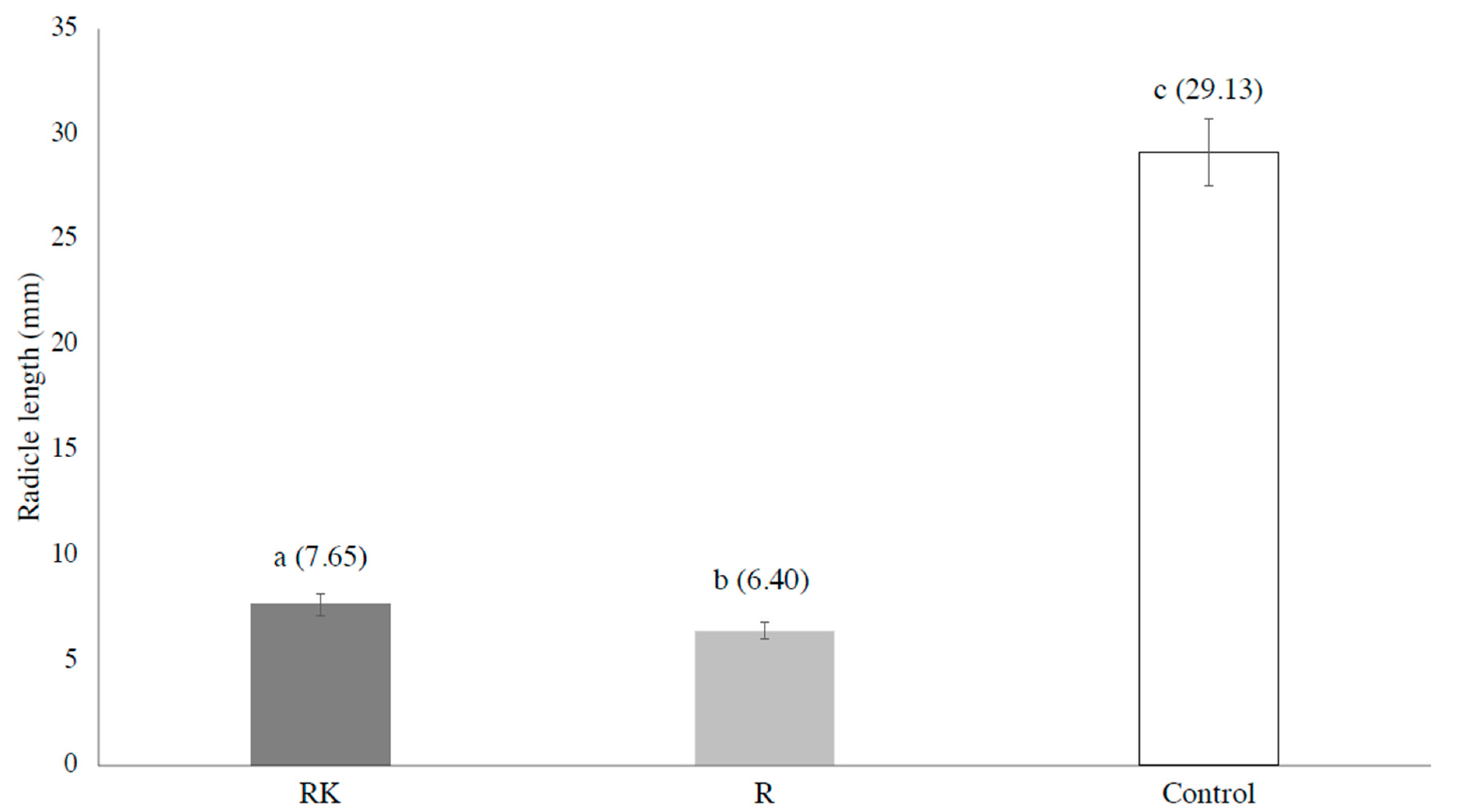
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holm, L.; Doll, J.; Holm, E.; Pancho, J.; Herberger, J. World Weeds: Natural Histories and Distribution.; Wiley-Blackwell. John Wiley and Sons: New York, NY, USA, 1997; p. 1129. [Google Scholar]
- International Union for Conservation of Nature (IUCN) Invasive Species Specialist Group. Available online: http://www.issg.org/worst100_species.html (accessed on 24 November 2019).
- Invasive Species Compendium (CABI) Ulex europaeus (gorse). Available online: https://www.cabi.org/ISC/datasheet/55561 (accessed on 24 November 2019).
- Weir, B.S.; Turner, S.J.; Silvester, W.B.; Park, D.C.; Young, J.M. Unexpectedly diverse Mesorhizobium strains and Rhizobium leguminosarum nodulate native legume genera of New Zealand, while introduced legume weeds are nodulated by Bradyrhizobium species. Appl. Environ. Microbiol. 2004, 70, 5980–5987. [Google Scholar] [CrossRef] [PubMed]
- Gage, D.J. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol. 2004, 68, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Leary, J.K.; Hue, N.V.; Singleton, P.W.; Borthakur, D. The major features of an infestation by the invasive weed legume gorse (Ulex europaeus) on volcanic soils in Hawaii. Biol. Fertil. Soils 2006, 42, 215–222. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and Exotic Plant Invasion: From Molecules and Genes to Species Interactions. Science 2003, 301, 1377. [Google Scholar] [CrossRef] [PubMed]
- Jarchow, M.E.; Cook, B.J. Allelopathy as a mechanism for the invasion of Typha angustifolia. Plant Ecol. 2009, 204, 113–124. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984; 422p. [Google Scholar]
- Hozawa, M.; Nawata, E. Variability and phenotypic plasticity of Ulex europaeus seeds in the Hawaiian Archipelago and California, USA: How do they support its invasiveness. In Proceeding of the 26th Asian-Pacific Weed Science Society Conference, Kyoto, Japan, 19–22 September 2017; p. 281. [Google Scholar]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soils 2003, 256, 67–83. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Geological Map of the State of Hawaii. Available online: https://pubs.usgs.gov/of/2007/1089/ (accessed on 17 October 2019).
- Fujii, Y.; Shibuya, T.; Nakatani, K.; Itani, T.; Hiradate, S.; Parvez, M. Assessment method for allelopathic effect from leaf litter leachates. Weed Biol. Manag. 2004, 4, 19–23. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 10 November 2019).
- Hilhorst, H.W.M.; Toorop, P.E. Review on dormancy, germinability and germination in crop and weed seeds. Adv. Agron. 1997, 61, 112–165. [Google Scholar]
| Estimate | Standard Error | t-Value | p-Value | |
|---|---|---|---|---|
| Close to Koa and control | −31.89 | 2.98 | −10.71 | <0.001 |
| Away from Koa and control | −33.59 | 2.98 | −11.28 | <0.001 |
| Close and away from Koa | −1.7 | 1.59 | −1.07 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hozawa, M.; Nawata, E. The Interaction between Leaf Allelopathy and Symbiosis with Rhizobium of Ulex europaeus on Hawaii Island. Plants 2020, 9, 226. https://doi.org/10.3390/plants9020226
Hozawa M, Nawata E. The Interaction between Leaf Allelopathy and Symbiosis with Rhizobium of Ulex europaeus on Hawaii Island. Plants. 2020; 9(2):226. https://doi.org/10.3390/plants9020226
Chicago/Turabian StyleHozawa, Mika, and Eiji Nawata. 2020. "The Interaction between Leaf Allelopathy and Symbiosis with Rhizobium of Ulex europaeus on Hawaii Island" Plants 9, no. 2: 226. https://doi.org/10.3390/plants9020226
APA StyleHozawa, M., & Nawata, E. (2020). The Interaction between Leaf Allelopathy and Symbiosis with Rhizobium of Ulex europaeus on Hawaii Island. Plants, 9(2), 226. https://doi.org/10.3390/plants9020226





