Effects of Arthrospira platensis Extract on Physiology and Berry Traits in Vitis vinifera
Abstract
1. Introduction
2. Results
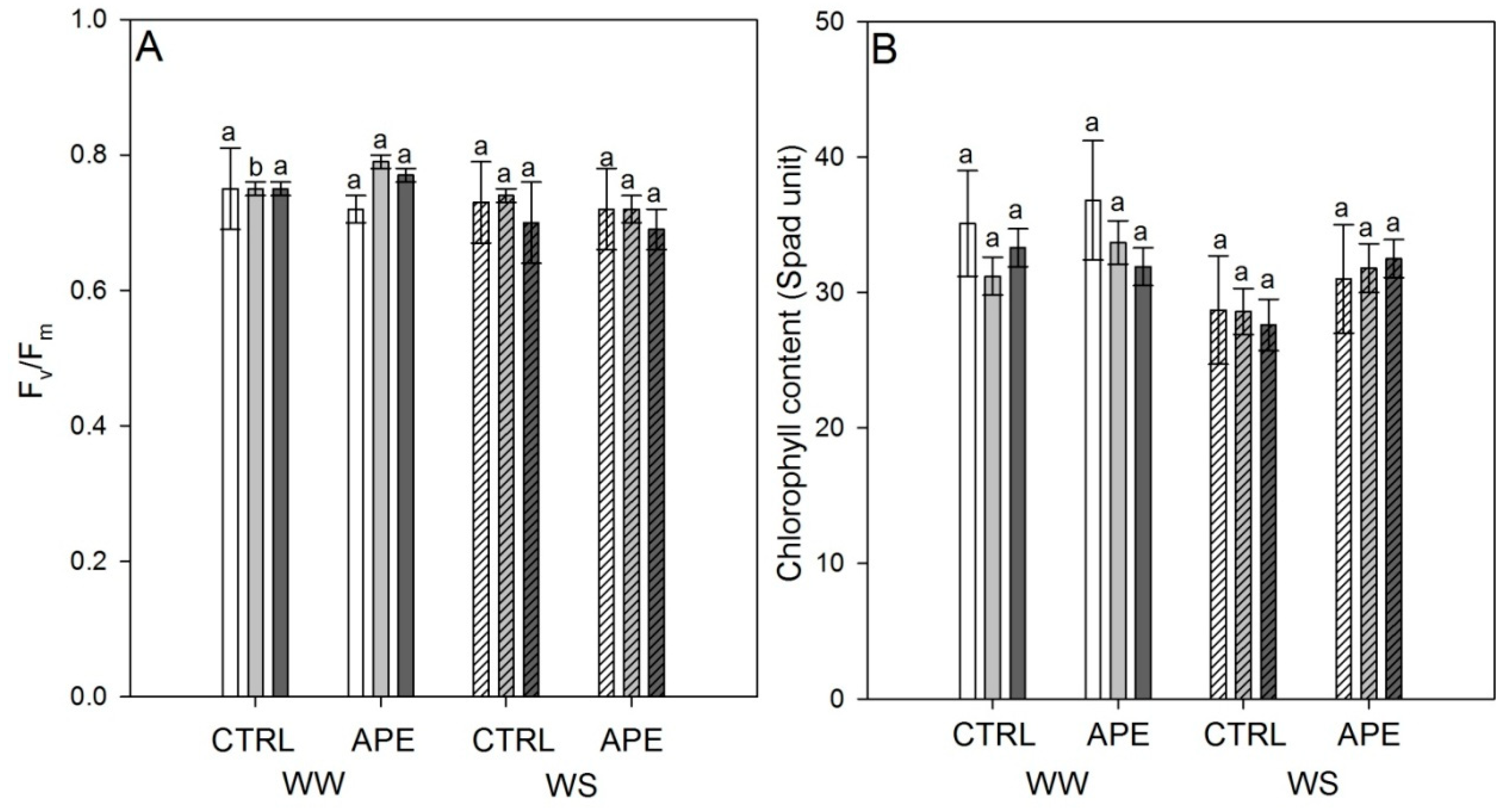
2.1. Leaf Gas Exchanges, Stem Water Potential, Leaf Chlorophyll a Fluorescence and Chlorophyll Content
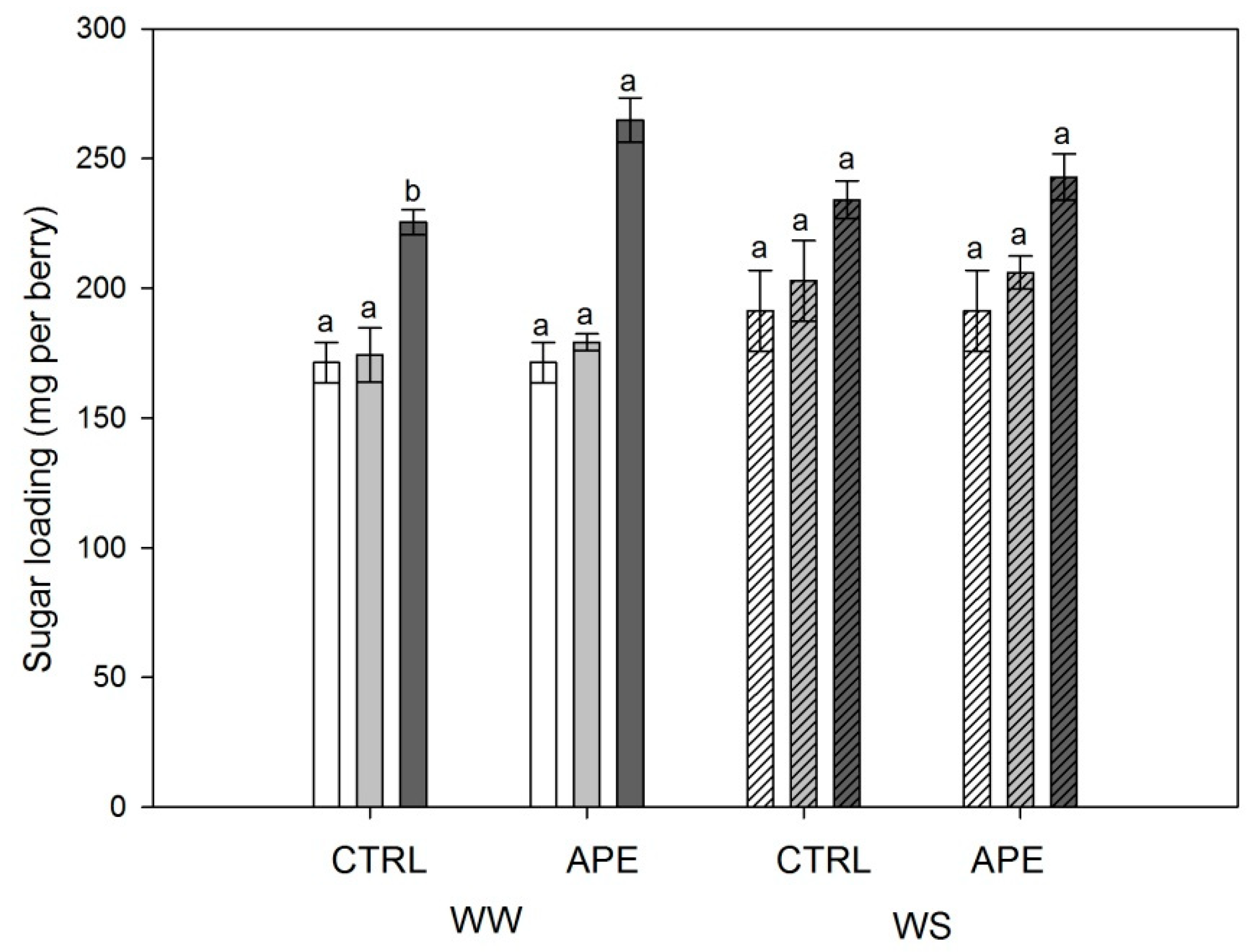
2.2. Berry Composition
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Settings
4.2. Leaf Gas Exchanges, Stem Water Potential, Leaf Chlorophyll a Fluorescence, and Content
4.3. Berry Composition
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dry, P.; Longbottom, M.; Mcloughlin, S.; Johnson, T.; Collins, C. Classification of reproductive performance of ten wine grape varieties. Aust. J. Grape Wine Res. 2010, 16, 47–55. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A.; Petrie, P.R. Resilience of grapevine yield in response to warming. OENO One 2017, 51. [Google Scholar] [CrossRef]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.): An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Chaves, M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Pou, A.; Flexas, J.; Alsina, M.; Bota, J.; Carambula, C.; de Herralde, F.; Galmés, J.; Lovisolo, C.; Jimenez, M.; Ribas-Carbó, M.; et al. Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri x V. rupestris). Physiol. Plant 2008, 134, 313–323. [Google Scholar] [CrossRef]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.M.; Galle, A.; Galmés, J.; Jimenez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri x V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef]
- Dunn, G.M.; Martin, S.R. Do temperature conditions at budburst affect flower number in Vitis vinifera L. cv. Cabernet Sauvignon? Aust. J. Grape Wine Res. 2000, 6, 116–124. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.T.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar]
- Chuine, I.; Yiou, P.; Viovy, N.; Seguin, B.; Daux, V.; Ladurie, E.L.R. Historical phenology: Grape ripening as a past climate indicator. Nature 2004, 432, 289–290. [Google Scholar] [CrossRef]
- Petrie, P.R.; Sadras, V.O. Advancement of grapevine maturity in Australia between 1993 and 2006: Putative causes, magnitude of trends and viticultural consequences. Aust. J. Grape Wine Res. 2008, 14, 33–45. [Google Scholar] [CrossRef]
- Xu, Y.; Castel, T.; Richard, Y.; Cuccia, C.; Bois, B. Burgundy regional climate change and its potential impact on grapevines. Climate Dynam. 2012, 39, 1613–1626. [Google Scholar] [CrossRef]
- Haselgrove, L.; Botting, D.; Van Heeswijck, R.; Høj, P.B.; Dry, P.R.; Ford, C.; Land, P.G.I. Canopy microclimate and berry composition: The effect of bunch exposure on the phenolic composition of Vitis vinifera L cv. Shiraz grape berries. Aust. J. Grape Wine Res. 2000, 6, 141–149. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Sadras, V.O.; Petrie, P.R.; Moran, M.A. Effects of elevated temperature in grapevine. II juice pH, titratable acidity and wine sensory attributes. Aust. J. Grape Wine Res. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality: A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- De Orduña, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertiliser. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species-A review. Food Ener. Secur. 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Materassi, R.; Tredici, M.; Balloni, W. Spirulina culture in sea-water. Appl. Microbiol. Biotechnol. 1984, 19, 384–386. [Google Scholar] [CrossRef]
- Tredici, M.; Biondi, N.; Ponis, E.; Rodolfi, L.; Zittelli, G.C. Advances in microalgal culture for aquaculture feed and other uses. In New Technologies in Aquaculture; Woodhead Publishing Limited: Combridge, UK, 2009; pp. 610–676. [Google Scholar]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Pszczółkowski, W.; Romanowska-Duda, Z.; Owczarczyk, A.; Grzesik, M.; Sakowicz, T.; Chojnacka, A. Influence of secondary metabolites from Cyanobacteria on plant growth and development. In Current Advances in Algal Taxonomy and Its Applications: Phylogenetic, Ecological and Applied Perspective; Wołowski, K., Kaczmarska, I., Ehrman, J.M., Wojtal, A.Z., Eds.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2012; pp. 195–203. [Google Scholar]
- Faheed, F.A.; Fattah, Z.A. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar]
- Oliveira, J.; Mógor, G.; Mógor, A. Produtividade de beterraba em função da aplicação foliar de biofertilisante. Cad. Agroecol. 2013, 8, 1–4. [Google Scholar]
- Seif, Y.I.A.; El-Miniawy, S.E.D.M.; El-Azm, N.A.A.; Hegazi, A.Z. Response of snap bean growth and seed yield to seed size, plant density and foliar application with algae extract. Ann. Agric. Sci. 2016, 61, 187–199. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal. Res. 2016, 15, 59–64. [Google Scholar]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalgae Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- El Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; El Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). Environ. Biol. Fishes 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Edmeades, D.C. The effects of liquid fertilisers derived from natural products on crop, pasture, and animal production: A review. Aust. J. Agric. Res. 2002, 53, 965–976. [Google Scholar] [CrossRef]
- Hodge, S.; Merfield, C.N.; Creasy, G.L. The effect of organically derived fertilisers on early growth of Pinot noir cuttings under glasshouse conditions. N. Z. J. Crop. Hortic. Sci. 2017, 45, 223–231. [Google Scholar] [CrossRef]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Quaglia, M.; Calderini, O.; Moretti, C.; Poni, S.; Palliotti, A. Metabolic and transcriptional changes associated with the use of Ascophyllum nodosum extracts as tools to improve the quality of wine grapes (Vitis vinifera cv. Sangiovese) and their tolerance to biotic stress. J. Sci. Food Agric. 2019, 99, 6350–6363. [Google Scholar] [CrossRef]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Storchi, P.; Mattii, G.B. Eco-Physiological Traits and Phenylpropanoid Profiling on Potted Vitis vinifera L. cv Pinot Noir Subjected to Ascophyllum nodosum Treatments under Post-Veraison Low Water Availability. Appl. Sci. 2020, 10, 4473. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Escalona, J.M.; Galmes, J.; Gulias, J.; Lefi, E.-K.; Martinez-Canellas, S.F.; Moreno, M.T.; Ribas-Carbo, M.; et al. Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2005, 144, 273–283. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar]
- Poni, S.; Lakso, A.N.; Turner, J.R.; Melious, R.E. The effects of pre-and post-véraison water stress on growth and physiology of potted Pinot Noir grapevines at varying crop levels. Vitis 1993, 32, 207–214. [Google Scholar]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Gómez-del-Campo, M.; Baeza, P.; Ruiz, C.; Sotés, V.; Lissarrague, J.R. Effect of previous water conditions on vine response to rewatering. Vitis 2007, 46, 51–55. [Google Scholar]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Matthews, M.A.; Nuzzo, V. Berry size and yield paradigms on grapes and wine quality. Acta Hortic. 2007, 754, 423–436. [Google Scholar] [CrossRef]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of irrigation and crop level on growth, yield and wine quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 1985, 36, 132–139. [Google Scholar]
- Dokoozlian, N.K.; Kliewer, W.M. Influence of light on grape berry growth and composition varies during fruit development. J. Am. Soc. Hortic. Sci. 1996, 121, 869–874. [Google Scholar] [CrossRef]
- Greer, D.H.; Weston, C. Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in a controlled environment. Funct. Plant Biol. 2010, 37, 206–214. [Google Scholar]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Kerridge, G.H.; Ruhl, E.H.; Nicholas, P.R. Shiraz berry size in relation to seed number and implications for juice and wine composition. Aust. J. Grape Wine Res. 2005, 11, 2–8. [Google Scholar] [CrossRef]
- Barbagallo, M.G.; Guidoni, S.; Hunter, J.J. Berry size and qualitative characteristics of Vitis vinifera L. cv. Syrah. S. Afr. J. Enol. Vitic. 2011, 32, 129–136. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Palliotti, A.; Tombesi, S.; Frioni, T.; Famiani, F.; Silvestroni, O.; Zamboni, M.; Poni, S. Morpho-structural and physiological response of container-grown Sangiovese and Montepulciano cvv.(Vitis vinifera) to re-watering after a pre-veraison limiting water deficit. Funct. Plant Biol. 2014, 41, 634–647. [Google Scholar] [CrossRef]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front. Plant Sci. 2017, 8, 1362. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Deloire, A. The concept of berry sugar loading. Wineland Wynboer 2011, 257, 93–95. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine-Stabilization and Treatments, 2nd ed.; John Wiley and Sons: Chichester, UK, 2006; Volume 2. [Google Scholar]
| Irrig. Regime | Stage | Pn (µmol CO2 m2 s−1) | gs (mmol H2O m2 s−1) | eWUE (µmol CO2/mmol H2O) | Ψm (MPa) | ||||
|---|---|---|---|---|---|---|---|---|---|
| CTRL | APE | CTRL | APE | CTRL | APE | CTRL | APE | ||
| t0 | 8.2 ± 1.0 a | 8.2 ± 0.8 a | 136.2 ± 21.2 a | 169.8 ± 19.7 a | 2.00 ± 0.20 a | 1.70 ± 0.10 a | −0.85 ± 0.05 a | −0.85 ± 0.05 a | |
| WW | t1 | 3.3 ± 0.3 b | 6.0 ± 0.9 a | 131.4 ± 21.6 a | 121.3 ± 15.3 a | 0.66 ± 0.12 b | 1.94 ± 0.20 a | −1.07 ± 0.04 b | −0.80 ± 0.06 a |
| t2 | 7.0 ± 0.5 a | 7.5 ± 0.7 a | 132.7 ± 27.4 a | 107.1 ± 15.0 a | 1.86 ± 0.51 a | 1.72 ± 0.22 a | −1.05 ± 0.04 a | −1.28 ± 0.06 a | |
| t0 | 6.9 ± 0.7 a | 7.5 ± 1.1 a | 127.0 ± 9.3 a | 121.4 ± 12.4 a | 1.70 ± 0.20 a | 1.70 ± 0.20 a | −1.41 ± 0.08 a | −1.41 ± 0.08 a | |
| WS | t1 | 4.0 ± 0.9 a | 4.5 ± 0.2 a | 146.7 ± 26.5 b | 194.2 ± 21.6 a | 1.66 ± 0.50 a | 3.26 ± 0.20 a | −1.24 ± 0.07 a | −1.19 ± 0.04 a |
| t2 | 8.8 ± 0.9 a | 8.9 ± 0.7 a | 110.0 ± 16.3 a | 129.6 ± 22.3 a | 3.10 ± 0.40 a | 2.40 ± 0.20 a | −1.25 ± 0.07 a | −1.27 ± 0.05 a | |
| Pn (µmol CO2 m2 s−1) | gs (mmol H2O m2 s−1) | eWUE (µmol CO2/mmol H2O) | Ψm (MPa) | Fv/Fm | Chlorophyll Content (Spad Unit) | |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| APE | 7.1 | 140.6 | 2.12 | −1.13 | 0.74 | 32.9 |
| CTRL | 6.4 | 130.7 | 1.83 | −1.15 | 0.74 | 30.7 |
| Irrig. Regime | ||||||
| WW | 6.7 | 133.1 | 1.65 | −0.98 | 0.76 | 33.7 |
| WS | 6.8 | 138.1 | 2.30 | −1.30 | 0.72 | 30.0 |
| Significance | ||||||
| Treatment | 0.573 | 0.550 | 0.480 | 0.904 | 0.901 | 0.050 |
| Irrig. Regime | 0.959 | 0.757 | 0.132 | 0.011 | 0.019 | 0.005 |
| Interaction | 0.796 | 0.522 | 0.980 | 0.986 | 0.396 | 0.220 |
| Irrig. Regime | Stage | Sugar Content (°Brix) | TA (mg L−1 Tartaric Acid) | pH | Berry Weight (g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| CTRL | APE | CTRL | APE | CTRL | APE | CTRL | APE | ||
| t0 | 14.5 ± 0.3 a | 14.5 ± 0.2 a | 8.6 ± 0.6 a | 8.4 ± 0.4 a | 3.03 ± 0.03 a | 3.01 ± 0.01 a | 1.18 ± 0.03 a | 1.17 ± 0.02 a | |
| WW | t1 | 16.8 ± 0.2 a | 17.0 ± 0.1 a | 5.7 ± 0.2 a | 6.0 ± 0.2 a | 3.35 ± 0.01 a | 3.33 ± 0.04 a | 1.03 ± 0.06 a | 1.05 ± 0.01 a |
| t2 | 19.0 ± 0.2 a | 20.0 ± 0.5 a | 5.0 ± 0.2 a | 5.6 ± 0.1 a | 3.44 ± 0.02 a | 3.42 ± 0.02 a | 1.19 ± 0.02 b | 1.32 ± 0.02 a | |
| t0 | 15.5 ± 0.3 a | 15.4 ± 0.3 a | 7.6 ± 0.3 a | 7.8 ± 0.3 a | 3.13 ± 0.01 a | 3.15 ± 0.01 a | 1.23 ± 0.09 a | 1.17 ± 0.05 a | |
| WS | t1 | 17.9 ± 0.7 a | 17.4 ± 0.3 a | 5.7 ± 0.2 a | 5.3 ± 0.3 a | 3.48 ± 0.02 a | 3.44 ± 0.05 a | 1.13 ± 0.05 a | 1.13 ± 0.03 a |
| t2 | 20.6 ± 0.2 a | 18.8 ± 0.2 b | 5.2 ± 0.1 a | 5.4 ± 0.1 a | 3.49 ± 0.02 a | 3.42 ± 0.02 a | 1.18 ± 0.04 b | 1.29 ± 0.04 a | |
| Irrig. Regime | Stage | Tot. Anth. (mg L−1) | Extr. Anth. (mg L−1) | Tot. Polyp. (mg L−1) | Extr. Polyp. (mg L−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| CTRL | APE | CTRL | APE | CTRL | APE | CTRL | APE | ||
| t0 | 707 ± 19 a | 700 ± 18 a | 271 ± 19 a | 270 ± 18 a | 2288 ± 58 a | 2276 ± 57 a | 1579 ± 100 a | 1489 ± 99 a | |
| WW | t1 | 813 ± 23 a | 774 ± 14 a | 309 ± 13 a | 316 ± 8 a | 2061 ± 55 a | 1783 ± 49 a | 1552 ± 74 a | 1468 ± 111 a |
| t2 | 784 ± 18 a | 740 ± 32 a | 335 ± 10 a | 337 ± 6 a | 1781 ± 35 a | 1691 ± 87 a | 1077 ± 17 a | 1117 ± 43 a | |
| t0 | 706 ± 18 a | 707 ± 19 a | 268 ± 19 a | 266 ± 17 a | 2268 ± 60 a | 2285 ± 58 a | 1588 ± 98 a | 1598 ± 100 a | |
| WS | t1 | 1074 ± 45 a | 955 ± 41 a | 411 ± 17 a | 385 ± 10 a | 1929 ± 113 a | 1744 ± 34 a | 1555 ± 103 a | 1322 ± 20 a |
| t2 | 921 ± 55 a | 837 ± 44 a | 448 ± 13 a | 362 ± 21 b | 2285 ± 45 a | 2220 ± 92 a | 2115 ± 52 a | 1980 ± 63 a | |
| Sugar Content (°Brix) | Sugar Loading (mg per Berry) | TA (mg L−1 Tartaric Acid) | pH | Berry Weight (g) | Tot. Anth. (mg L−1) | Extr. Anth. (mg L−1) | Tot. Polyp. (mg L−1) | Extr. Polyp. (mg L−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | |||||||||
| APE | 17.2 | 209.2 | 6.4 | 3.30 | 1.19 | 785 | 322 | 1999 | 1495 |
| CTRL | 17.4 | 199.9 | 6.3 | 3.32 | 1.16 | 834 | 340 | 2102 | 1577 |
| Irrig. Regime | |||||||||
| WW | 16.9 | 197.7 | 6.5 | 3.26 | 1.16 | 753 | 306 | 1980 | 1380 |
| WS | 17.6 | 211.4 | 6.2 | 3.35 | 1.19 | 866 | 356 | 2121 | 1693 |
| Significance | |||||||||
| Treatment | 0.886 | 0.653 | 0.899 | 0.835 | 0.578 | 0.489 | 0.633 | 0.530 | 0.635 |
| Irrig. Regime | 0.635 | 0.514 | 0.679 | 0.468 | 0.578 | 0.128 | 0.195 | 0.389 | 0.097 |
| Interaction | 0.670 | 0.795 | 0.899 | 0.967 | 0.790 | 0.788 | 0.584 | 0.879 | 0.828 |
| APE | Protein | Carbohydrate | Lipid | Moisture | Ash |
| 35.73 ± 1.20 | 28.02 ± 1.01 | 2.01 ± 0.20 | 5.43 ± 0.20 | 17.58 ± 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvi, L.; Niccolai, A.; Cataldo, E.; Sbraci, S.; Paoli, F.; Storchi, P.; Rodolfi, L.; Tredici, M.R.; Mattii, G.B. Effects of Arthrospira platensis Extract on Physiology and Berry Traits in Vitis vinifera. Plants 2020, 9, 1805. https://doi.org/10.3390/plants9121805
Salvi L, Niccolai A, Cataldo E, Sbraci S, Paoli F, Storchi P, Rodolfi L, Tredici MR, Mattii GB. Effects of Arthrospira platensis Extract on Physiology and Berry Traits in Vitis vinifera. Plants. 2020; 9(12):1805. https://doi.org/10.3390/plants9121805
Chicago/Turabian StyleSalvi, Linda, Alberto Niccolai, Eleonora Cataldo, Sofia Sbraci, Francesca Paoli, Paolo Storchi, Liliana Rodolfi, Mario R. Tredici, and Giovan Battista Mattii. 2020. "Effects of Arthrospira platensis Extract on Physiology and Berry Traits in Vitis vinifera" Plants 9, no. 12: 1805. https://doi.org/10.3390/plants9121805
APA StyleSalvi, L., Niccolai, A., Cataldo, E., Sbraci, S., Paoli, F., Storchi, P., Rodolfi, L., Tredici, M. R., & Mattii, G. B. (2020). Effects of Arthrospira platensis Extract on Physiology and Berry Traits in Vitis vinifera. Plants, 9(12), 1805. https://doi.org/10.3390/plants9121805








