Variability in the Capacity to Produce Damage-Induced Aldehyde Green Leaf Volatiles among Different Plant Species Provides Novel Insights into Biosynthetic Diversity
Abstract
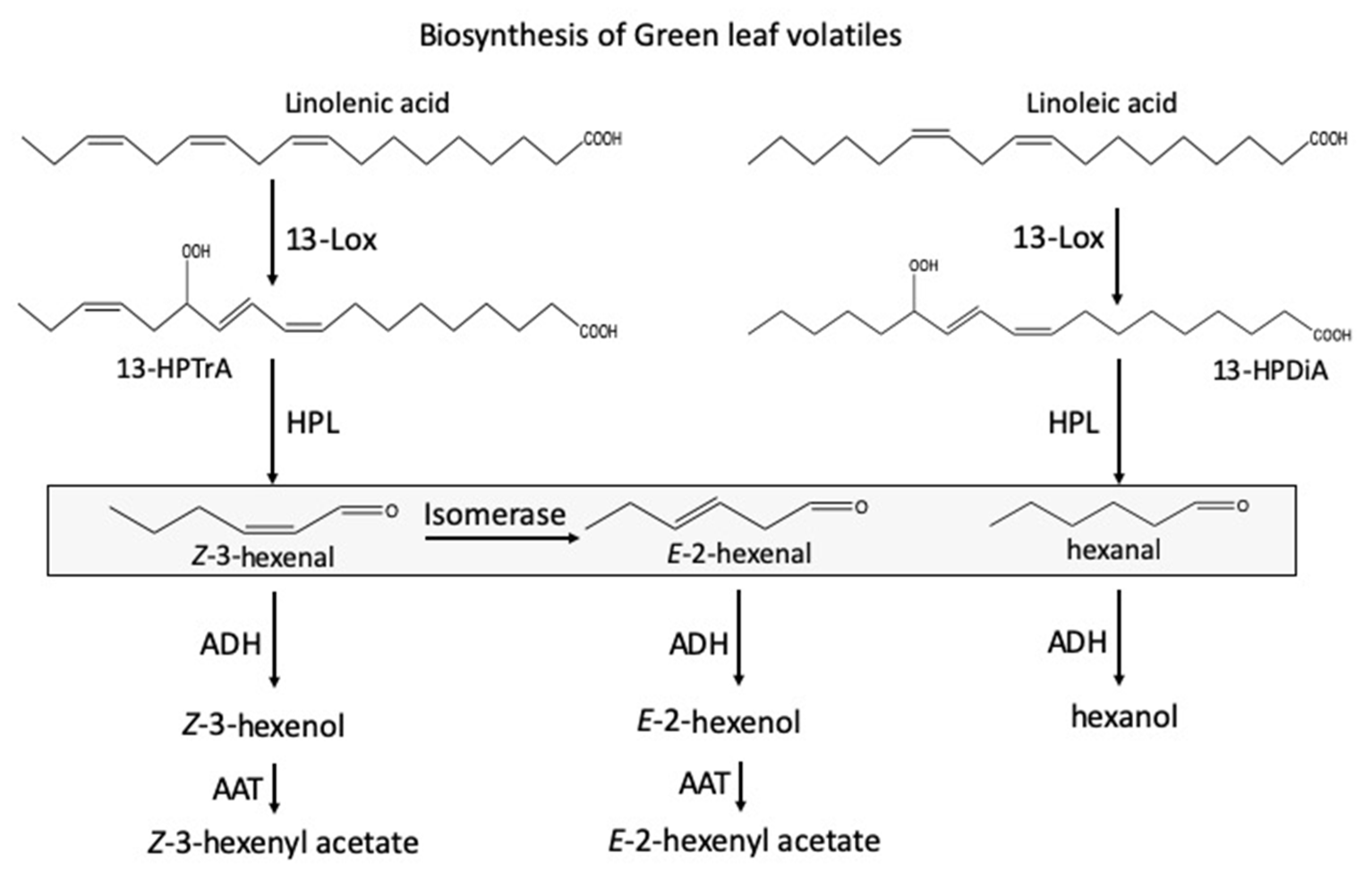
1. Introduction
2. Results
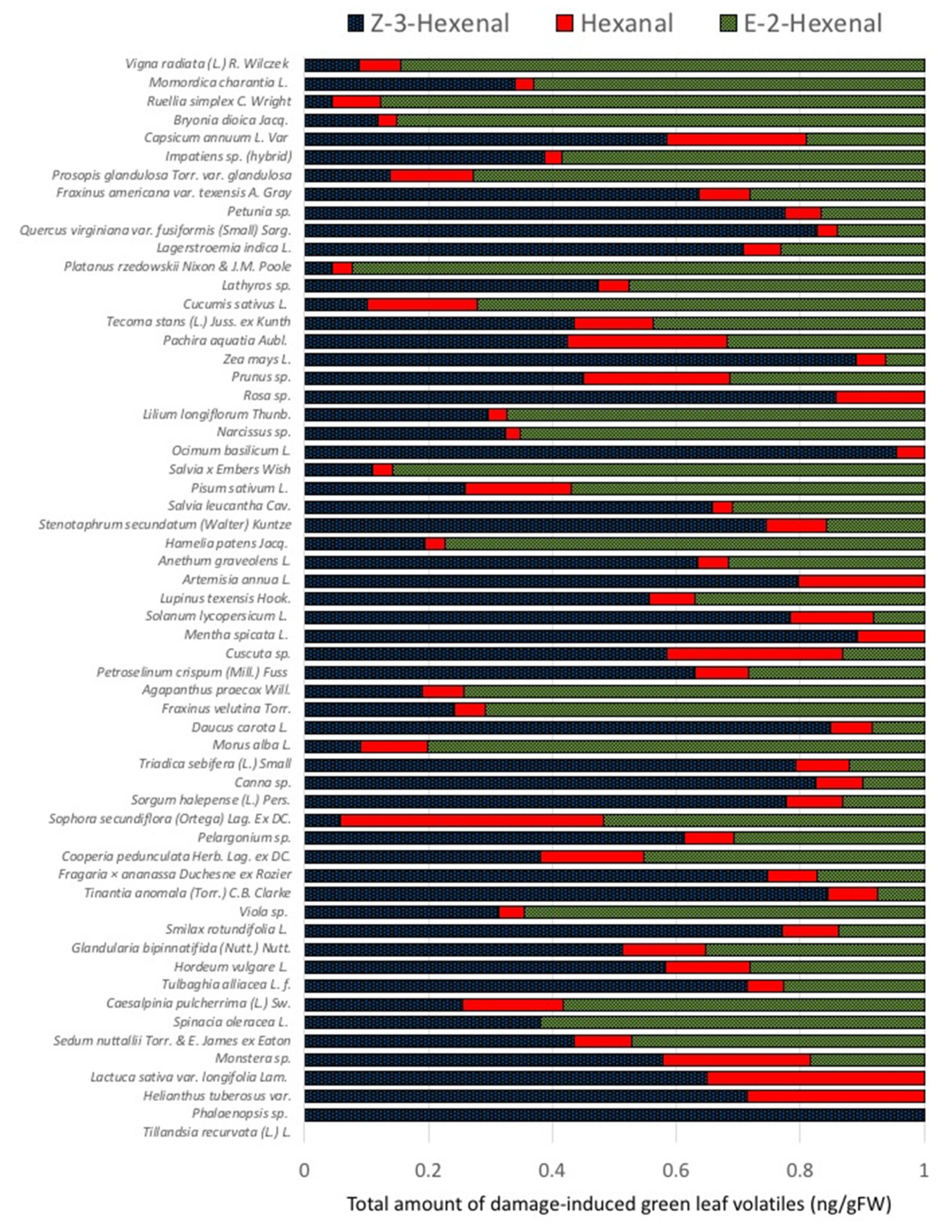
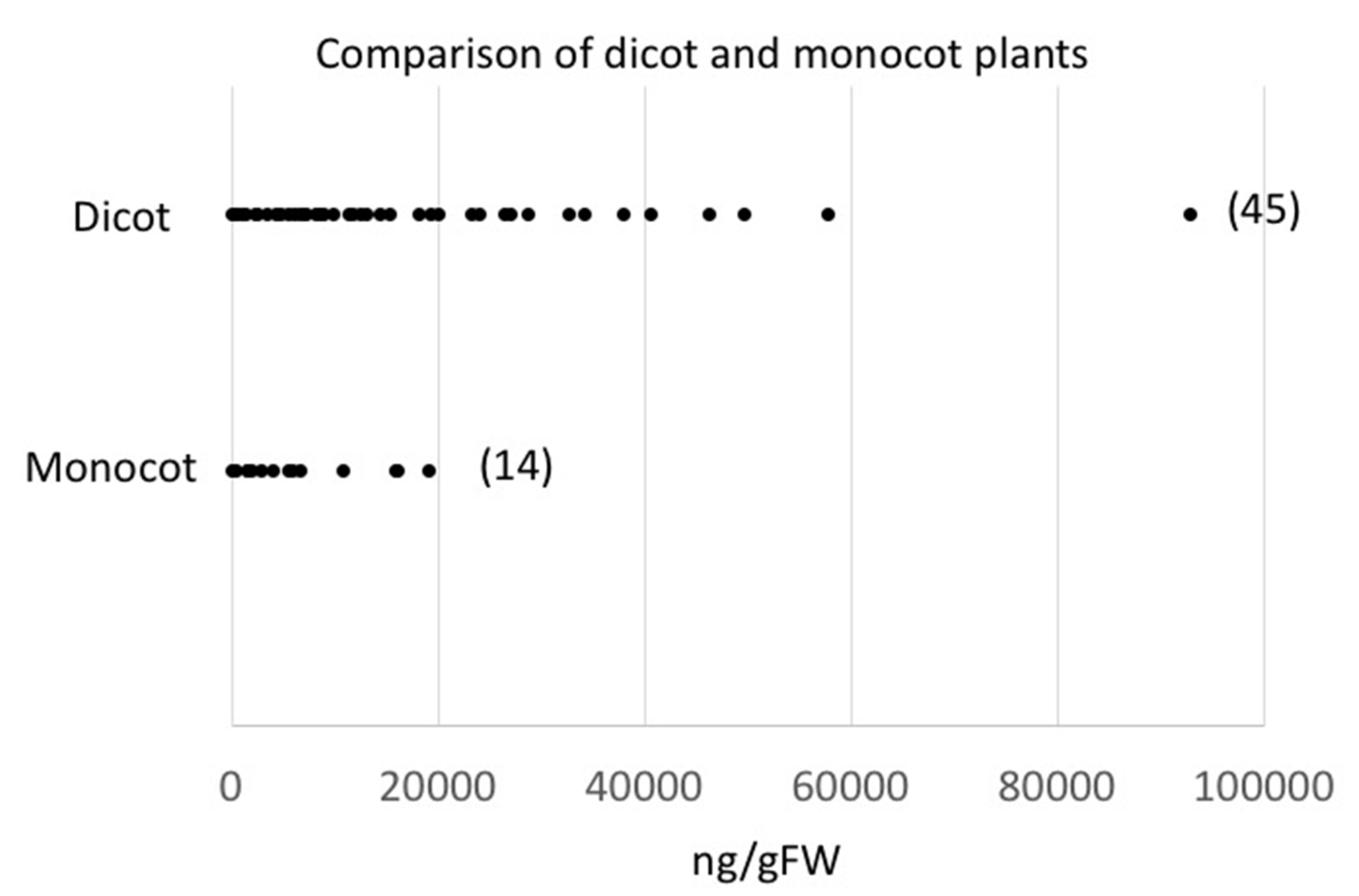
2.1. Quantities and Qualities of Damaged-Induced GLV Vary Significantly among Different Plant Species
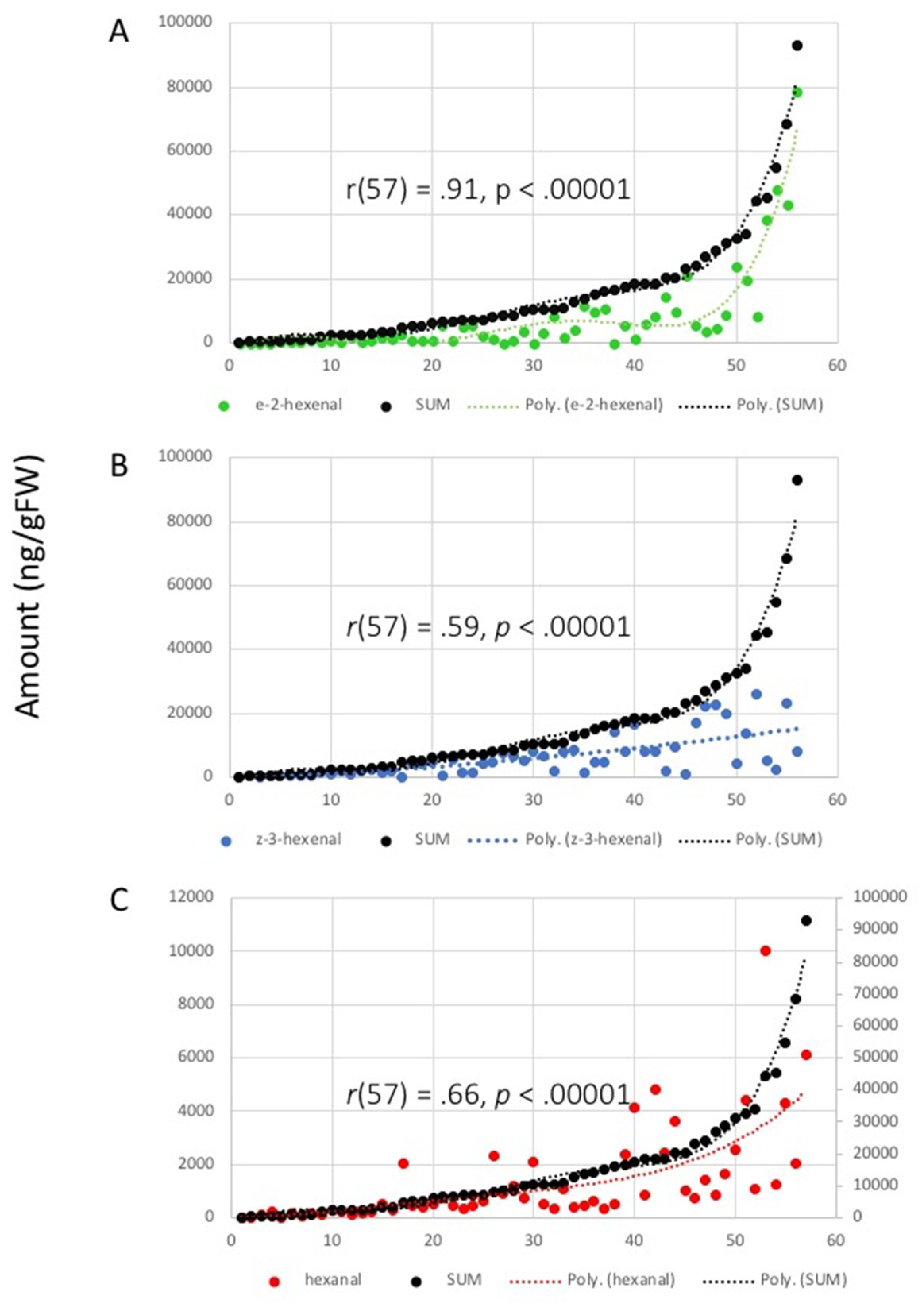
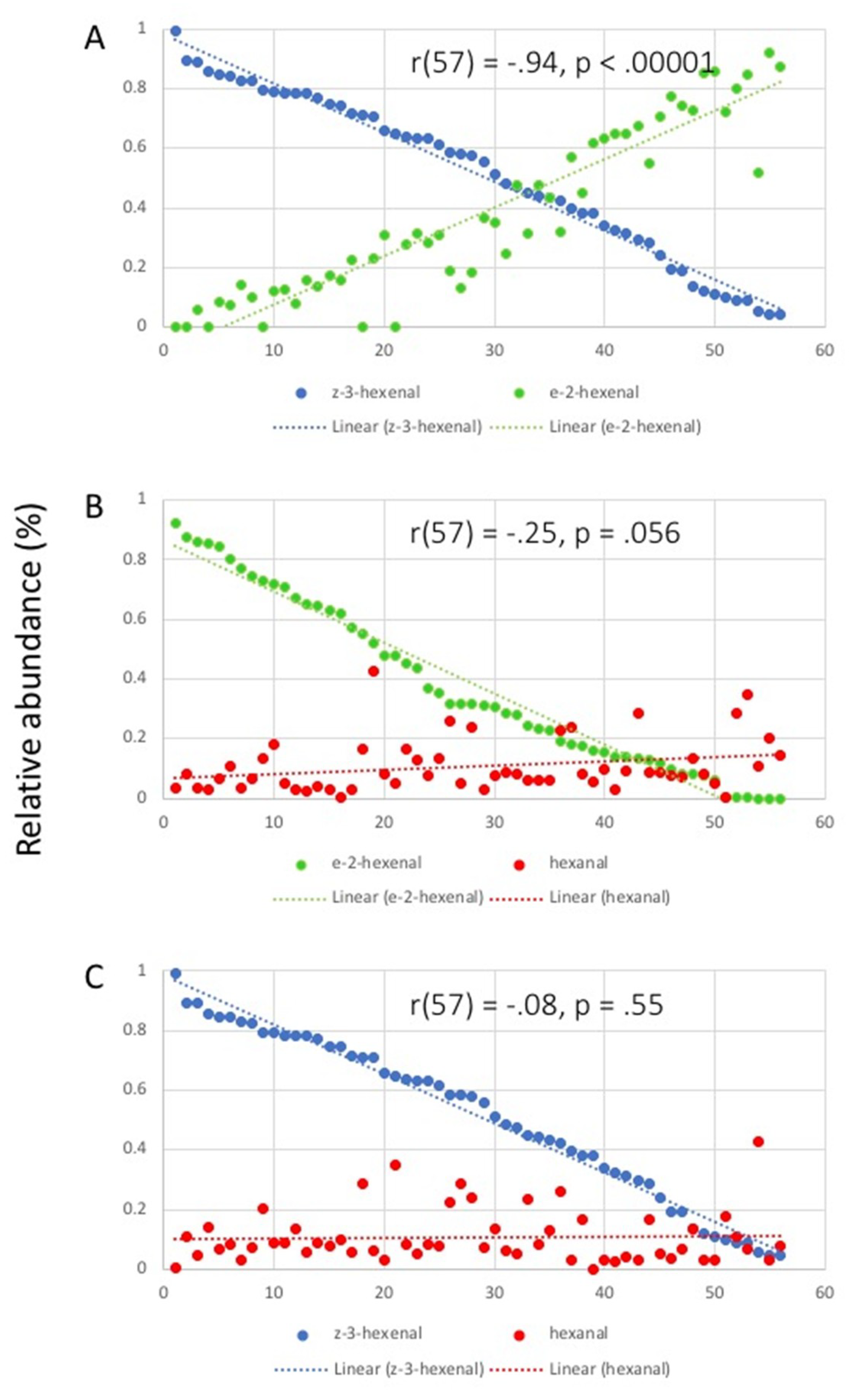
2.2. Correlation Analysis
2.3. Damage-Induced GLV Production and Plant Systematics
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Leave Damage and Analysis of Green Leaf Volatile Synthesis
4.4. Phylogenetic Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Shiojiri, K.; Kishimoto, K.; Ozawa, R.; Kugimiya, S.; Urashimo, S.; Arimura, G.; Horiuchi, J.; Nishioka, T.; Matsui, K.; Takabayashi, J. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 16672–16676. [Google Scholar] [CrossRef] [PubMed]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early Transcriptome Analyses of Z-3-Hexenol-Treated Zea mays Revealed Distinct Transcriptional Networks and Anti-Herbivore Defense Potential of Green Leaf Volatiles. PLoS ONE 2012, 8, e77465. [Google Scholar] [CrossRef] [PubMed]
- Cofer, T.M.; Engelberth, M.J.; Engelberth, J. Green leaf volatiles protect maize (Zea mays) seedlings against damage from cold stress. Plant Cell Environ. 2018, 41, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, M.; Selman, S.M.; Engelberth, J. In-cold exposure to Z-3-hexenal provides protection against ongoing cold stress in Zea mays. Plants 2019, 8, E165. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimoto, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015, 5, 8030. [Google Scholar] [CrossRef]
- Tian, S.; Guo, R.; Zou, X.; Zhang, X.; Yu, X.; Zhan, Y.; Ci, D.; Wang, M.; Si, T. Priming with the green leaf volatile (Z)-3-hexeny-1-yl acetate enhances salinity stress tolerance in peanut (Arachis hypogaea L.) seedlings. Front. Plant Sci 2019, 10, 785. [Google Scholar] [CrossRef]
- Lee, K.; Seo, P.J. Airborne signals from salt-stressed Arabidopsis plants trigger salinity tolerance in neighboring plants. Plant Sig. Behav. 2014, 9, e28392. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; D’Alessandro, M.; Jourdi, V.; Jakab, G.; Karlen, D.; Held, M.; Mauch-Mani, B.; Turlings, T.C.J. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007, 49, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; De Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochem 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Matsui, K.; Koeduka, T. Green leaf volatiles in plant signaling and response. Subcell Biochem. 2016, 86, 427–443. [Google Scholar]
- Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their application in biotechnology. Plant Biotech. 2015, 13, 727–739. [Google Scholar] [CrossRef]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Sobhy, I.; Bosak, L.; Erb, M.; Roberts, C.; Vaughn, K.A.; Göbel, C.; Tumlinson, J.; et al. The multi-tasking lipoxygenase, ZmLOX10, regulates GLV, JA, and HIPV production for defense against insect attack. Plant J. 2012, 74, 59–73. [Google Scholar] [CrossRef]
- Kunishima, M.; Yamauchi, Y.; Mizutani, M.; Kuse, M.; Takikawa, H.; Sugimoto, Y. Identification of (Z)-3:(E)-2-hexenal isomerases essential to the production of the leaf aldehyde in plants. J. Biol. Chem. 2016, 291, 14023–14033. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Dekker, H.L.; Steemers, L.; van Maarseveen, J.H.; de Koster, C.G.; Haring, M.A.; Schuurink, R.C.; Allmann, S. Identification and characterization of (3Z):(2E)-hexenal isomerases from cucumber. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikeda, A.; Shiojiri, K.; Ozawa, R.; Shiki, K.; Nagai-Kunihiro, N.; Fujita, K.; Sugimoto, K.; Yamato, K.T.; Dohra, H.; et al. Identification of a hexenal reductase that modulates the composition of green leaf volatiles. Plant Physiol. 2018, 178, 552–564. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007, 49, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolism of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef]
- Roese, U.; Manukian, A.; Heath, R.R.; Tumlinson, J.H. Volatile semiochemicals released from undamaged cotton leaves (a systemic response of living plants to caterpillar damage). Plant Physiol. 1996, 111, 487–495. [Google Scholar] [CrossRef]
- Chamberlain, K.; Khan, Z.R.; Pickett, J.A.; Toshova, T.; Wadhams, L.J. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J. Chem. Ecol. 2006, 32, 565–577. [Google Scholar] [CrossRef]
- Jardine, K.; Barron-Gafford, G.A.; Norman, J.P.; Abrell, L.; Monson, R.K.; Meyers, K.T.; Pavao-Zuckerman, M.; Dontsova, K.; Kleist, E.; Werner, C.; et al. Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light-dark transitions. Photosynth Res. 2012, 113, 321–333. [Google Scholar] [CrossRef]
- Farmaki, T.; Sanmartin, M.; Jiminez, P.; Paneque, M.; Sanz, C.; Vancanneyt, G.; Leon, J.; Sanchez-Serrano, J.J. Differential distribution of the lipoxygenase pathway enzymes within potato chloroplasts. J. Exp. Bot. 2007, 58, 555–568. [Google Scholar] [CrossRef]
- Blee, E.; Joyard, J. Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol. 1996, 110, 445–454. [Google Scholar] [CrossRef]
- Froehlich, J.E.; Itoh, A.; Howe, G.A. Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipins metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol. 2001, 125, 306–317. [Google Scholar] [CrossRef]
- Rustgi, S.; Springer, A.; Kang, C.; von Wettstein, D.; Reinbothe, C.; Reinbothe, S.; Pollmann, S. Allene oxide synthase and hydroperoxide lyase, two non-canonical cytochrome p450s in Arabidopsis thaliana and their different roles in plant defense. Int. J. Mol. Sci. 2019, 20, 3064. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, K.; Zdyb, A.; Feussner, I.; Pawlowski, K. Analysis of the subcellular localization of lipoxygenase in legume and actinorhizal nodules. Plant. Biol. 2012, 14, 46–63. [Google Scholar]
- Zhang, C.; Cao, S.; Jin, Y.; Chen, Q.; Xing, Q.; Qi, H. Melon 13-lipoxygenase CmLOX18 may be involved in C6 volatile biosynthesis in fruit. Sci. Rep. 2017, 7, 2816. [Google Scholar] [CrossRef] [PubMed]
- Weichert, H.; Kolbe, A.; Kraus, A.; Wasternack, C.; Feussner, I. Metabolic profiling of oxylipins in germinating cucumber seedlings—Lipoxygenase-dependent degradation of triacylglycerols and biosynthesis of volatile aldehydes. Planta 2002, 215, 612–619. [Google Scholar] [CrossRef]
- Matsui, K. Properties and structures of fatty acid hydroperoxide lyase. Belg. J. Bot. 1998, 131, 50–62. [Google Scholar]
- Hornostaj, A.R.; Robinson, D.S. Purification of hydroperoxide lyase from cucumber. Food Chem. 1999, 66, 173–180. [Google Scholar] [CrossRef]
- De Domenico, S.; Tsesmetzis, N.; Di Sansebastiano, G.P.; Hughes, R.K.; Casey, R.; Santino, A. Subcellular localization of Medicago truncatula 9/13-hydroperoxide lyase reveal a new localization pattern and activation mechanism for CYP74C enzymes. BMC Plant Biol. 2007, 7, 58. [Google Scholar] [CrossRef]
- Vick, B.A.; Zimmerman, D.C. Pathways of fatty acid hydroperoxide metabolism in spinach leaf chloroplasts. Plant Physiol. 1987, 85, 1073–1078. [Google Scholar] [CrossRef]
- Husson, F.; Belin, J.M. Purification of hydroperoxide lyase from green bell pepper (Capsicum annum) fruits for the generation of C6-aldehydes in vitro. J. Agric. Food Chem. 2002, 50, 1991–1995. [Google Scholar] [CrossRef]
- Nemchenko, A.; Kunze, S.; Feussner, I.; Kolomiets, M. Duplicate maize 13-lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J. Exp. Bot. 2006, 57, 3767–3779. [Google Scholar] [CrossRef]
- Jones, A.C.; Seidl-Adams, I.; Engelberth, J.; Hunter, C.T.; Alborn, H.; Tumlinson, J.H. Herbivorous caterpillars can utilize three different mechanisms to alter green leaf volatile emissions. Environ. Entomol. 2019, 48, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Ozawa, R.; Takabayashi, J.; Fujii, S.; Arai, K.; Ichiki, R.T.; Koeduka, T.; Dohra, H.; Ohnishi, T.; Taketazu, S.; et al. Silkworms suppress the release of green leaf volatiles by mulberry leaves with an enzyme from their spinnerets. Sci. Rep. 2018, 8, 11942. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Pearse, I.S.; Ignatia, L.; Karban, R.; Dehesh, K. Insect herbivores selectively suppress the HPL branch of the oxylipin pathway in host plants. Plant J. 2012, 73, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Dehesh, K. Insect herbivores selectively mute GLV production in plants. Plant Signal. Behav. 2013, 8, e24136. [Google Scholar] [CrossRef] [PubMed]
- Bremer, B.; Chase, M.W.; Fay, M.F.; Reveal, J.L.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Lin. Soc. 2009, 161, 105–121. [Google Scholar]
- More, N.; Daniel, R.M.; Petach, H.H. The effects of low temperature on enzyme activity. Biochem. J. 1995, 305, 17–20. [Google Scholar] [CrossRef]
- Nakashima, A.; von Russ, S.H.; Tasaka, H.; Nomura, M.; Mochizuki, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Boland, W.; Takabayashi, J.; et al. Traumatin- and dinortraumatin-containing galactolipids in Arabidopsis: Their formation in tissue-disrupted leaves as counterparts of green leaf volatiles. J. Biol. Chem. 2013, 288, 26078–26088. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelberth, J.; Engelberth, M. Variability in the Capacity to Produce Damage-Induced Aldehyde Green Leaf Volatiles among Different Plant Species Provides Novel Insights into Biosynthetic Diversity. Plants 2020, 9, 213. https://doi.org/10.3390/plants9020213
Engelberth J, Engelberth M. Variability in the Capacity to Produce Damage-Induced Aldehyde Green Leaf Volatiles among Different Plant Species Provides Novel Insights into Biosynthetic Diversity. Plants. 2020; 9(2):213. https://doi.org/10.3390/plants9020213
Chicago/Turabian StyleEngelberth, Jurgen, and Marie Engelberth. 2020. "Variability in the Capacity to Produce Damage-Induced Aldehyde Green Leaf Volatiles among Different Plant Species Provides Novel Insights into Biosynthetic Diversity" Plants 9, no. 2: 213. https://doi.org/10.3390/plants9020213
APA StyleEngelberth, J., & Engelberth, M. (2020). Variability in the Capacity to Produce Damage-Induced Aldehyde Green Leaf Volatiles among Different Plant Species Provides Novel Insights into Biosynthetic Diversity. Plants, 9(2), 213. https://doi.org/10.3390/plants9020213





