Peptone-Induced Physio-Biochemical Modulations Reduce Cadmium Toxicity and Accumulation in Spinach (Spinacia oleracea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Analysis
2.2. Experimental Design
2.3. Plant Harvesting and Sampling
2.4. Determination of Chlorophyll and Gas Exchange Parameters
2.5. Determination of Electrolyte Leakage, Hydrogen Peroxide (H2O2), and Malondialdehyde (MDA)
2.6. Determination of Superoxide Dismutase (SOD), Peroxidase (POD), and Catalase (CAT) Activity
2.7. Determination of the Contents of Ascorbic Acid and Proline
2.8. Determination of Cd Content
2.9. Statistical Analysis
3. Results
3.1. Plant Growth
3.2. Photosynthetic Pigments
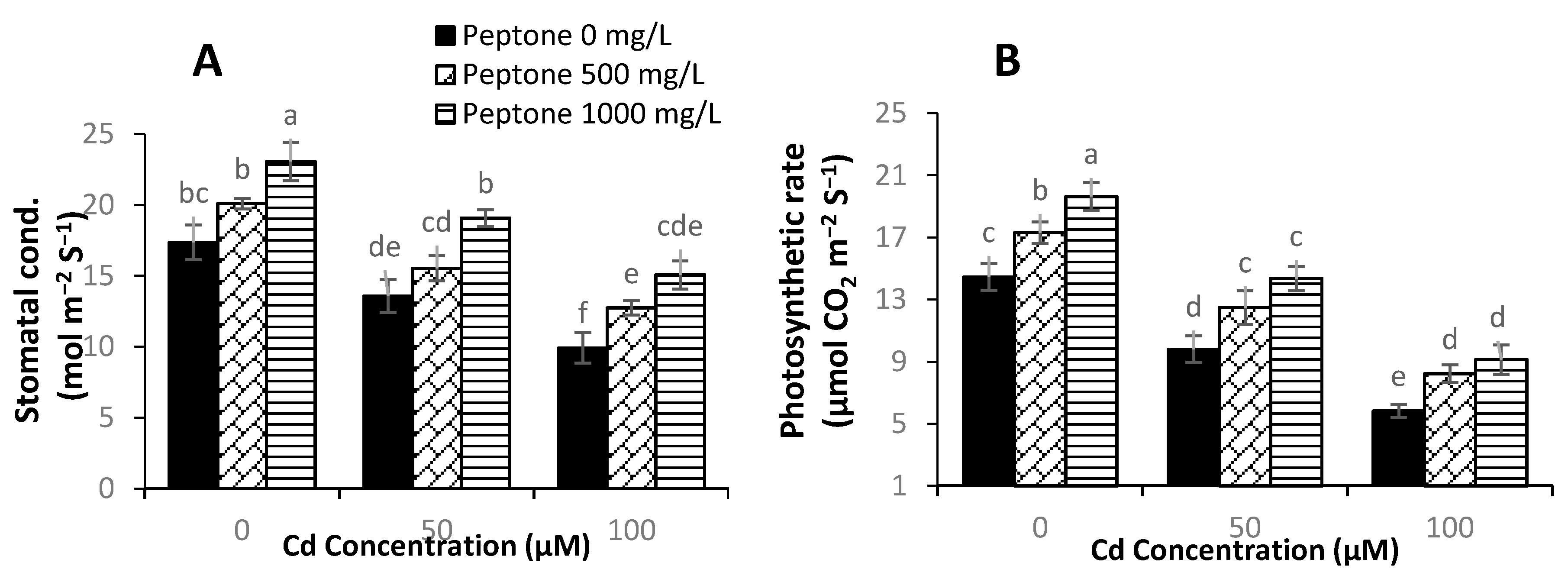
3.3. Gas Exchange Parameters
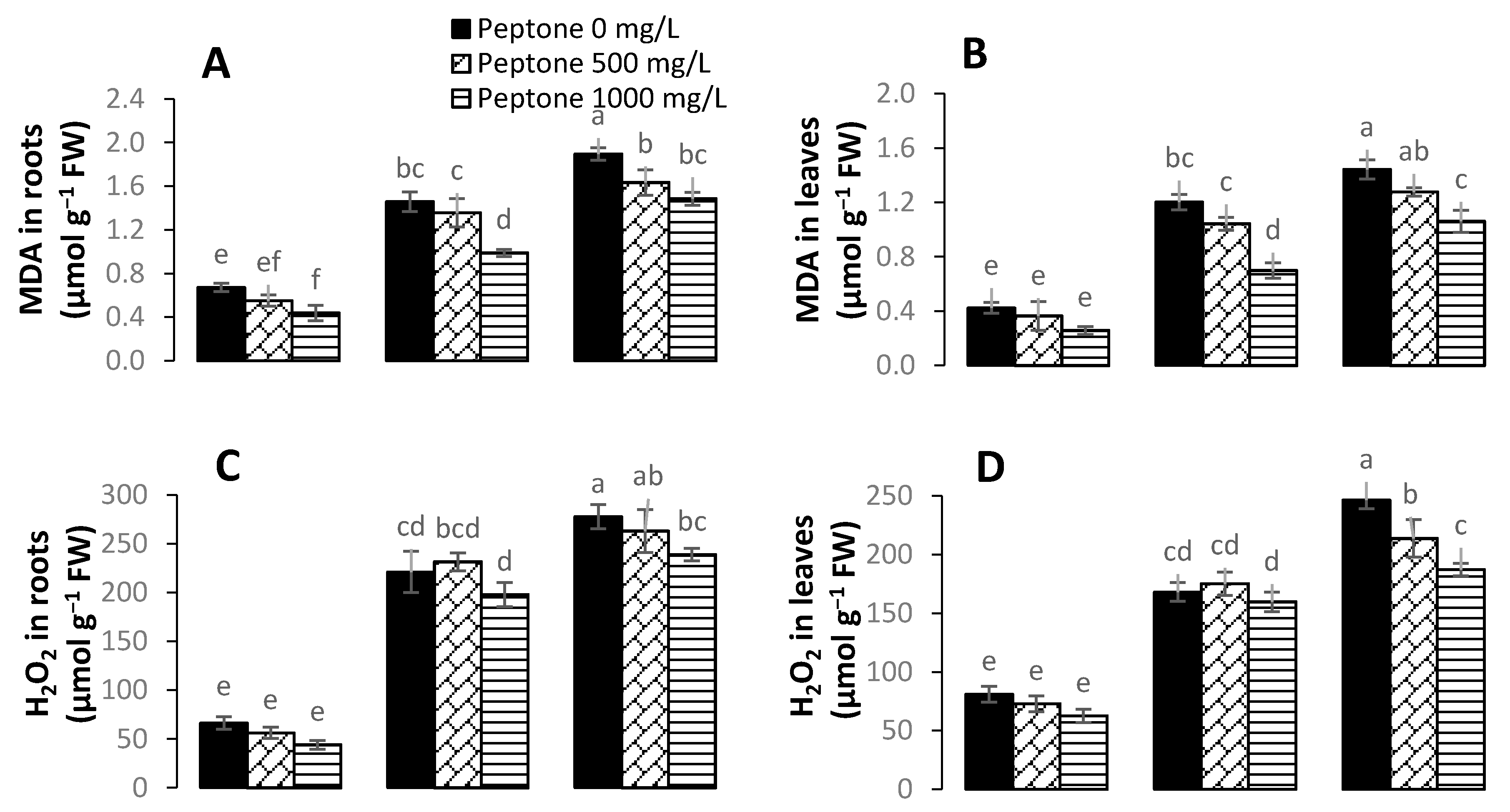
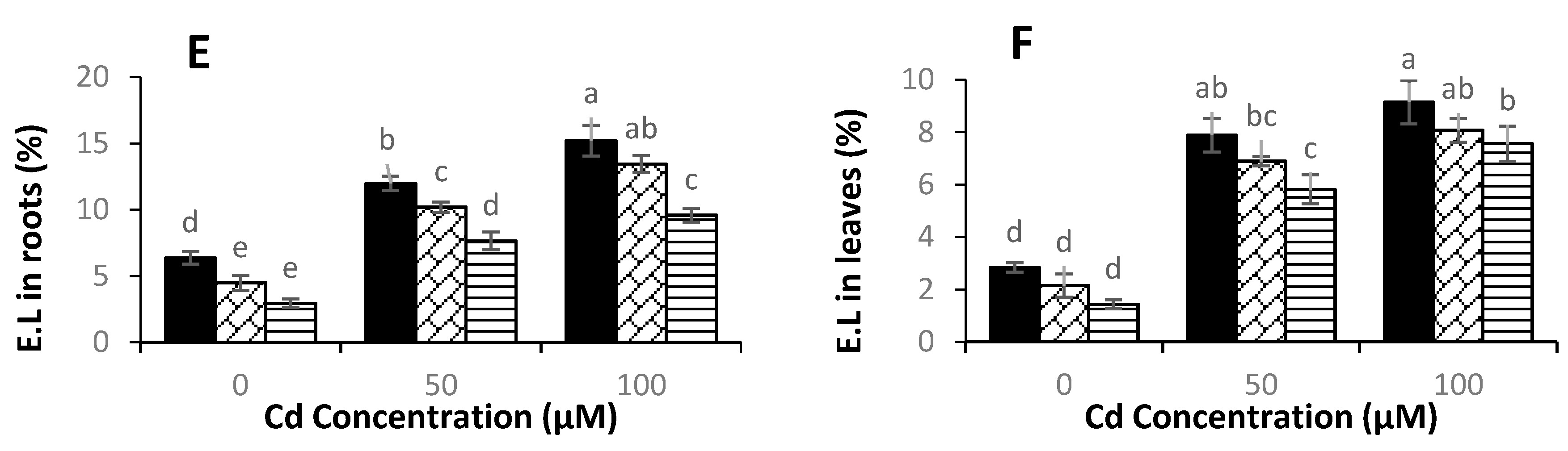
3.4. Levels of Hydrogen Peroxide, Malondialdehyde, and Electrolyte Leakage
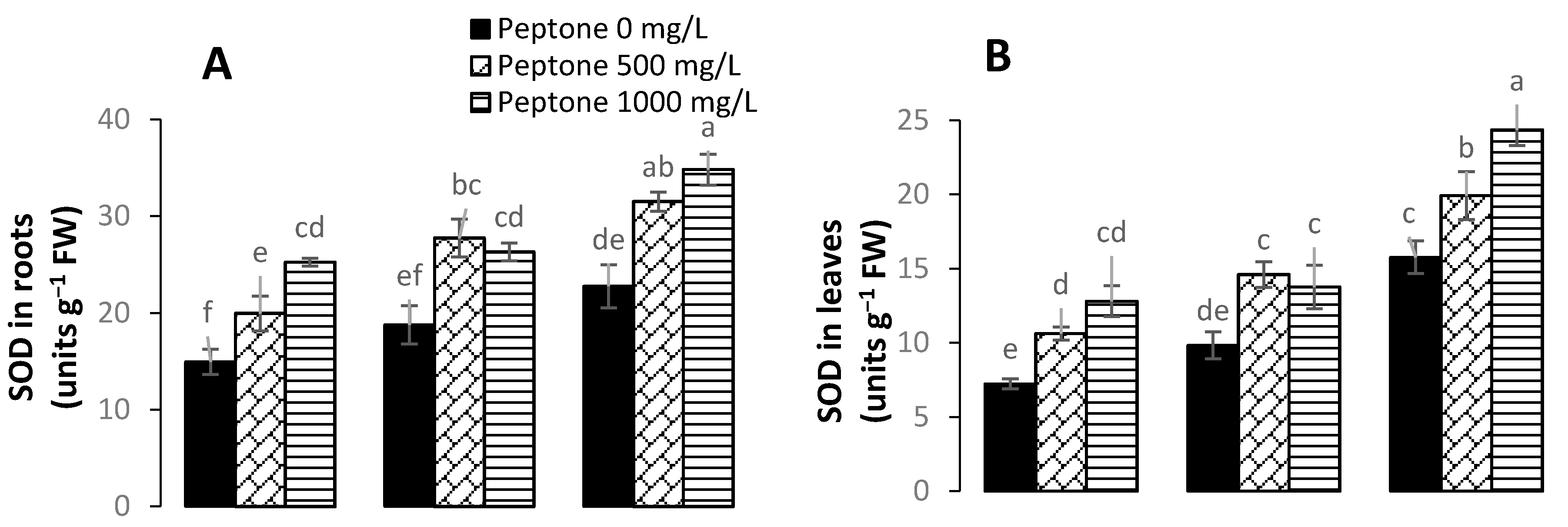
3.5. Activity of Antioxidant Enzymes
3.6. Proline and Ascorbic Acid Content
3.7. Cd Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, Q.L.; Liu, C.; Wu, H.Y.; Jin, Y.; Hua, M.; Zhu, B.W.; Chen, K.; Huang, L. Association of soil cadmium contamination with ceramic industry: A case study in a Chinese town. Sci. Total Environ. 2015, 514, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Feng, C.; Zeng, G.; Zhong, M.; Gao, X.; Li, X.; He, X.; Li, X.; Fang, Y.; Mo, D. Atmospheric deposition of mercury and cadmium impacts on topsoil in a typical coal mine city, Lianyuan, China. Chemosphere 2017, 189, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Dharma-Wardana, M.W.C. Fertilizer usage and cadmium in soils, crops and food. Environ. Geochem. Health 2018, 40, 2739–2759. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Kotaki, K.; Gunji, F.; Kubo, N.; Kobayashi, S.; Ito, T.; Itabashi, H. Suppression of cadmium uptake in rice using fermented bark as a soil amendment. Chemosphere 2016, 148, 487–494. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Martinez-Penalver, A.; Grana, E.; Reigosa, M.J.; Sanchez-Moreiras, A.M. The early response of Arabidopsis thaliana to cadmium-and copper-induced stress. Environ. Exp. Bot. 2012, 78, 1–9. [Google Scholar] [CrossRef]
- Rochayati, S.; Du Laing, G.; Rinklebe, J.; Meissner, R.; Verloo, M. Use of reactive phosphate rocks as fertilizer on acid upland soils in Indonesia: Accumulation of cadmium and zinc in soils and shoots of maize plants. J. Plant. Nutr. Soil Sci. 2011, 174, 186–194. [Google Scholar] [CrossRef]
- Garnier, J.; Cebron, A.; Tallec, G.; Billen, G.; Sebilo, M.; Martinez, A. Nitrogen behaviour and nitrous oxide emission in the tidal Seine River estuary (France) as influenced by human activities in the upstream watershed. Biogeochemistry 2006, 77, 305–326. [Google Scholar] [CrossRef]
- Mojiri, A. The potential of corn (Zea mays) for phytoremediation of soil contaminated with cadmium and lead. J. Biol. Environ. Sci. 2011, 5, 17–22. [Google Scholar]
- Alia, N.; Sardar, K.; Said, M.; Salma, K.; Sadia, A.; Sadaf, S.; Miklas, S. Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment. Int. J. Environ. Res. Public Health 2015, 12, 7400–7416. [Google Scholar] [CrossRef]
- Melquist, S.; Luff, B.; Bender, J. Arabidopsis PAI gene arrangements, cytosine methylation and expression. Genetics 1999, 153, 401–413. [Google Scholar] [PubMed]
- Soad, M.M.; Lobna, S.T.; Farahat, M.M. Influence of foliar application of pepton on growth, flowering and chemical composition of Helichrysum bracteatum L. plants under different irrigation intervals. Ozean J. Appl. Sci. 2010, 3, 143–155. [Google Scholar]
- Sadak, M.S.; Abdelhamid, M.T. Influence of amino acids mixture application on some biochemical aspects, antioxidant enzymes and endogenous polyamines of Vicia faba L.plant grown under seawater salinity stress. Gesunde Pflanz. 2015, 67, 119–129. [Google Scholar] [CrossRef]
- Sadak, M.S.H.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Ali, Q.; Perveen, R.; El-Esawi, M.A.; Ali, S.; Hussain, S.M.; Amber, M.; Iqbal, N.; Rizwan, M.; Alyemeni, M.N.; El-Serehy, H.A.; et al. Low Doses of Cuscuta reflexa Extract Act as Natural Biostimulants to Improve the Germination Vigor, Growth, and Grain Yield of Wheat Grown under Water Stress: Photosynthetic Pigments, Antioxidative Defense Mechanisms, and Nutrient Acquisition. Biomolecules 2020, 10, 1212. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Soliman, M.H.; Abdulmajeed, A.M.; Alhaithloul, H.; Alharbi, B.M.; El-Esawi, M.A.; Hasanuzzaman, M.; Elkelish, A. Saponin biopriming positively stimulates antioxidants defense, osmolytes metabolism and ionic status to confer salt stress tolerance in soybean. Acta Physiol. Plant. 2020, 42, 114. [Google Scholar] [CrossRef]
- Cerdan, M.; Sanchez-Sanchez, A.; Oliver, M.; Juarez, M.; Sanchez-Andreu, J.J. Effect of foliar and root applications of amino acids on iron uptake by tomato plants. Acta Hortic. 2009, 830, 481–488. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant. Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Lachhab, N.; Sanzani, S.M.; Adrian, M.; Chiltz, A.; Balacey, S.; Boselli, M. Soybean and casein hydrolysates induce grapevine immune responses and resistance against Plasmopara viticola. Front. Plant. Sci. 2014, 5, 716. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant. Sci. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of soil analysis. In Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; p. 9. [Google Scholar]
- Soltanpour, P.N. Use of AB-DTPA soil test to evaluate elemental availability and toxicity. Commun. Soil Sci. Plant. Anal. 1985, 16, 323–338. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris L. Plant Physiol. 1949, 2, 1–15. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Carmak, I.; Horst, J.H. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max L.). J. Plant Physiol. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Zhang, X.Z. Themeasurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In Research Methodology of Crop Physiology; Agriculture Press: Beijing, China, 1992; pp. 208–211. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Rehman, M.Z.U.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef] [PubMed]
- Hina, K.; Kanwal, S.S.; Arshad, M.; Gul, I. Effect of cadmium (Cd) stress on spinach (Spinacea oleracea) and its retention kinetics in soil in response to organic amendments. Pak. J. Agric. Sci. 2019, 56, 179–185. [Google Scholar]
- Mahnoor, A.; Arshid, P.; Usman, I.; Qaisar, M.; Rafiq, A. Melatonin and plant growth-promoting rhizobacteria alleviate the cadmium and arsenic stresses and increase the growth of Spinacia oleracea L. Plant. Soil Environ. 2020, 66, 234–241. [Google Scholar]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Soliman, M.H.; Alnusaire, T.S.; Abdelbaky, N.F.; Alayafi, A.A.; Hasanuzzaman, M.; Rowezak, M.M.; El-Esawi, M.; Elkelish, A. Trichoderma-Induced Improvement in Growth, Photosynthetic Pigments, Proline, and Glutathione Levels in Cucurbita pepo Seedlings under Salt Stress. Phyton 2020, 89, 473. [Google Scholar] [CrossRef]
- Shanying, H.E.; Xiaoe, Y.A.N.G.; Zhenli, H.E.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar]
- Saeed, M.R.; Kheir, A.M.; Al-Sayed, A.A. Supperssive effect of some amino acids against Meloidogyne incognita on soybeans. J. Agric. Sci. Mansoura Univ. 2005, 30, 1097–1103. [Google Scholar]
- Goss, J.A. Physiology of Plants and Their Cells; Pergamon Biological Sciences Series; Pergamon: New York, NY, USA, 1973. [Google Scholar]
- Youssef, A.A.; Khattab, M.E.; Omer, E.A. Effect of spraying of molybdenum and tyrosine on growth, yield and chemical composition of lemon basil plant. Egypt Pharm. J. 2004, 3, 87–106. [Google Scholar]
- Talaat, I.M.; Youssef, A.A. The role of the amino acids lysine and ornithine in growth and chemical constituents of Basil plants. Egypt J. Appl. Sci. 2002, 17, 83–95. [Google Scholar]
- Mona, H.M.; Talaat, I.M. Physiological response of Rose Geranium (Pelargonium gravealens L.) to phenylalanine and nicatonic Acid. Annal. Agr. Sci. Moshtohor 2005, 43, 807–822. [Google Scholar]
- El-Esawi, M.A.; Elkelish, A.; Soliman, M.; Elansary, H.O.; Zaid, A.; Wani, S.H. Serratia marcescens BM1 enhances cadmium stress tolerance and phytoremediation potential of soybean through modulation of osmolytes, leaf gas exchange, antioxidant machinery, and stress-responsive genes expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of StDREB2 Transcription Factor Enhances Drought Stress Tolerance in Cotton (Gossypium barbadense L.). Genes 2019, 10, 142. [Google Scholar] [CrossRef]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; El-Esawi, M.A.; Rizwan, M.; Azeem, M.; Hussain, A.I.; Perveen, R.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Foliar spray of Fe-Asp confers better drought tolerance in sunflower as compared with FeSO4: Yield traits, osmotic adjustment, and antioxidative defense mechanisms. Biomolecules 2020, 10, 1217. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef]
- Vwioko, E.; Adinkwu, O.; El-Esawi, M.A. Comparative physiological, biochemical and genetic responses to prolonged waterlogging stress in okra and maize given exogenous ethylene priming. Front. Physiol. 2017, 8, 632. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Ahmad, M. Overexpression of AtWRKY30 transcription factor enhances heat and drought stress tolerance in wheat (Triticum aestivum L.). Genes 2019, 10, 163. [Google Scholar] [CrossRef]
- De Araujo, R.P.; de Almeida, A.A.F.; Pereira, L.S.; Mangabeira, P.A.; Souza, J.O.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Photosynthetic, antioxidative, molecular and ultrastructural responses of young cacao plants to Cd toxicity in the soil. Ecotoxicol. Environ. Saf. 2017, 144, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.S.; Yang, Y.H.; Shao, Y.F.; Wu, K.W.; Liu, Z.L. The effects of exogenous salicylic acid on alleviating cadmium toxicity in Nymphaea tetragona Georgi. S. Afr. J. Bot. 2018, 114, 267–271. [Google Scholar] [CrossRef]
- Hassanein, R.A.M. Effect of Some Amino Acids, Trace Elements and Irradiation on Fennel (Foeniculum vulgare L.). Ph.D. Thesis, Faculty of Agriculture, Cairo University, Giza, Egypt, 2003. [Google Scholar]
- Nahed, G.A.; Balbaa, L.K. Influence of tyrosine and zinc on growth, flowering and chemical constituents of Salvia farinacea plants. J. Appl. Sci. Res. 2007, 3, 1479–1489. [Google Scholar]
- Tatjana, M.H.; Nesi, A.N.; Wagner, L.A.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant. 2015, 8, 1563–1579. [Google Scholar]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, G.S.; El-Esawi, M.A. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, S.S.; Mustafa, A.; Ditta, A.; Alamri, S.; El-Esawi, M.A.; Rafique, M.; Ashraf, S.; Siddiqui, M.H. Mitigation of nickel toxicity and growth promotion in sesame through the application of a bacterial endophyte and zeolite in nickel contaminated soil. Int. J. Environ. Res. Public Health 2020, 17, 8859. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Genetic variation and alleviation of salinity stress in barley (Hordeum vulgare L.). Molecules 2018, 23, 2488. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, S.; El-Esawi, M.A.; Rana, M.S.; Saleem, M.H.; Riaz, M.; Ashraf, U.; Potcho, M.P.; Duan, M.; Rajput, I.A.; et al. Molybdenum supply alleviates the cadmium toxicity in fragrant rice by modulating oxidative stress and antioxidant gene expression. Biomolecules 2020, 10, 1582. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of genetic variation and enhancement of salt tolerance in French pea (Pisum Sativum L.). Int. J. Mol. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef]
- Ali, Q.; Shahid, S.; Ali, S.; El-Esawi, M.A.; Hussain, A.I.; Perveen, R.; Iqbal, N.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A.; et al. Fertigation of Ajwain (Trachyspermum ammi L.) with Fe-Glutamate confers better plant performance and drought tolerance in comparison with FeSO4. Sustainability 2020, 12, 7119. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.-S.; El-Esawi, M.A.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.; Athar, H.R.; Ashraf, M.; Hameed, A. Glycinebetaine-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ. Exp. Bot. 2007, 60, 368–376. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Noor, I.; El-Esawi, M.A.; Hayat, K.; Rizwan, M.; Abbas, Z.; El-Sheikh, M.A.; Alyemeni, M.N.; et al. Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater. Sustainability 2020, 12, 6690. [Google Scholar] [CrossRef]
- Nazli, F.; Mustafa, A.; Ahmad, M.; Hussain, A.; Jamil, M.; Wang, X.; Shakeel, Q.; Imtiaz, M.; El-Esawi, M.A. A Review on Practical Application and Potentials of Phytohormone-Producing Plant Growth-Promoting Rhizobacteria for Inducing Heavy Metal Tolerance in Crops. Sustainability 2020, 12, 9056. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Nie, Z.; Sun, X. Effects of molybdenum on water utilization, antioxidative defense system and osmotic-adjustment ability in winter wheat (Triticum aestivum L.) under drought stress. Plant Physiol. Biochem. 2014, 83, 365–374. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, N.N.; Zhang, Y.H.; Feng, Q.Z.; Yang, C.W.; Liu, B. DNA methylation involved in proline accumulation in response to osmotic stress in rice (Oryza sativa L.). Genet. Mol. Res. 2013, 12, 1269–1277. [Google Scholar] [CrossRef]





| Soil Characteristics | Values |
|---|---|
| Texture | Sandy clay |
| Sand % | 48 |
| Silt % | 16 |
| Clay % | 36 |
| pH | 7.65 |
| EC dS m−1 | 0.811 |
| Soluble ions | |
| Ca2+ mmol L−1 | 7.71 |
| Na mmol L−1 | 9.46 |
| K mmol L−1 | 1.34 |
| Metal concentrations | |
| Cd mg kg−1 | 0.83 |
| Pb mg kg−1 | 6.64 |
| Zn mg kg−1 | 4.93 |
| Mn mg kg−1 | 6.37 |
| Fe mg kg−1 | 50.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emanuil, N.; Akram, M.S.; Ali, S.; El-Esawi, M.A.; Iqbal, M.; Alyemeni, M.N. Peptone-Induced Physio-Biochemical Modulations Reduce Cadmium Toxicity and Accumulation in Spinach (Spinacia oleracea L.). Plants 2020, 9, 1806. https://doi.org/10.3390/plants9121806
Emanuil N, Akram MS, Ali S, El-Esawi MA, Iqbal M, Alyemeni MN. Peptone-Induced Physio-Biochemical Modulations Reduce Cadmium Toxicity and Accumulation in Spinach (Spinacia oleracea L.). Plants. 2020; 9(12):1806. https://doi.org/10.3390/plants9121806
Chicago/Turabian StyleEmanuil, Naila, Muhammad Sohail Akram, Shafaqat Ali, Mohamed A. El-Esawi, Muhammad Iqbal, and Mohammed Nasser Alyemeni. 2020. "Peptone-Induced Physio-Biochemical Modulations Reduce Cadmium Toxicity and Accumulation in Spinach (Spinacia oleracea L.)" Plants 9, no. 12: 1806. https://doi.org/10.3390/plants9121806
APA StyleEmanuil, N., Akram, M. S., Ali, S., El-Esawi, M. A., Iqbal, M., & Alyemeni, M. N. (2020). Peptone-Induced Physio-Biochemical Modulations Reduce Cadmium Toxicity and Accumulation in Spinach (Spinacia oleracea L.). Plants, 9(12), 1806. https://doi.org/10.3390/plants9121806







