Symbiotic Regulatory Genes Controlling Nodule Development in Pisum sativum L.
Abstract
1. Introduction
2. Analyses of Natural Variation in the Nodulation Ability of Pea
3. Induced Mutagenesis, Identification of Symbiotic Regulatory Genes, and Phenotypic Characterization of Mutants
4. Mapping of Symbiotic Regulatory Genes in Pea
5. Identification of Symbiotic Regulatory Genes in Pea
5.1. Receptor Kinases
5.1.1. PsSym10
5.1.2. Pssym37
5.1.3. PsK1
5.1.4. PsLykX
5.1.5. Interactions among PsSYM10, PsSYM37, and PsK1
5.1.6. PsSym19/PsSym41
5.1.7. PsSym28
5.1.8. PsSym29
5.2. Ion Channels
PsSym8
5.3. Calcium/Calmodulin-Dependent Protein Kinase
PsSym9
5.4. Transcription and Co-Transcriptional Factors
5.4.1. PsSym33
5.4.2. PsSym40
5.4.3. PsSym7
5.4.4. PsSym34
5.4.5. PsSym35
5.4.6. PsKNOTTED1-Related Homeobox3 (PsKNOX3)
5.4.7. PsWUSCHEL-Related Homeobox (PsWOX5)
5.4.8. PsCochleata (PsCoch)
5.5. Transporters
PsSym13
5.6. Enzymes
PsNod3
6. Unidentified Pea Symbiotic Genes of Great Interest
6.1. PsSym5
6.2. PsSym16
6.3. PsSym26
6.4. PsSym31
6.5. PsSym42
6.6. PsBrz
7. Analysis of Types of Interactions among Symbiotic Genes in Pea
8. Symbiotic Mutants in Pea as Adequate Genetic Models for Studying Nodule Development and Functioning
8.1. Cytokinins and Nodulation
8.2. Endoplasmic Reticulum Organization
8.3. Analyses of Nodule Senescence
8.4. Analyses of AON
8.5. Analyses of Nodulin Gene Expression
8.6. Analyses of Nitrogen Nutrition and the Yield Relationship
8.7. Analyses of Nod Factor Induction of Nod Factor Cleaving Enzymes
8.8. Rhizobial Gene Expression
9. Comparative Cell Biology
10. Use of Non-Symbiotic Mutants to Study Nodulation
11. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A plant regulator controlling development of symbiotic root nodules. Nature 1999, 402, 191–195. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of geneticdiscoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Govorov, L.I. The peas of Afghanistan. Bull. Appl. Bot. 1928, 19, 497–522. [Google Scholar]
- Razumovskaya, Z.G. Nodule formation in various pea cultivars. Mikrobiologiya 1937, 6, 321–328. [Google Scholar]
- Gelin, O.; Blixt, S. Root nodulation in peas. Agr. Hort. Genet. 1964, 22, 149–159. [Google Scholar]
- Borisov, A.Y.; Barmicheva, E.M.; Jacobi, L.M.; Tsyganov, V.E.; Voroshilova, V.A.; Tikhonovich, I.A. Pea (Pisum sativum L.) mendelian genes controlling development of nitrogen-fixing nodules and arbuscular mycorrhiza. Czech J. Genet. Plant Breed. 2000, 36, 106–110. [Google Scholar]
- Borisov, A.Y.; Danilova, T.N.; Koroleva, T.A.; Naumkina, T.S.; Pavlova, Z.B.; Pinaev, A.G.; Shtark, O.Y.; Tsyganov, V.E.; Voroshilova, V.A.; Zhernakov, A.I.; et al. Pea (Pisum sativum L.) regulatory genes controlling development of nitrogen-fixing nodule and arbuscular mycorrhiza: Fundamentals and application. Biologia 2004, 59, 137–144. [Google Scholar]
- Borisov, A.Y.; Danilova, T.N.; Koroleva, T.A.; Kuznetsova, E.V.; Madsen, L.; Mofett, M.; Naumkina, T.S.; Nemankin, T.A.; Ovchinnikova, E.S.; Pavlova, Z.B.; et al. Regulatory genes of garden pea (Pisum sativum L.) controlling the development of nitrogen-fixing nodules and arbuscular mycorrhiza: A review of basic and applied aspects. Appl. Biochem. Microbiol. 2007, 43, 237–243. [Google Scholar] [CrossRef]
- Macas, J.; Novák, P.; Pellicer, J.; Čížková, J.; Koblížková, A.; Neumann, P.; Fuková, I.; Doležel, J.; Kelly, L.J.; Leitch, I.J. In depth characterization of repetitive DNA in 23 plant genomes reveals sources of genome size variation in the legume tribe Fabeae. PLoS ONE 2015, 10, e0143424. [Google Scholar] [CrossRef]
- LaRue, T.A.; Weeden, N.F. The symbiosis genes of pea. Pisum Genet. 1992, 24, 5–12. [Google Scholar]
- Stougaard, J. Genetics and genomics of root symbiosis. Curr. Opin. Plant Biol. 2001, 4, 328–335. [Google Scholar] [CrossRef]
- Barker, D.G.; Bianchi, S.; Blondon, F.; Dattée, Y.; Duc, G.; Essad, S.; Flament, P.; Gallusci, P.; Génier, G.; Guy, P. Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol. Biol. Rep. 1990, 8, 40–49. [Google Scholar] [CrossRef]
- Handberg, K.; Stougaard, J. Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J. 1992, 2, 487–496. [Google Scholar] [CrossRef]
- Stracke, S.; Kistner, C.; Yoshida, S.; Mulder, L.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N.; Stougaard, J.; Szczyglowski, K. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 2002, 417, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.Y.; Madsen, L.H.; Tsyganov, V.E.; Umehara, Y.; Voroshilova, V.A.; Batagov, A.O.; Sandal, N.; Mortensen, A.; Schauser, L.; Ellis, N.; et al. The Sym35 gene required for root nodule development in pea is an ortholog of Nin from Lotus japonicus. Plant Physiol. 2003, 131, 1009–1017. [Google Scholar] [CrossRef]
- Lie, T.A. Temperature-dependent root-nodule formation in pea cv. Iran. Plant Soil 1971, 34, 751–752. [Google Scholar] [CrossRef]
- Holl, F.B. Host plant control of the inheritance of dinitrogen fixation in the Pisum-Rhizobium symbiosis. Euphytica 1975, 24, 767–770. [Google Scholar] [CrossRef]
- Lie, T.A. Symbiotic specialisation in pea plants: The requirement of specific Rhizobium strains for peas from Afghanistan. Ann. Appl. Biol 1978, 88, 462–465. [Google Scholar] [CrossRef]
- Lie, T.A. Host genes in Pisum sativum L. conferring resistance to European Rhizobium leguminosarum strains. Plant Soil 1984, 82, 415–425. [Google Scholar] [CrossRef]
- Kneen, B.E.; Larue, T.A. Peas (Pisum sativum L.) with strain specificity for Rhizobium leguminosarum. Heredity 1984, 52, 383–389. [Google Scholar] [CrossRef]
- Kozik, A.; Heidstra, R.; Horvath, B.; Kulikova, O.; Tikhonovich, I.; Ellis, T.H.N.; van Kammen, A.; Lie, T.A.; Bisseling, T. Pea lines carrying syml or sym2 can be nodulated by Rhizobium strains containing nodX; sym1 and sym2 are allelic. Plant Sci. 1995, 108, 41–49. [Google Scholar] [CrossRef]
- Geurts, R.; Heidstra, R.; Hadri, A.E.; Downie, J.A.; Franssen, H.; van Kammen, A.; Bisseling, T. Sym2 of pea is involved in a nodulation factor-perception mechanism that controls the infection process in the epidermis. Plant Physiol. 1997, 115, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lie, T.A.; Timmermans, P.C.J.M. Host-genetic control of nitrogen fixation in the legume-Rhizobium symbiosis: Complication in the genetic analysis due to maternal effects. Plant Soil 1983, 75, 449–453. [Google Scholar] [CrossRef]
- Lie, T.A.; Göktan, D.; Engin, M.; Pijnenborg, J.; Anlarsal, E. Co-evolution of the legume-Rhizobium association. Plant Soil 1987, 100, 171–181. [Google Scholar] [CrossRef]
- Young, J.P.W. Linkage of sym-2, the symbiotic specificity locus of Pisum sativum. J. Hered. 1985, 76, 207–208. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A. Another source of the sym2 mutant determining the resistance to nodulation with European strains of Rhizobium leguminosarum bv. viciae. Pisum Genet. 1998, 30, 28. [Google Scholar]
- Kneen, B.E.; LaRue, T.A. Nodulation resistant mutant of Pisum sativum (L.). J. Hered. 1984, 75, 238–240. [Google Scholar] [CrossRef]
- Kneen, B.E.; LaRue, T.A. Induced symbiosis mutants of pea (Pisum sativum) and sweetclover (Melilotus alba annua). Plant Sci. 1988, 58, 177–182. [Google Scholar] [CrossRef]
- Weeden, N.F.; Kneen, B.E.; LaRue, T.A. Genetic analysis of sym genes and other nodule-related genes in Pisum sativum. In Nitrogen Fixation: Achievements and Objectives, 1st ed.; Gresshoff, P.M., Roth, L.E., Stacey, G., Newton, W.E., Eds.; Chapman and Hall: New York, NY, USA, 1990; pp. 323–330. [Google Scholar]
- Sidorova, K.K.; Shumnyi, V.K. A collection of symbiotic mutants in pea Pisum sativum L.: Creation and genetic study. Russ. J. Genet. 2003, 39, 406–413. [Google Scholar] [CrossRef]
- Engvild, K.C. Nodulation and nitrogen fixation mutants of pea, Pisum sativum. Theor. Appl. Genet. 1987, 74, 711–713. [Google Scholar] [CrossRef]
- Kneen, B.E.; Weeden, N.F.; LaRue, T.A. Non-nodulating mutants of Pisum sativum (L.) cv. Sparkle. J. Hered. 1994, 85, 129–133. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Voroshilova, V.A.; Borisov, A.Y.; Tikhonovich, I.A.; Rozov, S.M. Four more symbiotic mutants obtained using EMS mutagenesis of line SGE. Pisum Genet. 2000, 32, 63. [Google Scholar]
- Borisov, A.Y.; Rozov, S.; Tsyganov, V.; Kulikova, O.; Kolycheva, A.; Yakobi, L.; Ovtsyna, A.; Tikhonovich, I. Identification of symbiotic genes in pea (Pisum sativum L.) by means of experimental mutagenesis. Genetika 1994, 30, 1484–1494. [Google Scholar]
- Novák, K. Allelic relationships of pea nodulation mutants. J. Hered. 2003, 94, 191–193. [Google Scholar] [CrossRef]
- Duc, G.; Messager, A. Mutagenesis of pea (Pisum sativum L.) and the isolation of mutants for nodulation and nitrogen fixation. Plant Sci. 1989, 60, 207–213. [Google Scholar] [CrossRef]
- Schneider, A.; Walker, S.; Sagan, M.; Duc, G.; Ellis, T.; Downie, J. Mapping of the nodulation loci sym9 and sym10 of pea (Pisum sativum L.). Theor. Appl. Genet. 2002, 104, 1312–1316. [Google Scholar] [CrossRef]
- Lévy, J.; Bres, C.; Geurts, R.; Chalhoub, B.; Kulikova, O.; Duc, G.; Journet, E.-P.; Ané, J.-M.; Lauber, E.; Bisseling, T.; et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 2004, 303, 1361–1364. [Google Scholar] [CrossRef]
- Postma, J.G.; Jacobsen, E.; Feenstra, W.J. Three pea mutants with an altered nodulation studied by genetic analysis and grafting. J. Plant Physiol. 1988, 132, 424–430. [Google Scholar] [CrossRef]
- Kneen, B.E.; LaRue, T.A.; Hirsch, A.M.; Smith, C.A.; Weeden, N.F. sym 13—A gene conditioning ineffective nodulation in Pisum sativum. Plant Physiol. 1990, 94, 899–905. [Google Scholar] [CrossRef]
- Sagan, M.; Huguet, T.; Barker, D.; Duc, G. Characterization of two classes of non-fixing mutants of pea plants (Pisum sativum L.). Plant Sci. 1993, 95, 55–66. [Google Scholar] [CrossRef]
- Kulaeva, O.A.; Zhernakov, A.I.; Afonin, A.M.; Boikov, S.S.; Sulima, A.S.; Tikhonovich, I.A.; Zhukov, V.A. Pea Marker Database (PMD)—A new online database combining known pea (Pisum sativum L.) gene-based markers. PLoS ONE 2017, 12, e0186713. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, V.E.; Voroshilova, V.A.; Kukalev, A.S.; Azarova, T.S.; Yakobi, L.M.; Borisov, A.Y.; Tikhonovich, I.A. Pisum sativum L. genes sym14 and sym35 control infection thread growth initiation during the development of symbiotic nodules. Genetika 1999, 35, 352–360. [Google Scholar]
- LaRue, T.A.; Temnykh, S.; Weeden, N.F. sym18. A novel gene conditioning altered strain specificity in Pisum sativum cv. ‘Sparkle’. Plant Soil 1996, 180, 191–195. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A. Fix− mutants RisFixA and RisFixV carry mutations in newly identified pea genes sym41 and sym42, respectively. Pisum Genet. 2001, 33, 36. [Google Scholar]
- Zhukov, V.A.; Borisov, A.Y.; Tikhonovich, I.A. Pea mutant line Sprint-2Nod-3 represents a new mutant allele of pea symbiotic gene sym19. Pisum Genet. 2007, 39, 27–28. [Google Scholar]
- Ovchinnikova, E. Genetic Analysis of Symbiosome Formation. PhD dissertation, Wageningen University, Wageningen, The Netherlands, 2012. [Google Scholar]
- Markwei, C.M.; LaRue, T.A. Phenotypic characterization of sym 21, a gene conditioning shoot-controlled inhibition of nodulation in Pisum sativum cv. Sparkle. Physiol. Plant. 1997, 100, 927–932. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Voroshilova, V.; Rozov, S.; Borisov, A.Y.; Tikhonovich, I. A new series of pea symbiotic mutants induced in the line SGE. Russ. J. Genet. Appl. Res. 2013, 3, 156–162. [Google Scholar] [CrossRef]
- Rozov, S.M.; Borisov, A.Y.; Tsyganov, V.E.; Kosterin, O.E. The history of the pea gene map: Last revolutions and the new symbiotic genes. Pisum Genet. 1999, 31, 55–57. [Google Scholar]
- Sagan, M.; Duc, G. Sym28 and Sym29, two new genes involved in regulation of nodulation in pea (Pisum sativum L.). Symbiosis 1996, 20, 229–245. [Google Scholar]
- Sinjushin, A.A.; Konovalov, F.A.; Gostimskii, S.A. Sym28, a gene controlling stem architecture and nodule number, is localized on linkage group V. Pisum Genet. 2008, 40, 15–18. [Google Scholar]
- Krusell, L.; Sato, N.; Fukuhara, I.; Koch, B.E.V.; Grossmann, C.; Okamoto, S.; Oka-Kira, E.; Otsubo, Y.; Aubert, G.; Nakagawa, T.; et al. The Clavata2 genes of pea and Lotus japonicus affect autoregulation of nodulation. Plant J. 2011, 65, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Tayeh, N.; Aluome, C.; Falque, M.; Jacquin, F.; Klein, A.; Chauveau, A.; Bérard, A.; Houtin, H.; Rond, C.; Kreplak, J.; et al. Development of two major resources for pea genomics: The GenoPea 13.2K SNP Array and a high-density, high-resolution consensus genetic map. Plant J. 2015, 84, 1257–1273. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.Y.; Morzhina, E.V.; Kulikova, O.A.; Tchetkova, S.A.; Lebsky, V.K.; Tikhonovich, I.A. New symbiotic mutants of pea (Pisum sativum L.) affecting either nodule initiation or symbiosome development. Symbiosis 1992, 14, 297–313. [Google Scholar]
- Tsyganov, V.E.; Rozov, S.M.; Knox, M.; Borisov, A.Y.; Ellis, T.H.N.; Tikhonovich, I.A. Fine localization of locus Sym31 in pea linkage group III. Russ. J. Genet. Appl. Res. 2013, 3, 114–119. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Borisov, A.Y.; Rozov, S.M.; Tikhonovich, I.A. New symbiotic mutants of pea obtained after mutagenesis of laboratory line SGE. Pisum Genet. 1994, 26, 36–37. [Google Scholar]
- Tsyganov, V.E.; Morzhina, E.V.; Stefanov, S.Y.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol. Gen. Genet. 1998, 259, 491–503. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Rozov, S.M.; Borisov, Y.; Tikhonovich, I.A. Symbiotic gene Sym33 is located on linkage group I. Pisum Genet. 2006, 38, 21–22. [Google Scholar]
- Zhernakov, A.I.; Shtark, O.Y.; Kulaeva, O.A.; Fedorina, J.V.; Afonin, A.M.; Kitaeva, A.B.; Tsyganov, V.E.; Afonso-Grunz, F.; Hoffmeier, K.; Rotter, B.; et al. Mapping-by-sequencing using NGS-based 3′-MACE-Seq reveals a new mutant allele of the essential nodulation gene Sym33 (IPD3) in pea (Pisum sativum L.). PeerJ 2019, 7, e6662. [Google Scholar] [CrossRef]
- Koroleva, T.A.; Voroshilova, V.A.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A. Symbiotic locus Sym38 is localized in linkage group V. Pisum Genet. 2001, 33, 30–31. [Google Scholar]
- Sagan, M.; Huguet, T.; Duc, G. Phenotypic characterization and classification of nodulation mutants of pea (Pisum sativum L.). Plant Sci. 1994, 100, 59–70. [Google Scholar] [CrossRef]
- Nemankin, N. Analysis of pea (Pisum sativum L.) genetic system, controlling development of arbuscular mycorrhiza and nitrogen-fixing symbiosis. Ph.D. Thesis, Saint Petersburg State University, Saint Petersburg, Russia, 2011. (In Russian). [Google Scholar]
- Kneen, B.E.; LaRue, T.A.; Welch, R.M.; Weeden, N.F. Pleiotropic effects of brz: A mutation in Pisum sativum (L.) cv ‘Sparkle’ conditioning decreased nodulation and increased iron uptake and leaf necrosis. Plant Physiol. 1990, 93, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Wellensiek, S.J. The linkage relations of the cochleata mutant in Pisum. Genetica 1963, 33, 145–153. [Google Scholar] [CrossRef]
- Gottschalk, W. Die wirkung mutierter gene auf die morphologie und funktion pflanzlicher organe, dargestellt an strahleninduzierten mutanten von Pisum sativum. In Botanische Studien, 1st ed.; Troll, W., von Guttenberg, H., Eds.; Gustav Fischer Verlag: Jena, Germany, 1963; Volume 14, p. 359. [Google Scholar]
- Blixt, S. Linkage studies in Pisum. VII: The manifestation of the genes Cri and Coch and the double-recessive in Pisum. Agr. Hort. Genet. 1967, 25, 121–144. [Google Scholar]
- Swiecicki, W.K. A new gene heterophylus (het) on chromosome 7. Pisum Newsl. 1989, 21, 75–76. [Google Scholar]
- Rozov, S.M.; Gorel, F.L.; Berdnikov, V.A. coch and het are allelic. Pisum Genet. 1992, 24, 82. [Google Scholar]
- Rozov, S.M.; Voroshilova, V.A.; Tsyganov, V.E.; Priefer, U.B.; Borisov, A.Y.; Tikhonovish, I.A. The Coch gene controls the subsequent differentiation of pea axial meristems into lateral structures. Pisum Genet. 2011, 43, 6–10. [Google Scholar]
- Couzigou, J.-M.; Zhukov, V.; Mondy, S.; Abu el Heba, G.; Cosson, V.; Ellis, T.H.N.; Ambrose, M.; Wen, J.; Tadege, M.; Tikhonovich, I.; et al. NODULE ROOT and COCHLEATA maintain nodule development and are legume orthologs of Arabidopsis BLADE-ON-PETIOLE genes. Plant Cell 2012, 24, 4498–4510. [Google Scholar] [CrossRef]
- Sulima, A.S.; Zhukov, V.A.; Afonin, A.A.; Zhernakov, A.I.; Tikhonovich, I.A.; Lutova, L.A. Selection signatures in the first exon of paralogous receptor kinase genes from the Sym2 region of the Pisum sativum L. genome. Front. Plant Sci. 2017, 8, 1957. [Google Scholar] [CrossRef]
- Kirienko, A.N.; Porozov, Y.B.; Malkov, N.V.; Akhtemova, G.A.; Le Signor, C.; Thompson, R.; Saffray, C.; Dalmais, M.; Bendahmane, A.; Tikhonovich, I.A.; et al. Role of a receptor-like kinase K1 in pea Rhizobium symbiosis development. Planta 2018, 248, 1101–1120. [Google Scholar] [CrossRef]
- Postma, J.G.; Jager, D.; Jacobsen, E.; Feenstra, W.J. Studies on a non-fixing mutant of pea (Pisum sativum L.). I. Phenotypical description and bacteriod activity. Plant Sci. 1990, 68, 151–161. [Google Scholar] [CrossRef]
- Jacobsen, E.; Feenstra, W.J. A new pea mutant with efficient nodulation in the presence of nitrate. Plant Sci. Lett. 1984, 33, 337–344. [Google Scholar] [CrossRef]
- Temnykh, S.; Kneen, B.; Weeden, N.; Larue, T. Localization of nod-3, a gene conditioning hypernodulation, and identification of a novel translocation in Pisum sativum L. cv. Rondo. J. Hered. 1995, 86, 303–305. [Google Scholar] [CrossRef][Green Version]
- Novák, K. Early action of pea symbiotic gene NOD3 is confirmed by adventitious root phenotype. Plant Sci. 2010, 179, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, K.K.; Uzhintseva, P. Use of mutants to detect genes controlling symbiotic characteristics in the pea. Sov. Genet. 1992, 28, 494–500. [Google Scholar]
- Sidorova, K.K.; Uzhintseva, L.P. Mapping of nod-4, a new hypernodulating mutant in pea. Pisum Genet. 1995, 27, 21. [Google Scholar]
- Sidorova, K.K.; Shumnyi, V.K.; Vlasova, E.Y. Study of pea symbiotic mutants. Russ. J. Genet. 1997, 33, 546–548. [Google Scholar]
- Jacobsen, E. Modification of symbiotic interaction of pea (Pisum sativum L.) and Rhizobium leguminosarum by induced mutations. Plant Soil 1984, 82, 427–438. [Google Scholar] [CrossRef]
- Novák, K.; Šlajs, M.; Biedermannová, E.; Vondrys, J. Development of an asymbiotic reference line for pea cv. Bohatýr by de novo mutagenesis. Crop Sci. 2005, 45, 1837–1843. [Google Scholar] [CrossRef]
- Duc, G.; Trouvelot, A.; Gianinazzi-Pearson, V.; Gianinazzi, S. First report of non-mycorrhizal plant mutants (Myc−) obtained in pea (Pisum sativum L.) and fababean (Vicia faba L.). Plant Sci. 1989, 60, 215–222. [Google Scholar] [CrossRef]
- Markmann, K.; Giczey, G.; Parniske, M. Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria. PLoS Biol. 2008, 6, e68. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Guinel, F.C.; Geil, R.D. A model for the development of the rhizobial and arbuscular mycorrhizal symbioses in legumes and its use to understand the roles of ethylene in the establishment of these two symbioses. Can. J. Bot. 2002, 80, 695–720. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Voroshilova, V.A.; Priefer, U.B.; Borisov, A.Y.; Tikhonovich, I.A. Genetic dissection of the initiation of the infection process and nodule tissue development in the Rhizobium-pea (Pisum sativum L.) symbiosis. Ann. Bot. 2002, 89, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Voroshilova, V.A.; Demchenko, K.N.; Brewin, N.J.; Borisov, A.Y.; Tikhonovich, I.A. Initiation of a legume nodule with an indeterminate meristem involves proliferating host cells that harbour infection threads. New Phytol. 2009, 181, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, V.; Radutoiu, S.; Madsen, L.H.; Rychagova, T.; Ovchinnikova, E.; Borisov, A.; Tikhonovich, I.; Stougaard, J. The pea Sym37 receptor kinase gene controls infection-thread initiation and nodule development. Mol. Plant Microbe Interact. 2008, 21, 1600–1608. [Google Scholar] [CrossRef]
- Walker, S.A.; Viprey, V.; Downie, J.A. Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 2000, 97, 13413–13418. [Google Scholar] [CrossRef]
- Madsen, E.B.; Madsen, L.H.; Radutoiu, S.; Olbryt, M.; Rakwalska, M.; Szczyglowski, K.; Sato, S.; Kaneko, T.; Tabata, S.; Sandal, N. A receptor kinase gene of the LysM type is involved in legumeperception of rhizobial signals. Nature 2003, 425, 637–640. [Google Scholar] [CrossRef]
- Arrighi, J.-F.; Barre, A.; Amor, B.B.; Bersoult, A.; Soriano, L.C.; Mirabella, R.; de Carvalho-Niebel, F.; Journet, E.-P.; Ghérardi, M.; Huguet, T.; et al. The Medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006, 142, 265–279. [Google Scholar] [CrossRef]
- Yacobi, L.M.; Voroshilova, V.A.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A. Pea mutants K5, K24, FN1 and nod3 induced in cv. Rondo are able to form arbuscular endomycorrhiza. Mutant K24 is not an allele of sym19. Pisum Genet. 1998, 30, 30. [Google Scholar]
- Kirienko, A.N.; Vishnevskaya, N.A.; Kitaeva, A.B.; Shtark, O.Y.; Kozyulina, P.Y.; Thompson, R.; Dalmais, M.; Bendahmane, A.; Tikhonovich, I.A.; Dolgikh, E.A. Structural variations in LysM domains of LysM-RLK PsK1 may result in a different effect on pea—Rhizobial symbiosis development. Int. J. Mol. Sci. 2019, 20, 1624. [Google Scholar] [CrossRef]
- Sulima, A.S.; Zhukov, V.A.; Kulaeva, O.A.; Vasileva, E.N.; Borisov, A.Y.; Tikhonovich, I.A. New sources of Sym2A allele in the pea (Pisum sativum L.) carry the unique variant of candidate LysM-RLK gene LykX. PeerJ 2019, 7, e8070. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Walker, S.; Poyser, S.; Sagan, M.; Ellis, T.; Downie, J. Genetic mapping and functional analysis of a nodulation-defective mutant (sym19) of pea (Pisum sativum L.). Mol. Gen. Genet. 1999, 262, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Endre, G.; Kereszt, A.; Kevei, Z.; Mihacea, S.; Kaló, P.; Kiss, G.B. A receptor kinase gene regulating symbiotic nodule development. Nature 2002, 417, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Morzhina, E.V.; Tsyganov, V.E.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. Four developmental stages identified by genetic dissection of pea (Pisum sativum L.) root nodule morphogenesis. Plant Sci. 2000, 155, 75–83. [Google Scholar] [CrossRef]
- Hastwell, A.H.; Corcilius, L.; Williams, J.T.; Gresshoff, P.M.; Payne, R.J.; Ferguson, B.J. Triarabinosylation is required for nodulation-suppressive CLE peptides to systemically inhibit nodulation in Pisum sativum. Plant Cell Environ. 2019, 42, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Krusell, L.; Madsen, L.H.; Sato, S.; Aubert, G.; Genua, A.; Szczyglowski, K.; Duc, G.; Kaneko, T.; Tabata, S.; de Bruijn, F.; et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 2002, 420, 422–426. [Google Scholar] [CrossRef]
- Searle, I.R.; Men, A.E.; Laniya, T.S.; Buzas, D.M.; Iturbe-Ormaetxe, I.; Carroll, B.J.; Gresshoff, P.M. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 2003, 299, 109–112. [Google Scholar] [CrossRef]
- Edwards, A.; Heckmann, A.B.; Yousafzai, F.; Duc, G.; Downie, J.A. Structural implications of mutations in the pea SYM8 symbiosis gene, the DMI1 ortholog, encoding a predicted ion channel. Mol. Plant Microbe Interact. 2007, 20, 1183–1191. [Google Scholar] [CrossRef]
- Markwei, C.M.; LaRue, T.A. Phenotypic characterization of sym8 and sym9, two genes conditioning non-nodulation in Pisum sativum ‘Sparkle’. Can. J. Microbiol. 1992, 38, 548–554. [Google Scholar] [CrossRef]
- Zhukov, V.A.; Tsyganov, V.E.; Borisov, A.Y. “Drum sticks” is a trait associated with the Sym8 locus in pea. Pisum Genet. 2004, 36, 20–22. [Google Scholar]
- Chovanec, P.; Novák, K. Visualization of nodulation gene activity on the early stages of Rhizobium leguminosarum bv. viciae symbiosis. Folia Microbiol. 2005, 50, 323. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M.; Bredemeier, R.; Wanner, G.; Takeda, N.; Schleiff, E.; Parniske, M. Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. Plant Cell 2008, 20, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Novák, K.; Felsberg, J.; Biedermannová, E.; Vondrys, J. A mutation affecting symbiosis in the pea line Risnod27 changes the ion selectivity filter of the DMI1 homolog. Biol. Plant. 2009, 53, 451–460. [Google Scholar] [CrossRef]
- Kolycheva, A.N.; Jakobi, L.M.; Borisov, A.Y.; Filatov, A.A.; Tikhonovich, I.A.; Muromtsev, G.S. Pea gene sym8 affects symbiosis both with Rhizobium and with endomycorrhizal fungi. Pisum Genet. 1993, 25, 22. [Google Scholar]
- Albrecht, C.; Geurts, R.; Lapeyrie, F.; Bisseling, T. Endomycorrhizae and rhizobial Nod factors both require SYM8 to induce the expression of the early nodulin genes PsENOD5 and PsENOD12A. Plant J. 1998, 15, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Katzer, K.; Lambert, J.; Cerri, M.; Parniske, M. CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 2014, 15, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, E.; Journet, E.-P.; Chabaud, M.; Cosson, V.; Ratet, P.; Duc, G.; Fedorova, E.; Liu, W.; den Camp, R.O.; Zhukov, V.; et al. IPD3 controls the formation of nitrogen-fixing symbiosomes in pea and Medicago Spp. Mol. Plant Microbe Interact. 2011, 24, 1333–1344. [Google Scholar] [CrossRef]
- Cerri, M.R.; Wang, Q.; Stolz, P.; Folgmann, J.; Frances, L.; Katzer, K.; Li, X.; Heckmann, A.B.; Wang, T.L.; Downie, J.A. The ERN 1 transcription factor gene is a target of the CC a MK/CYCLOPS complex and controls rhizobial infection in Lotus japonicus. New Phytol. 2017, 215, 323–337. [Google Scholar] [CrossRef]
- Voroshilova, V.A.; Boesten, B.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Priefer, U.B. Effect of mutations in Pisum sativum L. genes blocking different stages of nodule development on the expression of late symbiotic genes in Rhizobium leguminosarum bv. viciae. Mol. Plant Microbe Interact. 2001, 14, 471–476. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Ivanova, K.A.; Tsyganov, V.E. Histological and ultrastructural nodule organization of the pea (Pisum sativum) mutant SGEFix−-5 in the Sym33 gene encoding the transcription factor PsCYCLOPS/PsIPD3. Ekol. Genet. 2019, 17, 65–70. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Seliverstova, E.; Voroshilova, V.; Tsyganova, A.; Pavlova, Z.; Lebskii, V.; Borisov, A.Y.; Brewin, N.; Tikhonovich, I. Double mutant analysis of sequential functioning of pea (Pisum sativum L.) genes Sym13, Sym33, and Sym40 during symbiotic nodule development. Russ. J. Genet. Appl. Res. 2011, 1, 343. [Google Scholar] [CrossRef]
- Ivanova, K.A.; Tsyganova, A.V.; Brewin, N.J.; Tikhonovich, I.A.; Tsyganov, V.E. Induction of host defences by Rhizobium during ineffective nodulation of pea (Pisum sativum L.) carrying symbiotically defective mutations sym40 (PsEFD), sym33 (PsIPD3/PsCYCLOPS) and sym42. Protoplasma 2015, 252, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Tsyganova, A.V.; Seliverstova, E.V.; Brewin, N.J.; Tsyganov, V.E. Bacterial release is accompanied by ectopic accumulation of cell wall material around the vacuole in nodules of Pisum sativum sym33–3 allele encoding transcription factor PsCYCLOPS/PsIPD3. Protoplasma 2019, 256, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, L.M.; Petrova, O.S.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A. Effect of mutations in the pea genes Sym33 and Sym40 I. Arbuscular mycorrhiza formation and function. Mycorrhiza 2003, 13, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, L.M.; Zubkova, L.A.; Barmicheva, E.M.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A. Effect of mutations in the pea genes Sym33 and Sym40 II. Dynamics of arbuscule development and turnover. Mycorrhiza 2003, 13, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.R.; Poole, P.S.; Hudman, J.F. Succinate uptake by free-living and bacteroid forms of Rhizobium leguminosarum. Microbiology 1980, 119, 267–271. [Google Scholar] [CrossRef][Green Version]
- Safronova, V.I.; Novikova, N.I. Comparison of two methods for root nodule bacteria preservation: Lyophilization and liquid nitrogen freezing. J. Microbiol. Methods 1996, 24, 231–237. [Google Scholar] [CrossRef]
- Vernié, T.; Moreau, S.; de Billy, F.; Plet, J.; Combier, J.-P.; Rogers, C.; Oldroyd, G.; Frugier, F.; Niebel, A.; Gamas, P. EFD is an ERF transcription factor involved in the control of nodule number and differentiation in Medicago truncatula. Plant Cell 2008, 20, 2696–2713. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Tsyganov, V.; Borisov, A.Y.; Tikhonovich, I.A.; Brewin, N.J. Comparative cytochemical analysis of hydrogen peroxide distribution in pea ineffective mutant SGEFix−-1 (sym40) and initial line SGE. Ekol. Genet. 2009, 7, 3–9. [Google Scholar] [CrossRef]
- Provorov, N.A.; Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E.; Vorobyov, N.I. Evolution of symbiotic bacteria within the extra- and intra-cellular plant compartments: Experimental evidence and mathematical simulation (Mini-review). Symbiosis 2012, 58, 39–50. [Google Scholar] [CrossRef]
- Kaló, P.; Gleason, C.; Edwards, A.; Marsh, J.; Mitra, R.M.; Hirsch, S.; Jakab, J.; Sims, S.; Long, S.R.; Rogers, J.; et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 2005, 308, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, A.B.; Lombardo, F.; Miwa, H.; Perry, J.A.; Bunnewell, S.; Parniske, M.; Wang, T.L.; Downie, J.A. Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol. 2006, 142, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Miwa, H.; Imaizumi-Anraku, H.; Kouchi, H.; Downie, J.A.; Kawaguchi, M.; Kawasaki, S. Positional cloning identifies Lotus japonicus NSP2, a putative transcription factor of the GRAS family, required for NIN and ENOD40 gene expression in nodule initiation. DNA Research 2006, 13, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Dolgikh, E.A.; Leppyanen, I.V.; Osipova, M.A.; Savelyeva, N.V.; Borisov, A.Y.; Tsyganov, V.E.; Geurts, R.; Tikhonovich, I.A. Genetic dissection of Rhizobium-induced infection and nodule organogenesis in pea based on ENOD12A and ENOD5 expression analysis. Plant Biol. 2011, 13, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Shtark, O.Y.; Sulima, A.S.; Zhernakov, A.I.; Kliukova, M.S.; Fedorina, J.V.; Pinaev, A.G.; Kryukov, A.A.; Akhtemova, G.A.; Tikhonovich, I.A.; Zhukov, V.A. Arbuscular mycorrhiza development in pea (Pisum sativum L.) mutants impaired in five early nodulation genes including putative orthologs of NSP1 and NSP2. Symbiosis 2016, 68, 129–144. [Google Scholar] [CrossRef]
- Hirsch, S.; Kim, J.; Muñoz, A.; Heckmann, A.B.; Downie, J.A.; Oldroyd, G.E.D. GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell 2009, 21, 545–557. [Google Scholar] [CrossRef]
- Vernié, T.; Kim, J.; Frances, L.; Ding, Y.; Sun, J.; Guan, D.; Niebel, A.; Gifford, M.L.; de Carvalho-Niebel, F.; Oldroyd, G.E.D. The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula root. Plant Cell 2015, 27, 3410–3424. [Google Scholar] [CrossRef]
- Azarakhsh, M.; Kirienko, A.N.; Zhukov, V.A.; Lebedeva, M.A.; Dolgikh, E.A.; Lutova, L.A. KNOTTED1-LIKE HOMEOBOX 3: A new regulator of symbiotic nodule development. J. Exp. Bot. 2015, 66, 7181–7195. [Google Scholar] [CrossRef]
- Osipova, M.A.; Mortier, V.; Demchenko, K.N.; Tsyganov, V.E.; Tikhonovich, I.A.; Lutova, L.A.; Dolgikh, E.A.; Goormachtig, S. WUSCHEL-RELATED HOMEOBOX5 gene expression and interaction of CLE peptides with components of the systemic control add two pieces to the puzzle of autoregulation of nodulation. Plant Physiol. 2012, 158, 1329–1341. [Google Scholar] [CrossRef]
- Voroshilova, V.A.; Tsyganov, V.E.; Rozov, S.M.; Priefer, U.B.; Borisov, A.Y.; Tikhonovich, I.A. A unique pea (Pisum sativum L.) mutant impaired in nodule, leaf and flower development. In Biology of Plant-Microbe Interactions, Volume 4 Molecular Plant–Microbe Interactions: New Bridges Between Past and Future, 1st ed.; Tikhonovich, I.A., Lugtenberg, B., Provorov, N.A., Eds.; APS Press: International Society for Molecular Plant–Microbe Interactions: St Paul, MN, USA, 2004; pp. 376–379. [Google Scholar]
- Ferguson, B.J.; Reid, J.B. Cochleata: Getting to the root of legume nodules. Plant Cell Physiol. 2005, 46, 1583–1589. [Google Scholar] [CrossRef]
- Suganuma, N.; LaRue, T.A. Comparison of enzymes involved in carbon and nitrogen metabolism in normal nodules and ineffective nodules induced by a pea mutant E135 (sym 13). Plant Cell Physiol. 1993, 34, 761–765. [Google Scholar] [CrossRef]
- Suganuma, N.; Tamaoki, M.; Takaki, M. Comparison of the protein composition and enzymatic activities during development between effective and plant-determined ineffective nodules in pea. Plant Cell Physiol. 1993, 34, 781–788. [Google Scholar] [CrossRef]
- Romanov, V.I.; Gordon, A.J.; Minchin, F.R.; Witty, J.F.; Skøt, L.; James, C.L.; Borisov, A.Y.; Tikhonovich, I.A. Carbon and nitrogen metabolism in plant-derived ineffective nodules of pea (Pisum sativum L.). In Biological Fixation of Nitrogen for Ecology and Sustainable Agriculture NATO ASI Series (Series G: Ecological Sciences), 1st ed.; Legocki, A., Bothe, H., Pühler, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 39. [Google Scholar]
- Rosov, F.N.; Shleev, S.V.; Petrova, N.E.; Tsyganov, V.E.; Borisov, A.Y.; Topunov, A.F.; Tikhonovich, I.A. The Sym31 gene responsible for bacteroid differentiation is involved in nitrate-dependent nodule formation in pea plants. Russ. J. Plant Physiol. 2001, 48, 459–463. [Google Scholar] [CrossRef]
- Hakoyama, T.; Niimi, K.; Yamamoto, T.; Isobe, S.; Sato, S.; Nakamura, Y.; Tabata, S.; Kumagai, H.; Umehara, Y.; Brossuleit, K.; et al. The integral membrane protein SEN1 is required for symbiotic nitrogen fixation in Lotus japonicus nodules. Plant Cell Physiol. 2012, 53, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.E.; Ferguson, B.J.; Hayashi, S.; Lin, Y.-H.; Gresshoff, P.M. Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 2011, 108, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, E.L.; Kassaw, T.K.; Smith, L.S.; Marsh, J.F.; Oldroyd, G.E.; Long, S.R.; Frugoli, J.A. The ROOT DETERMINED NODULATION1 gene regulates nodule number in roots of Medicago truncatula and defines a highly conserved, uncharacterized plant gene family. Plant Physiol. 2011, 157, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Fearn, J.C.; LaRue, T.A. Ethylene inhibitors restore nodulation to sym 5 mutants of Pisum sativum L. cv Sparkle. Plant Physiol. 1991, 96, 239–244. [Google Scholar] [CrossRef]
- Guinel, F.C.; LaRue, T.A. Light microscopy study of nodule initiation in Pisum sativum L. cv Sparkle and in its low-nodulating mutant E2 (sym 5). Plant Physiol. 1991, 97, 1206–1211. [Google Scholar] [CrossRef]
- Guinel, F.C.; Sloetjes, L.L. Ethylene is involved in the nodulation phenotype of Pisum sativum R50 (sym 16), a pleiotropic mutant that nodulates poorly and has pale green leaves. J. Exp. Bot. 2000, 51, 885–894. [Google Scholar] [CrossRef][Green Version]
- Pepper, A.N.; Morse, A.P.; Guinel, F.C. Abnormal root and nodule vasculature in R50 (sym16), a pea nodulation mutant which accumulates cytokinins. Ann. Bot. 2007, 99, 765–776. [Google Scholar] [CrossRef][Green Version]
- Lorteau, M.-A.; Ferguson, B.J.; Guinel, F.C. Effects of cytokinin on ethylene production and nodulation in pea (Pisum sativum) cv. Sparkle. Physiol. Plant. 2001, 112, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Ross, J.J.; Reid, J.B. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol. 2005, 138, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Held, M.; Pepper, A.N.; Bozdarov, J.; Smith, M.D.; Emery, R.J.N.; Guinel, F.C. The pea nodulation mutant R50 (sym16) displays altered activity and expression profiles for cytokinin dehydrogenase. J. Plant Growth Regul. 2008, 27, 170–180. [Google Scholar] [CrossRef]
- Long, C.; Held, M.; Hayward, A.; Nisler, J.; Spíchal, L.; Neil Emery, R.J.; Moffatt, B.A.; Guinel, F.C. Seed development, seed germination and seedling growth in the R50 (sym16) pea mutant are not directly linked to altered cytokinin homeostasis. Physiol. Plant. 2012, 145, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Serova, T.A.; Tsyganova, A.V.; Tsyganov, V.E. Early nodule senescence is activated in symbiotic mutants of pea (Pisum sativum L.) forming ineffective nodules blocked at different nodule developmental stages. Protoplasma 2018, 255, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Novák, K.; Skrdleta, V.; Nemcova, M.; Lisá, L. Symbiotic traits, growth, and classification of pea nodulation mutants Rost. Vyroba 1993, 39, 157–170. [Google Scholar]
- Novák, K.; Pešina, K.; Nebesářová, J.; Škrdleta, V.; Lisá, L.; Našinec, V. Symbiotic tissue degradation pattern in the ineffective nodules of three nodulation mutants of pea (Pisum sativum L.). Ann. Bot. 1995, 76, 303–313. [Google Scholar] [CrossRef]
- Borisov, A.Y.; Rozov, S.M.; Tsyganov, V.E.; Morzhina, E.V.; Lebsky, V.K.; Tikhonovich, I.A. Sequential functioning of Sym-13 and Sym-31, two genes affecting symbiosome development in root nodules of pea (Pisum sativum L). Mol. Gen. Genet. 1997, 254, 592–598. [Google Scholar] [CrossRef]
- Sherrier, D.J.; Borisov, A.Y.; Tikhonovich, I.A.; Brewin, N.J. Immunocytological evidence for abnormal symbiosome development in nodules of the pea mutant line Sprint-2Fix− (sym31). Protoplasma 1997, 199, 57–68. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Brewin, N.J.; Tsyganov, V.E. Comparative analysis of remodelling of the plant—Microbe interface in Pisum sativum and Medicago truncatula symbiotic nodules. Protoplasma 2019, 256, 983–996. [Google Scholar] [CrossRef]
- Dahiya, P.; Sherrier, D.J.; Kardailsky, I.V.; Borisov, A.Y.; Brewin, N.J. Symbiotic gene Sym31 controls the presence of a lectinlike glycoprotein in the symbiosome compartment of nitrogen-fixing pea nodules. Mol. Plant Microbe Interact. 1998, 11, 915–923. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Voroshilova, V.A.; Herrera-Cervera, J.A.; Sanjuan-Pinilla, J.M.; Borisov, A.Y.; Tikhonovich, I.A.; Priefer, U.B.; Olivares, J.; Sanjuan, J. Developmental downregulation of rhizobial genes as a function of symbiosome differentiation in symbiotic root nodules of Pisum sativum. New Phytol. 2003, 159, 521–530. [Google Scholar] [CrossRef]
- Romanov, V.I.; Gordon, A.J.; Minchin, F.R.; Witty, J.F.; Skøt, L.; James, C.L.; Borisov, A.Y.; Tikhonovich, I.A. Anatomy, physiology and biochemistry of root nodules of Sprint-2Fix−, a symbiotically defective mutant of pea (Pisum sativum L.). J. Exp. Bot. 1995, 46, 1809–1816. [Google Scholar] [CrossRef]
- Novák, K.; Škrdleta, V.; Němcová, M.; Lisá, L. Behavior of pea nodulation mutants as affected by increasing nitrate level. Symbiosis 1993, 15, 195–206. [Google Scholar]
- Welch, R.M.; LaRue, T.A. Physiological characteristics of Fe accumulation in the ‘Bronze’ mutant of Pisum sativum L., cv ‘Sparkle’ E107 (brz brz). Plant Physiol. 1990, 93, 723–729. [Google Scholar] [CrossRef]
- Guinel, F.C.; LaRue, T.A. Excessive aluminium accumulation in the pea mutant E107 (brz). Plant Soil 1993, 157, 75–82. [Google Scholar] [CrossRef]
- Novak, K.; Skrdleta, V.; Kropacova, M.; Lisa, L.; Nemcova, M. Interaction of two genes controlling symbiotic nodule number in pea (Pisum sativum L.). Symbiosis 1997, 23, 43–62. [Google Scholar]
- Tsyganov, V.E.; Tsyganova, A.V.; Voroshilova, V.A.; Borisov, A.Y.; Tikhonovich, I.A. Analysis of the interaction of pea (Pisum sativum L.) symbiotic genes Sym33 and Sym42 whose mutations result in abnormalities during infection thread development. Russ. J. Genet. Appl. Res. 2014, 4, 83–87. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.; Tsyganov, V.E. Analysis of epitope distribution of arabinogalactan protein-extensins in pea (Pisum sativum) nodules of wild-type and mutants impaired in infection thread growth. Ekol. Genet. 2019, 17, 5–12. [Google Scholar] [CrossRef]
- Foo, E.; Ferguson, B.J.; Reid, J.B. The potential roles of strigolactones and brassinosteroids in the autoregulation of nodulation pathway. Ann. Bot. 2014, 113, 1037–1045. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Yoneyama, K.; Hugill, C.J.; Quittenden, L.J.; Reid, J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant 2013, 6, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.C.; Clairmont, L.; Macdonald, E.S.; Weiner, C.A.; Emery, R.J.N.; Guinel, F.C. E151 (sym15), a pleiotropic mutant of pea (Pisum sativum L.), displays low nodule number, enhanced mycorrhizae, delayed lateral root emergence, and high root cytokinin levels. J. Exp. Bot. 2015, 66, 4047–4059. [Google Scholar] [CrossRef] [PubMed]
- Dolgikh, E.A.; Kusakin, P.G.; Kitaeva, A.B.; Tsyganova, A.V.; Kirienko, A.N.; Leppyanen, I.V.; Dolgikh, A.V.; Ilina, E.L.; Demchenko, K.N.; Tikhonovich, I.A.; et al. Mutational analysis indicates that abnormalities in rhizobial infection and subsequent plant cell and bacteroid differentiation in pea (Pisum sativum) nodules coincide with abnormal cytokinin responses and localization. Ann. Bot. 2020, 125, 905–923. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Tsyganov, V.E. Organization of the endoplasmic reticulum in cells of effective and ineffective pea nodules (Pisum sativum L.). Ekol. Genet. 2019, 17, 5–14. [Google Scholar] [CrossRef]
- Serova, T.A.; Tikhonovich, I.A.; Tsyganov, V.E. Analysis of nodule senescence in pea (Pisum sativum L.) using laser microdissection, real-time PCR, and ACC immunolocalization. J. Plant Physiol. 2017, 212, 29–44. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins inhibit nodule senescence and stimulate nodule meristem bifurcation in pea (Pisum sativum L.). Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Li, D.; Kinkema, M.; Gresshoff, P.M. Autoregulation of nodulation (AON) in Pisum sativum (pea) involves signalling events associated with both nodule primordia development and nitrogen fixation. J. Plant Physiol. 2009, 166, 955–967. [Google Scholar] [CrossRef]
- Huynh, C.A.; Guinel, F.C. Shoot extracts from two low nodulation mutants significantly reduce nodule number in pea. Plants 2020, 9, 1505. [Google Scholar] [CrossRef]
- Sagan, M.; Ney, B.; Duc, G. Plant symbiotic mutants as a tool to analyse nitrogen nutrition and yield relationship in field-growth peas (Pisum sativum L.). Plant Soil 1993, 153, 33–45. [Google Scholar] [CrossRef]
- Ovtsyna, A.O.; Dolgikh, E.A.; Kilanova, A.S.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Staehelin, C. Nod factors induce Nod factor cleaving enzymes in pea roots. Genetic and pharmacological approaches indicate different activation mechanisms. Plant Physiol. 2005, 139, 1051–1064. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitaeva, A.B.; Demchenko, K.N.; Tikhonovich, I.A.; Timmers, A.C.J.; Tsyganov, V.E. Comparative analysis of the tubulin cytoskeleton organization in nodules of Medicago truncatula and Pisum sativum: Bacterial release and bacteroid positioning correlate with characteristic microtubule rearrangements. New Phytol. 2016, 210, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Foo, E.; Ross, J.J.; Reid, J.B. Relationship between gibberellin, ethylene and nodulation in Pisum sativum. New Phytol. 2011, 189, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, V.E.; Pavlova, Z.B.; Kravchenko, L.V.; Rozov, S.M.; Borisov, A.Y.; Lutova, L.A.; Tikhonovich, I.A. New gene Crt (Curly roots) controlling pea (Pisum sativum L.) root development. Ann. Bot. 2000, 86, 975–981. [Google Scholar] [CrossRef][Green Version]
- Zhernakov, A.I.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A. The pea gene CRT, which controls root morphogenetic reactions, is involved in the regulation of ACC-oxidase activity. Russ. J. Genet. Appl. Res. 2013, 3, 127–137. [Google Scholar] [CrossRef]
- Pavlova, Z.B.; Ischenko, N.V.; Voroshilova, V.A.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Lutova, L.A. Use of pea (Pisum sativum L.) mutants impaired in root formation to study the role of auxin in nodule development. In Nitrogen Fixation: From Molecules to Crop Productivity; Current Plant Science and Biotechnology in Agriculture; Volume 38, Pedrosa, F.O., Hungria, M., Yates, G., Newton, W.E., Eds.; Springer: Dordrecht, The Netherland, 2002; p. 244. [Google Scholar] [CrossRef]
- Kulaeva, O.A.; Tsyganov, V.E. Molecular-genetic basis of cadmium tolerance and accumulation in higher plants. Russ. J. Genet. Appl. Res. 2011, 1, 349. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Belimov, A.A.; Borisov, A.Y.; Safronova, V.I.; Georgi, M.; Dietz, K.-J.; Tikhonovich, I.A. A chemically induced new pea (Pisum sativum) mutant SGECdt with increased tolerance to, and accumulation of, cadmium. Ann. Bot. 2007, 99, 227–237. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Zhernakov, A.I.; Khodorenko, A.V.; Kisutin, P.Y.; Belimov, A.A.; Safronova, V.I.; Naumikina, T.S.; Borisov, A.Y.; Lindblad, P.; Dietz, K.J.; et al. Mutational analysis to study the role of genetic factors in pea adaptation to stresses during development its symbioses with Rhizobium and mycorrhizal fungi. In Biological Nitrogen Fixation, Sustainable Agriculture and the Environment, 1st ed.; Wang, Y.P., Lin, M., Tian, Z.X., Elmerich, C., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 41, pp. 279–281. [Google Scholar]
- Tsyganov, V.E.; Tsyganova, A.V.; Gorshkov, A.P.; Seliverstova, E.V.; Kim, V.E.; Chizhevskaya, E.P.; Belimov, A.A.; Serova, T.A.; Ivanova, K.A.; Kulaeva, O.A.; et al. Efficacy of a plant-microbe system: Pisum sativum (L.) cadmium-tolerant mutant and Rhizobium leguminosarum strains, expressing pea metallothionein genes PsMT1 and PsMT2, for cadmium phytoremediation. Front. Microbiol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Tsyganov, V.E. Influence of mutation in pea (Pisum sativum L.) cdt (cadmium tolerance) gene on histological and ultrastructural nodule organization. Ekol. Genet. 2019, 17, 71–80. [Google Scholar] [CrossRef]
- Alves-Carvalho, S.; Aubert, G.; Carrère, S.; Cruaud, C.; Brochot, A.-L.; Jacquin, F.; Klein, A.; Martin, C.; Boucherot, K.; Kreplak, J.; et al. Full-length de novo assembly of RNA-seq data in pea (Pisum sativum L.) provides a gene expression atlas and gives insights into root nodulation in this species. Plant J. 2015, 84, 1–19. [Google Scholar] [CrossRef]
- Zhukov, V.A.; Zhernakov, A.I.; Kulaeva, O.A.; Ershov, N.I.; Borisov, A.Y.; Tikhonovich, I.A. De Novo assembly of the pea (Pisum sativum L.) nodule transcriptome. Int. J. Genom. 2015, 2015, 695947. [Google Scholar] [CrossRef]
- Kreplak, J.; Madoui, M.-A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/assembly/GCA_003013575.1 (accessed on 3 December 2020).
- Shirasawa, K.; Sasaki, K.; Hirakawa, H.; Isobe, S. Genomic region associated with pod color variation in pea (Pisum sativum). bioRxiv 2020. [Google Scholar] [CrossRef]
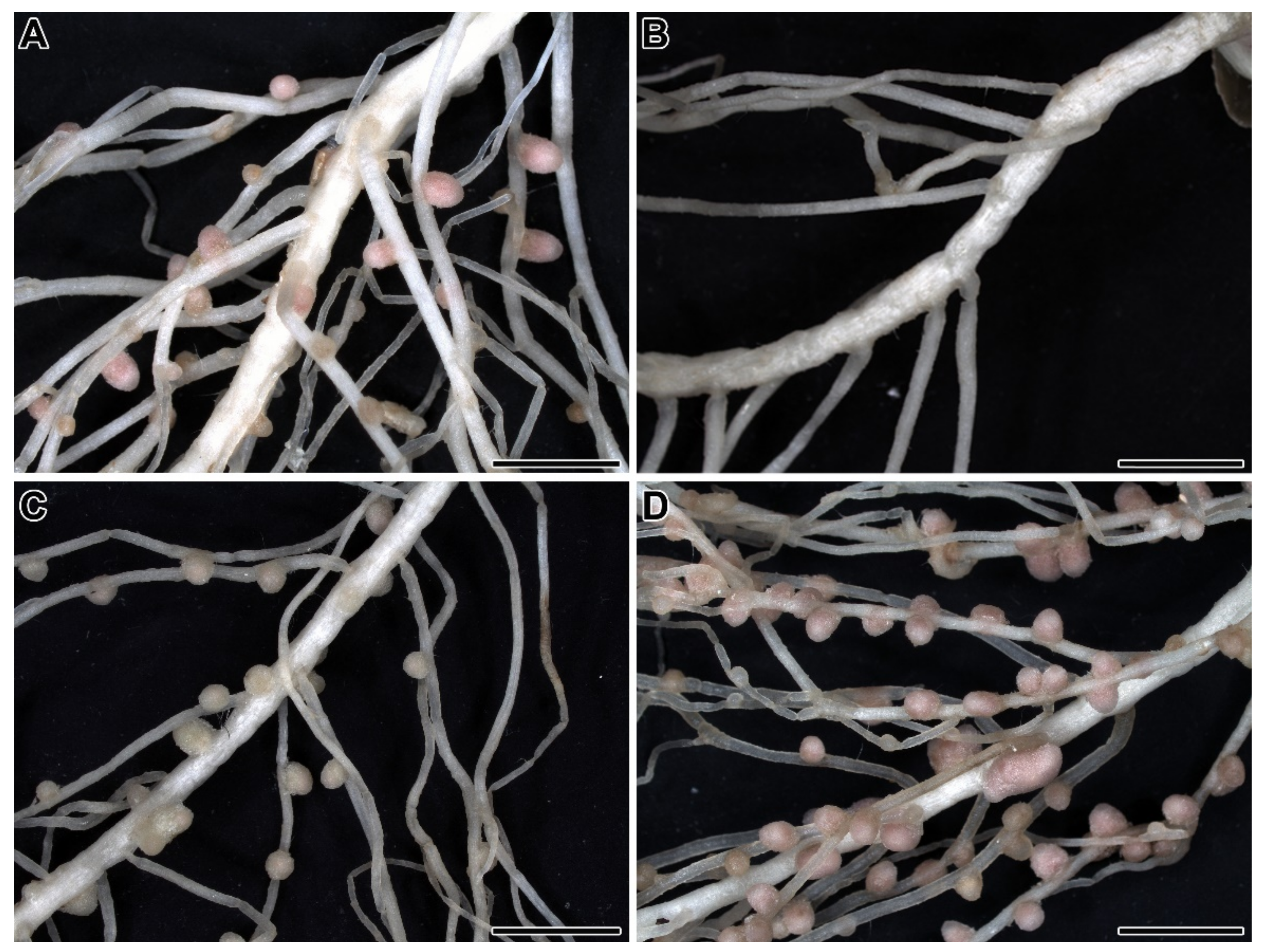
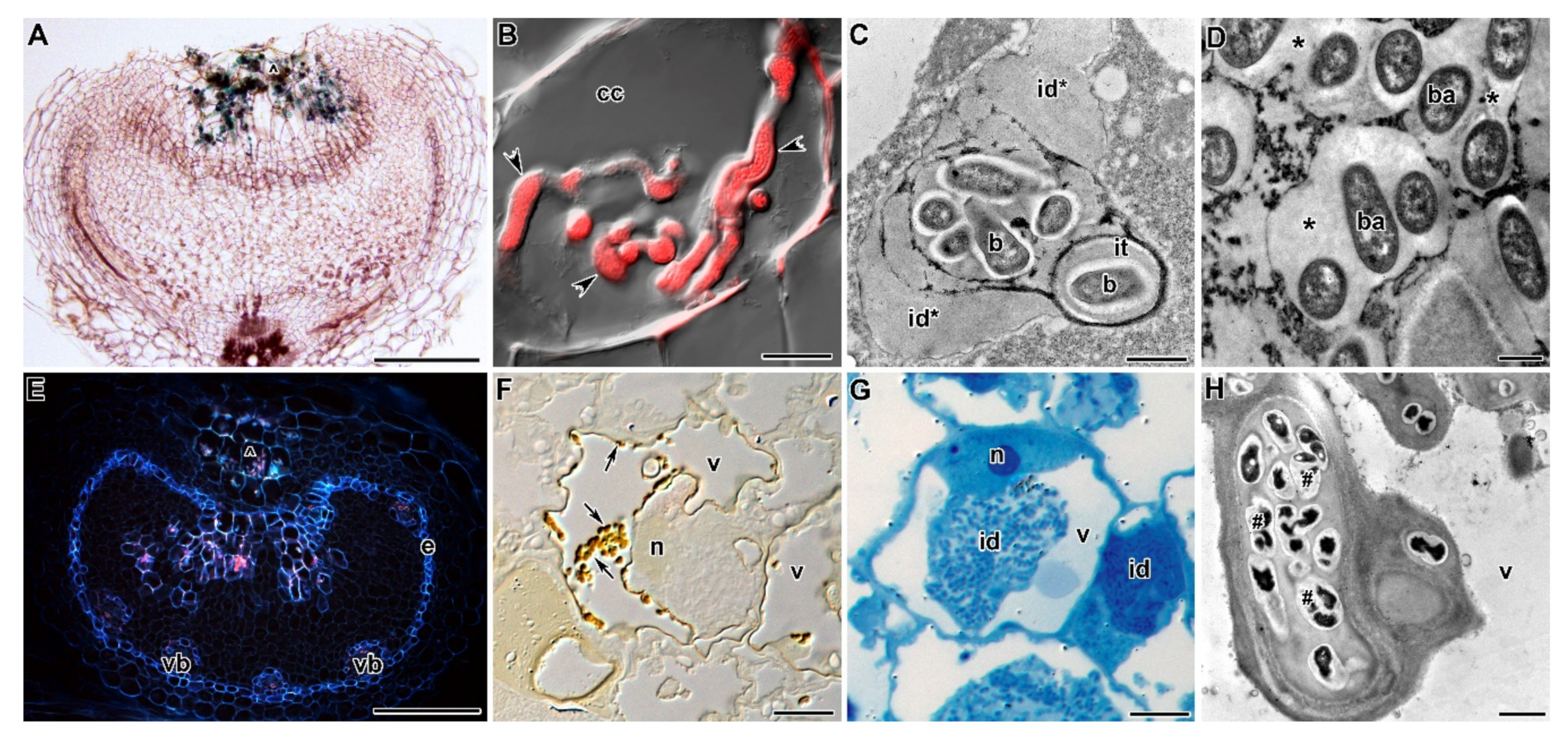
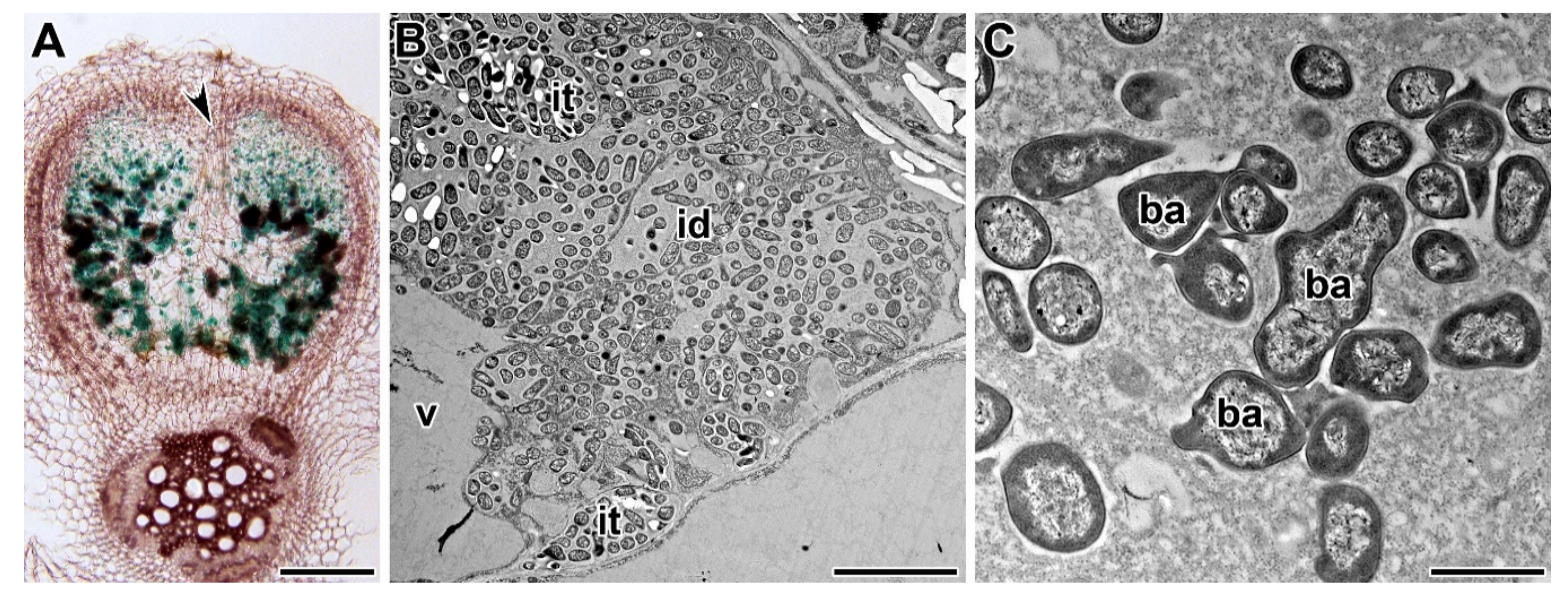
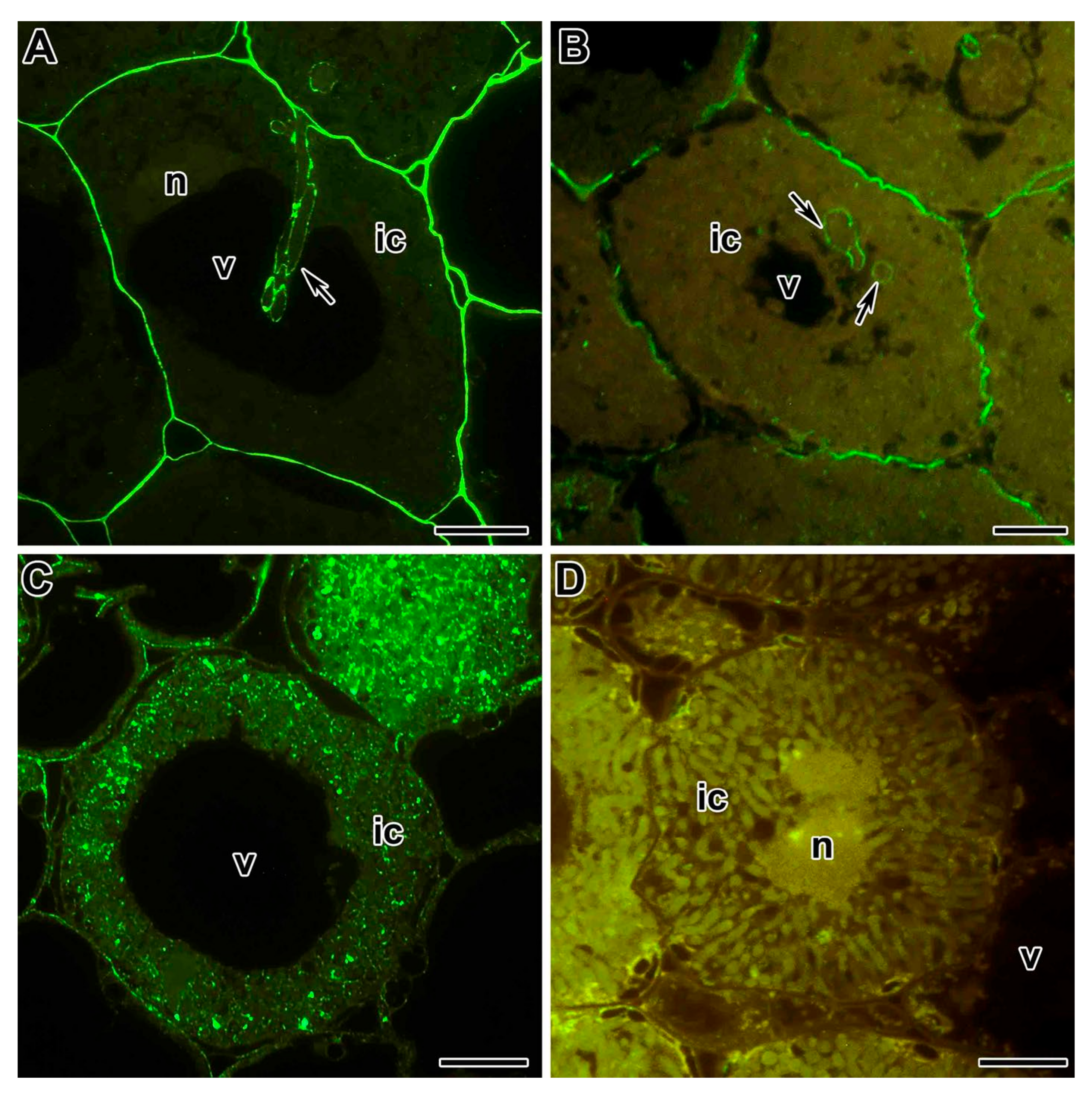
| Symbiotic Locus * | Linkage Group | Phenotypes | Mutant Lines | References |
|---|---|---|---|---|
| Sym1 = sym2 | I | Nod+/− | JI 1357 (registered type line), VIR K320-1 | [16,17,20,21,25,26] |
| sym3 | - | Fix− | JI 1357 (registered type line) | [17] |
| Sym4 | - | Nod− | JI 261 | [19] |
| sym5 | I | Nod− | E2, R88, E77, E111, E143, E166, E169, K20a | [27,28,29,30] |
| sym6 | - | Fix− | JI 1357 (registered type line) | [23,24] |
| sym7 | III | Nod− | E69, N12, RisNod14, SGENod−-2 | [7,31,32,33] |
| sym8 = sym20 | VI | Nod− | E14, R19, R25, R80, RisNod10, RisNod13, RisNod19, RisNod21, RisNod25, Sprint-2Nod−-1, Sprint-2Nod−-2 | [7,31,32,34,35] |
| sym9 = sym30 | IV | Nod− | R72, P54, P1, P2, P3, P53, RisNod6, RisNod9, RisNod22 | [7,8,31,32,35,36,37,38] |
| sym10 | I | Nod− | P5, P7, P8, P9, P10, P56, RisFixG | [7,31,32,36] |
| sym12 | Nod+/− | K5 | [39] | |
| sym13 | VII | Fix− | E135f, E136, P58 | [40,41,42] |
| sym14 | III | Nod− | E135n, SGENod−-2 | [29,40,43] |
| sym15 | VII | Nod+/− | E151 | [32] |
| sym16 | V | Nod− | R50 | [32] |
| sym17 | VI | Nod+/− | R82 | [32] |
| sym18 | II | Nod+/− | E54 | [29,44] |
| sym19 = sym41 | I | Nod−/Fix− | P4, P6, P55, NEU5, NMU1, RisNod2, RisNod7, RisNod16, RisNod20, Sprint-2Nod−-3, RisFixA | [7,28,31,36,45,46,47] |
| sym21 | - | Nod+/− | E132 | [48] |
| Sym22 | II | Nod+/− | JI 1794 | [10] |
| sym23 | - | Fix− | P59 | [7,36,41] |
| sym24 | - | Fix− | P60 | [7,36] |
| sym25 | - | Fix− | P14, P17, P19, P61, SGEFix−-8 | [7,36,49] |
| sym26 | - | Fix− | P63, RisFixM, RisFixT, SGEFix−-3 | [7,31,33,36] |
| sym27 | V | Fix− | P12, RisFixQ, SGEFix−-7, | [7,31,36,42,49,50] |
| sym28 | V | Nod++ | P64, P77, P109. P110, P113, P120 | [51,52,53] |
| sym29 | VII | Nod++ | P87, P88, P89, P90, P91, P93, P94 | [51,54] |
| sym31 | III | Fix− | Sprint-2Fix− | [55,56] |
| sym32 | - | Fix− | RisFixL, RisFixO | [7,31] |
| sym33 = sym11 | I | Fix−/Nod− | RisFixU, SGEFix−-2, SGEFix−-5, N24 | [7,31,32,49,57,58,59,60] |
| sym34 | - | Nod− | RisNod1, RisNod23, RisNod30 | [7,31] |
| sym35 | I | Nod− | RisNod8, SGENod−-1, SGENod−-3 | [15,31,43] |
| sym36 | - | Nod− | RisNod24, RisNod26 | [7,31] |
| sym37 | - | Nod+/− | RisNod4, K24 | [7,31,39] |
| sym38 | V | Nod− | RisFixF, SGENod−-4, SGENod−-8 | [7,31,57,61] |
| sym39 | - | Nod+/− | P57 | [7,62] |
| sym40 | VII | Fix− | SGEFix−-1, SGEFix−-6 | [49,58,63] |
| sym42 | - | Fix− | RisFixV | [31,45] |
| brz | IV | Nod− | E107 | [64] |
| coch | - | - | JI1824, JI3121, JI2165, JI2757, Wt11304, SGRcoch, SGEapm, FN3185/1325 | [7,65,66,67,68,69,70,71] |
| LykX | - | - | JI 1357 (registered type line) | [72] |
| k1 | I | Nod−/Nod+/− | Cameor 817, Cameor 885, Cameor 2265 | [73] |
| nof1 | - | Fix− | FN1 | [74] |
| nod1 | - | Nod++ | Parvus | [5] |
| nod2 | - | Nod++ | Parvus | [5] |
| nod3 | I | Nod++ | nod3, P79, K10a, K11a, K12a, P79, RisFixC | [30,75,76,77] |
| nod4 | V | Nod++ | K301 | [78,79] |
| Nod5 | - | Nod++ | Torsdag | [80] |
| nod6 | VII | Nod++ | K21a, K22a | [30,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganov, V.E.; Tsyganova, A.V. Symbiotic Regulatory Genes Controlling Nodule Development in Pisum sativum L. Plants 2020, 9, 1741. https://doi.org/10.3390/plants9121741
Tsyganov VE, Tsyganova AV. Symbiotic Regulatory Genes Controlling Nodule Development in Pisum sativum L. Plants. 2020; 9(12):1741. https://doi.org/10.3390/plants9121741
Chicago/Turabian StyleTsyganov, Viktor E., and Anna V. Tsyganova. 2020. "Symbiotic Regulatory Genes Controlling Nodule Development in Pisum sativum L." Plants 9, no. 12: 1741. https://doi.org/10.3390/plants9121741
APA StyleTsyganov, V. E., & Tsyganova, A. V. (2020). Symbiotic Regulatory Genes Controlling Nodule Development in Pisum sativum L. Plants, 9(12), 1741. https://doi.org/10.3390/plants9121741







