Genotype × Environment Interaction for Wheat Yield Traits Suitable for Selection in Different Seed Priming Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Field Emergence
2.2. Grain Weight Per Plant
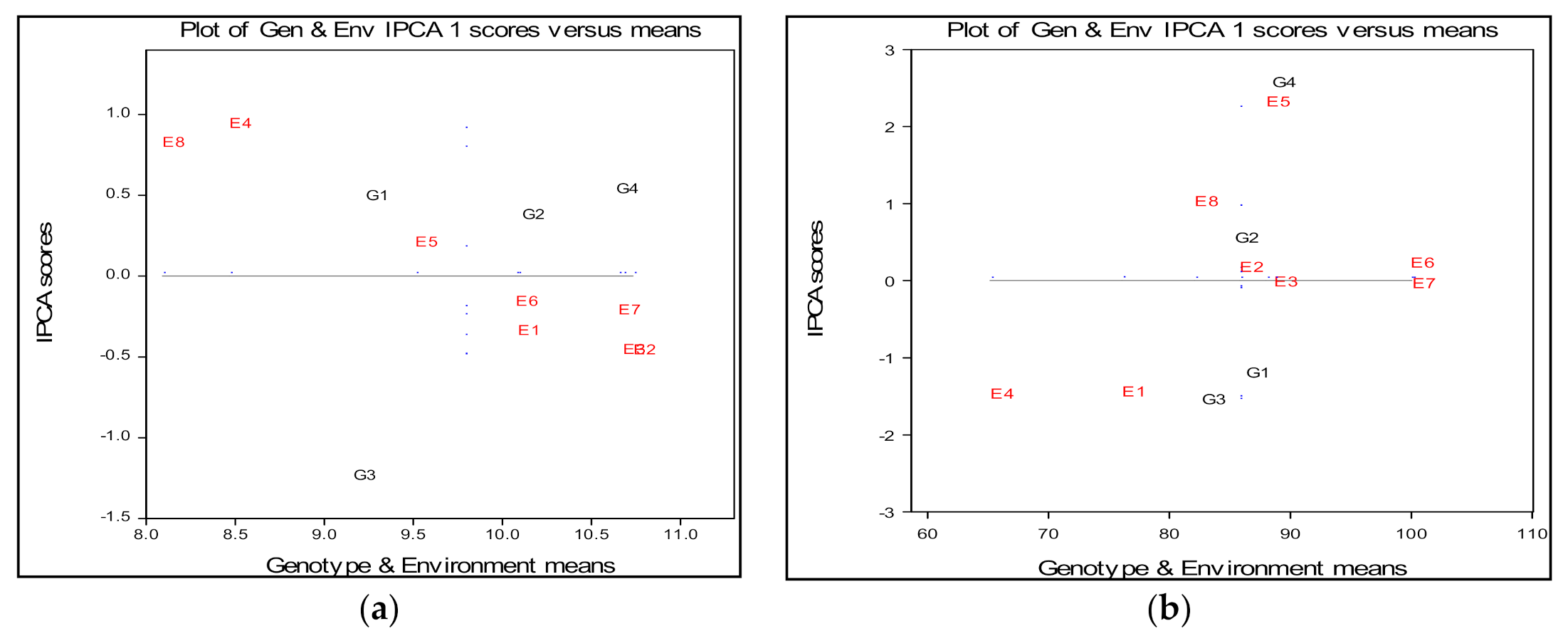
2.3. Spike Length
2.4. Plant Height
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Listman, M.; Ordóñez, R. Ten Things You Should Know about Maize and Wheat; CIMMYT: Batan, Mexico, 2019. [Google Scholar]
- Kneževic, D.; Zečcević, V.; Dukić, N.; Dodig, D. Genetic and phenotypic variability of grain mass per spike of winter wheat genotypes (Triticum aestivum L.). Kragujev. J. Sci. 2008, 30, 131–136. [Google Scholar]
- Foroozanfar, M.; Zeynali, H. Inheritance of some correlated traits in bread wheat using generation mean analysis. Adv. Crop Sci. 2013, 3, 436–443. [Google Scholar]
- Ljubičič, N.; Petrović, S.; Dimitrijević, M.; Hristov, N. Gene actions involved in the inheritance of yield related traits in bread wheat (Triticum aestivum L.). Emir. J. Food Agric. 2016, 28, 477–484. [Google Scholar]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nano-priming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostruct. 2018, 13, 315–323. [Google Scholar]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Neto, E.M.; Britt, D.W.; Lara, L.M.; Cartwright, A.; dos Santos, R.F.; Inoue, T.T.; Batista, M.A. Initial development of corn seedlings after seed priming with nanoscale synthetic zinc oxide. Agronomy 2020, 10, 307. [Google Scholar] [CrossRef]
- Solanki, P.; Laura, J.S. Effect of ZnO nanoparticles on seed germination and seedling growth in wheat (Triticum aestivum). J. Pharmacogn. Phytochem. 2018, 7, 2048–2052. [Google Scholar]
- Ariyo, O.J. Ayo-Vaughan Effectiveness and relative discriminatory abilities of techniques measuring genotype x environment interaction and stability in Okra. Euphytica 2000, 7, 99–105. [Google Scholar]
- Aremu, C.O.; Ariyo, O.J. Adewale Assessments of selection techniques in genotype by environment interaction in cowpea [Vigna unguiculata (L.) Walp]. Afr. J. Res. 2007, 2, 352–355. [Google Scholar]
- Kang, M. Using Genotype-by-Environment Interaction for Crop Cultivar Development. Adv. Agron. 1998, 62, 199–252. [Google Scholar]
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Gauch, H.G. Statistical analysis of yield trials by AMMI and GGE. CropScience 2006, 46, 1488–1500. [Google Scholar]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 643–655. [Google Scholar] [CrossRef]
- Gauch, H.G.; Zobel, R.W. AMMI analyses of yield trials. In Genotype by Environment Interaction; Kang, M.S., Gauch, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 85–122. [Google Scholar]
- Rodrigues, P.C.; Malosetti, M.; Gauch, H.G.; Van Eeuwijk, F.A. A weighted AMMI algorithm to study genotype-by-environment interaction and QTL-by-environment interaction. Crop Sci. 2014, 54, 1555–1570. [Google Scholar] [CrossRef]
- Popović, V.; Glamočlija, Đ.; Malešević, M.; Ikanović, J.; Dražić, G.; Spasić, M.; Stanković, S. Genotype specificity in nitrogen nutrition of malting barley. Genetika 2011, 43, 197–204. [Google Scholar] [CrossRef]
- Ugrenović, V.; Bodroža Solarov, M.; Pezo, L.; Đisalov, J.; Popović, V.; Marić, B.; Filipović, V. Analysis of spelt variability (Triticum spelta L.) grown in different conditions of Serbia by organic conditions. Genetika 2018, 50, 635–646. [Google Scholar]
- Vasileva, V.; Vasilev, V. Agronomic characterization and the possibility for potential use of subterranean clover (Trifolium subterraneum L.) in the forage production in Bulgaria. Pak. J. Bot. 2020, 52, 1–5. [Google Scholar] [CrossRef]
- 23. Zečević; V.; Knežević; D.; Mićanović; D.; Pavlović; M.; Urošević; D. The inheritance of plant height in winter wheat (Triticum aestivum L.). Genetika 2005, 37, 173–179. [CrossRef]
- Al-Baldawi, M.H.; Hamza, J.H. Seed priming effect on field emergence and grain yield in sorghum. J. Cent. Eur. Agric. 2017, 18, 404–423. [Google Scholar] [CrossRef]
- Forcella, F.; Arnold, R.L.B.; Sanchez, R.; Ghersa, C.M. Modeling seedling emergence. Field Crop. Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- Wang, H.; Cutforth, H.; McCaig, T.; McLeod, G.; Brandt, K.; Lemke, R.; Goddard, T.; Sprout, C. Predicting the time to 50% seedling emergence in wheat using a Beta model. NJAS Wagening. J. Life Sci. 2009, 57, 65–71. [Google Scholar] [CrossRef]
- Gan, Y.; Stobbe, E.H.; Moes, J. Relative Date of Wheat Seedling Emergence and Its Impact on Grain Yield. Crop Sci. 1992, 32, 1275–1281. [Google Scholar] [CrossRef]
- Rad MR, N.; Kadir, M.A.; Rafii, M.Z.; Jaafar HZ, E.; Naghavi, M.R.; Ahmadi, F. Genotype environment interaction by AMMI and GGE biplot analysis in three consecutive generations of wheat (Triticum aestivum) under normal and drought stress conditions. Aust. J. Crop Sci. 2013, 7, 956–961. [Google Scholar]
- Mohammadi, R.; Aemion, M.; Zadhasan, E.; Ahmadi, M.M.; Amri, A. The use of AMMI model for interpreting genotype x environment interaction in durum wheat. Exp. Agric. 2018, 54, 670–683. [Google Scholar] [CrossRef]
- Zečević, V.; Knežević, D.; Mićanović, D.; Madić, M. Genetic and phenotypic variability of spike length and plant height in wheat. Kragujev. J. Sci. 2008, 30, 125–130. [Google Scholar]
- Mladenov, V.; Dimitrijević, M.; Petrović, S.; Boćanski, J.; Banjac, B.; Kondić-Špika, A.; Trkulja, D. Genetic analysis of spike length in wheat. Genetika 2019, 51, 167–178. [Google Scholar] [CrossRef]
- Brbaklić, L.J.; Trkulja, D.; Kondić-Špika, A.; Mikić, S.; Tomičić, A.; Kobiljski, B. Determination of population structure of wheat core collection for association mapping. Cereal Res. Comm. 2015, 43, 45–54. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Petrović, S.; Belić, M.; Ljubičić (Vuković), N.; Vukosavljev, M. AMMI analyzed genotypes by environmental interactions in bread wheat. In Proceedings of the III Congress of Ecologists of the Republic of Macedonia with International Participation, Macedonian Ecological Society, Skopje, Macedonia, 6–9 October 2007; pp. 277–283. [Google Scholar]
- Zobel, R.W. A powerful statistical model for understanding genotype by environment interaction. In Genotype by Environment Interaction and Plant Breeding; Kang, M.S., Ed.; Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 1990; pp. 126–140. [Google Scholar]
- Božović, D.; Popović, , V.; Rajičić, V.; Kostić, M.; Filipović, V.; Kolarić, L.; Ugrenović, V.; Spalević, V. Stability of the expression of the maize productivity parameters by AMMI models and GGE-biplot analysis. Notulae Botanicae Horti Agrobot. Cluj Napoca. 2020, 48, 1387–1397. [Google Scholar] [CrossRef]
- Kaya, Y.; Palta, C.; Taner, S. Additive main effects and multiplicative interactions analysis of yield performances in bread wheat genotypes across environments. Turk. J. Agric. 2002, 26, 275–279. [Google Scholar]
- Božović, D.; Živanović, T.; Popović, V.; Tatić, M.; Gospavić, Z.; Miloradović, Z.; Stanković, G.; Đokić, M. Assessment stability of maize lines yield by GGE-biplot analysis. Genetika 2018, 50, 755–770. [Google Scholar] [CrossRef]
- Popović, V.; Vučković, S.; Jovović, Z.; Rakaščan, N.; Kostić, M.; Ljubičić, N.; Mladenović, G.M.; Ikanović, J. Genotype by year interaction effects on soybean morpho-productive traits and biogas production. Genetika 2020, 52, 1055–1073. [Google Scholar] [CrossRef]
- Banjac, B.; Mladenov, V.; Dimitrijević, M.; Petrović, S.; Boćanski, J. Genotype× environment interactions and phenotypic stability for wheat grown in stressful conditions. Genetika 2014, 46, 799–806. [Google Scholar] [CrossRef]
- Grausgruber, H.; Oberforster, M.; Werteker, M.; Ruckenbauer, P.; Vollmann, J. Stability of quality traits in Austrian-grown winter wheats. Field Crop. Res. 2000, 66, 257–267. [Google Scholar] [CrossRef]
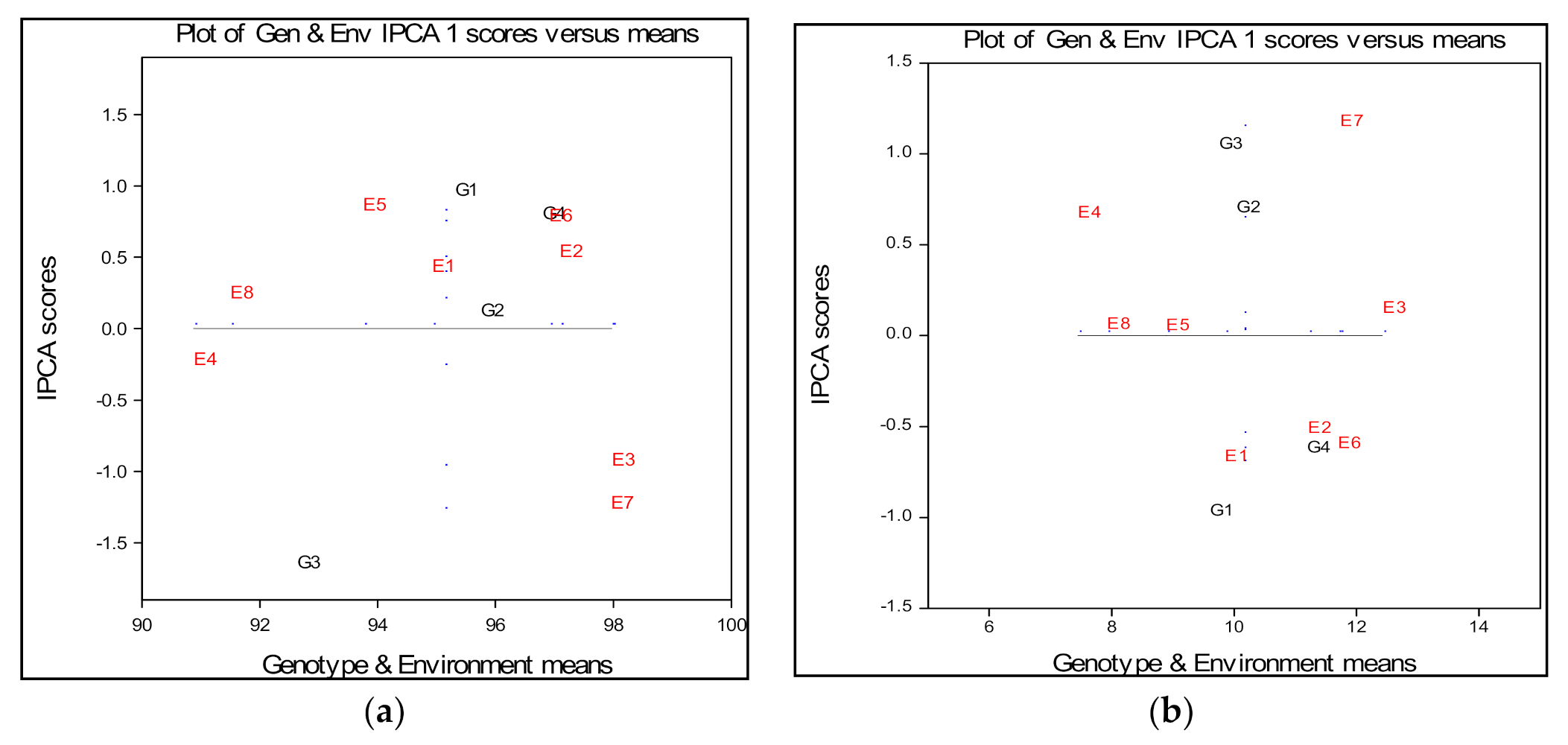
| Field Emergence (%) | |||||||
|---|---|---|---|---|---|---|---|
| Genotypes | |||||||
| Environments | G1 | G2 | G3 | G4 | Em | IPCAe [1] | Variance |
| E1 | 95.44 | 95.57 | 91.79 | 96.86 | 94.92 | 0.37112 | 4.45 |
| E2 | 97.71 | 97.74 | 93.79 | 99.11 | 97.09 | 0.47355 | 5.16 |
| E3 | 97.27 | 98.54 | 97.16 | 98.92 | 97.97 | −0.98531 | 1.28 |
| E4 | 90.81 | 91.48 | 88.86 | 92.34 | 90.87 | −0.28110 | 2.73 |
| E5 | 94.67 | 94.42 | 89.90 | 96.01 | 93.75 | 0.80115 | 6.21 |
| E6 | 97.75 | 97.57 | 93.18 | 99.11 | 96.9 | 0.72378 | 5.60 |
| E7 | 96.98 | 98.50 | 97.66 | 98.68 | 97.95 | −1.28690 | 0.60 |
| E8 | 91.86 | 92.14 | 88.70 | 93.31 | 91.50 | 0.18372 | 4.82 |
| IPCAg [1] | 0.90567 | 0.05992 | −1.70292 | 0.73733 | 95.12 | 10.68 | |
| Gm | 95.31 | 95.75 | 92.63 | 96.79 | |||
| Source 1 | df | SS | MS | F-Value | Share of Total Variation % |
|---|---|---|---|---|---|
| Total | 95 | 1014.4 | 10.68 | - | - |
| Treatments | 31 | 974.9 | 31.45 | 65.77 ** | 96.11 |
| Genotypes | 3 | 226.2 | 75.42 | 157.73 ** | 23.20 |
| Environments | 7 | 675.1 | 96.45 | 93.32 ** | 69.25 |
| Block | 16 | 16.5 | 1.03 | 2.16 * | 1.69 |
| Interactions | 21 | 73.6 | 3.5 | 7.33 ** | 7.55 |
| IPCA [1] | 9 | 54.6 | 6.07 | 12.7 ** | 74.18 |
| IPCA [2] | 7 | 16.7 | 2.39 | 4.99 ** | 22.69 |
| Residuals | 5 | 2.2 | 0.44 | 0.92 ns | 2.99 |
| Error | 48 | 23 | 0.48 | - | - |
| Grain Weight Per Plant (g) | |||||||
|---|---|---|---|---|---|---|---|
| Genotypes | |||||||
| Environments | G1 | G2 | G3 | G4 | Em | PCAe [1] | Variance |
| E1 | 10.02 | 9.30 | 8.80 | 11.40 | 9.88 | −0.71003 | 2.24 |
| E2 | 11.22 | 10.74 | 10.25 | 12.62 | 11.21 | −0.55240 | 2.10 |
| E3 | 11.77 | 12.38 | 12.13 | 13.40 | 12.42 | 0.10500 | 1.45 |
| E4 | 6.27 | 7.75 | 7.69 | 8.08 | 7.45 | 0.62931 | 1.04 |
| E5 | 8.34 | 8.79 | 8.51 | 9.93 | 8.89 | 0.01144 | 1.08 |
| E6 | 11.79 | 11.16 | 10.65 | 13.15 | 11.69 | −0.63667 | 1.92 |
| E7 | 10.04 | 12.37 | 12.48 | 12.02 | 11.72 | 1.13472 | 3.31 |
| E8 | 7.36 | 7.83 | 7.55 | 8.96 | 7.92 | 0.01864 | 0.94 |
| IPCAg [1] | −1.00917 | 0.65919 | 1.01033 | −0.66035 | 10.14 | 4.792 | |
| Gm | 9.60 | 10.04 | 9.75 | 11.19 | |||
| Source 1 | df | SS | MS | F-Value | Share of Total Variation % |
|---|---|---|---|---|---|
| Total | 95 | 455.2 | 4.792 | * | |
| Treatments | 31 | 378.7 | 12.215 | 10.73 ** | 83.19 |
| Genotypes | 3 | 37.4 | 12.463 | 10.95 ** | 9.88 |
| Environments | 7 | 300.8 | 42.964 | 31.3 ** | 79.43 |
| Block | 16 | 22 | 1.373 | 1.21 ns | 5.81 |
| Interactions | 21 | 40.5 | 1.929 | 1.70 ns | 10.69 |
| IPCA [1] | 9 | 25.4 | 2.822 | 2.48 * | 62.72 |
| IPCA [2] | 7 | 13.5 | 1.935 | 1.70 ns | 33.33 |
| Residuals | 5 | 1.6 | 0.313 | 0.28 ns | 0.42 |
| Error | 48 | 54.6 | 1.138 | * | 0.00 |
| Spike Length (cm) | |||||||
|---|---|---|---|---|---|---|---|
| Genotypes | |||||||
| Environments | G1 | G2 | G3 | G4 | Em | IPCAe [1] | Variance |
| E1 | 9.36 | 10.28 | 9.95 | 10.75 | 10.083 | −0.38314 | 0.36 |
| E2 | 9.96 | 10.89 | 10.75 | 11.34 | 10.733 | −0.50046 | 0.31 |
| E3 | 9.90 | 10.83 | 10.69 | 11.28 | 10.675 | −0.49779 | 0.38 |
| E4 | 8.32 | 9.09 | 6.70 | 9.76 | 8.467 | 0.89740 | 1.66 |
| E5 | 9.03 | 9.89 | 8.68 | 10.44 | 9.508 | 0.16308 | 0.63 |
| E6 | 9.43 | 10.33 | 9.71 | 10.83 | 10.075 | −0.20415 | 0.60 |
| E7 | 9.98 | 10.89 | 10.35 | 11.38 | 10.65 | −0.25478 | 0.34 |
| E8 | 7.89 | 8.68 | 6.47 | 9.33 | 8.092 | 0.77983 | 1.42 |
| IPCAg [1] | 0.45054 | 0.33458 | −1.27816 | 0.49304 | 9.785 | 1.58 | |
| Gm | 9.233 | 10.108 | 9.162 | 10.637 | |||
| Source 1 | df | SS | MS | F-Value | Share of Total Variation % |
|---|---|---|---|---|---|
| Total | 95 | 150.28 | 1.58 | * | |
| Treatments | 31 | 142.59 | 4.60 | 41.67 ** | 94.88 |
| Genotypes | 3 | 36.56 | 12.19 | 110.39 ** | 25.64 |
| Environments | 7 | 87.54 | 12.51 | 83.78 ** | 61.39 |
| Block | 16 | 2.39 | 0.15 | 1.35 ns | 1.68 |
| Interactions | 21 | 18.50 | 0.88 | 7.98 * | 12.97 |
| IPCA [1] | 9 | 14.41 | 1.601 | 14.51 ** | 77.89 |
| IPCA [2] | 7 | 3.99 | 0.57 | 5.16 ** | 21.57 |
| Residuals | 5 | 0.1 | 0.02 | 0.18 ns | 0.07 |
| Error | 48 | 5.3 | 0.11 | * |
| Plant Height (cm) | |||||||
|---|---|---|---|---|---|---|---|
| Genotypes | |||||||
| Environments | G1 | G2 | G3 | G4 | Em | IPCAe [1] | Variance |
| E1 | 78.68 | 75.07 | −− | 75.05 | 76.08 | −1.53868 | 7.17 |
| E2 | 86.34 | 85.55 | 82.65 | 88.79 | 85.83 | 0.07406 | 9.61 |
| E3 | 89.41 | 88.30 | 85.79 | 91.16 | 88.67 | −0.11068 | 14.24 |
| E4 | 67.80 | 64.14 | 64.68 | 64.06 | 65.17 | −1.57010 | 5.24 |
| E5 | 85.74 | 88.70 | 81.30 | 96.26 | 88.00 | 2.21890 | 37.09 |
| E6 | 100.35 | 99.66 | 96.64 | 103.01 | 99.92 | 0.12898 | 20.99 |
| E7 | 100.90 | 99.70 | 97.20 | 102.50 | 100.08 | −0.13462 | 8.81 |
| E8 | 81.48 | 82.20 | 77.49 | 87.16 | 82.08 | 0.93215 | 29.36 |
| IPCAg [1] | −1.29245 | 0.45801 | −1.63818 | 2.47262 | 85.73 | 135.38 | |
| Gm | 86.33 | 85.42 | 82.67 | 88.5 | |||
| Source 1 | df | SS | MS | F-Value | Share of Total Variation % |
|---|---|---|---|---|---|
| Total | 95 | 12,861 | 135.4 | - | - |
| Treatments | 31 | 12,393 | 399.8 | 52.17 ** | 96.36 |
| Genotypes | 3 | 420 | 140.2 | 18.29 ** | 3.39 |
| Environments | 7 | 11,403 | 1629 | 260.21 ** | 92.01 |
| Block | 16 | 100 | 6.3 | 0.82 ns | 0.81 |
| Interactions | 21 | 569 | 27.1 | 3.54 * | 4.59 |
| IPCA [1] | 9 | 342 | 38 | 4.96 ** | 60.11 |
| IPCA [2] | 7 | 162 | 23.2 | 3.02 ** | 28.47 |
| Residuals | 5 | 65 | 13 | 1.7 ns | 11.42 |
| Error | 48 | 368 | 7.7 | - | - |
| Environments Code | Growing Season | Zno Nps Seed Priming Treatment |
|---|---|---|
| E1 | 2018/2019 | 0 mg L−1–control |
| E2 | 2018/2019 | 10 mg L−1 |
| E3 | 2018/2019 | 100 mg L−1 |
| E4 | 2018/2019 | 1000 mg L−1 |
| E5 | 2019/2020 | 0 mg L−1–control |
| E6 | 2019/2020 | 10 mg L−1 |
| E7 | 2019/2020 | 100 mg L−1 |
| E8 | 2019/2020 | L−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, V.; Ljubičić, N.; Kostić, M.; Radulović, M.; Blagojević, D.; Ugrenović, V.; Popović, D.; Ivošević, B. Genotype × Environment Interaction for Wheat Yield Traits Suitable for Selection in Different Seed Priming Conditions. Plants 2020, 9, 1804. https://doi.org/10.3390/plants9121804
Popović V, Ljubičić N, Kostić M, Radulović M, Blagojević D, Ugrenović V, Popović D, Ivošević B. Genotype × Environment Interaction for Wheat Yield Traits Suitable for Selection in Different Seed Priming Conditions. Plants. 2020; 9(12):1804. https://doi.org/10.3390/plants9121804
Chicago/Turabian StylePopović, Vera, Nataša Ljubičić, Marko Kostić, Mirjana Radulović, Dragana Blagojević, Vladan Ugrenović, Dragana Popović, and Bojana Ivošević. 2020. "Genotype × Environment Interaction for Wheat Yield Traits Suitable for Selection in Different Seed Priming Conditions" Plants 9, no. 12: 1804. https://doi.org/10.3390/plants9121804
APA StylePopović, V., Ljubičić, N., Kostić, M., Radulović, M., Blagojević, D., Ugrenović, V., Popović, D., & Ivošević, B. (2020). Genotype × Environment Interaction for Wheat Yield Traits Suitable for Selection in Different Seed Priming Conditions. Plants, 9(12), 1804. https://doi.org/10.3390/plants9121804







