Opportunities for Increased Nitrogen Use Efficiency in Wheat for Forage Use
Abstract
1. Introduction
2. Results and Discussion
2.1. Greenhouse and Hoop House Evaluations
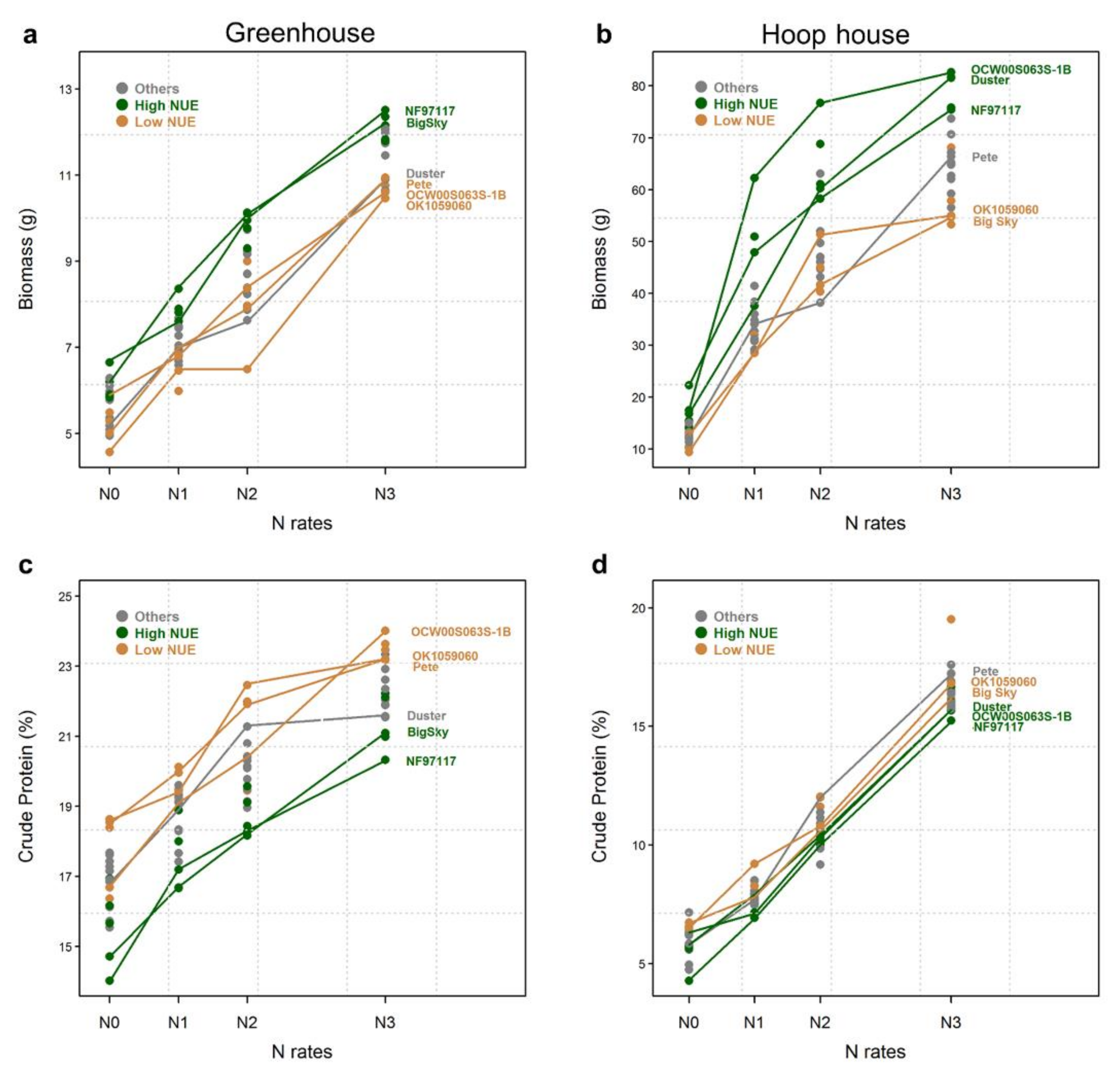
2.2. Biomass Production
2.3. Crude Protein Content
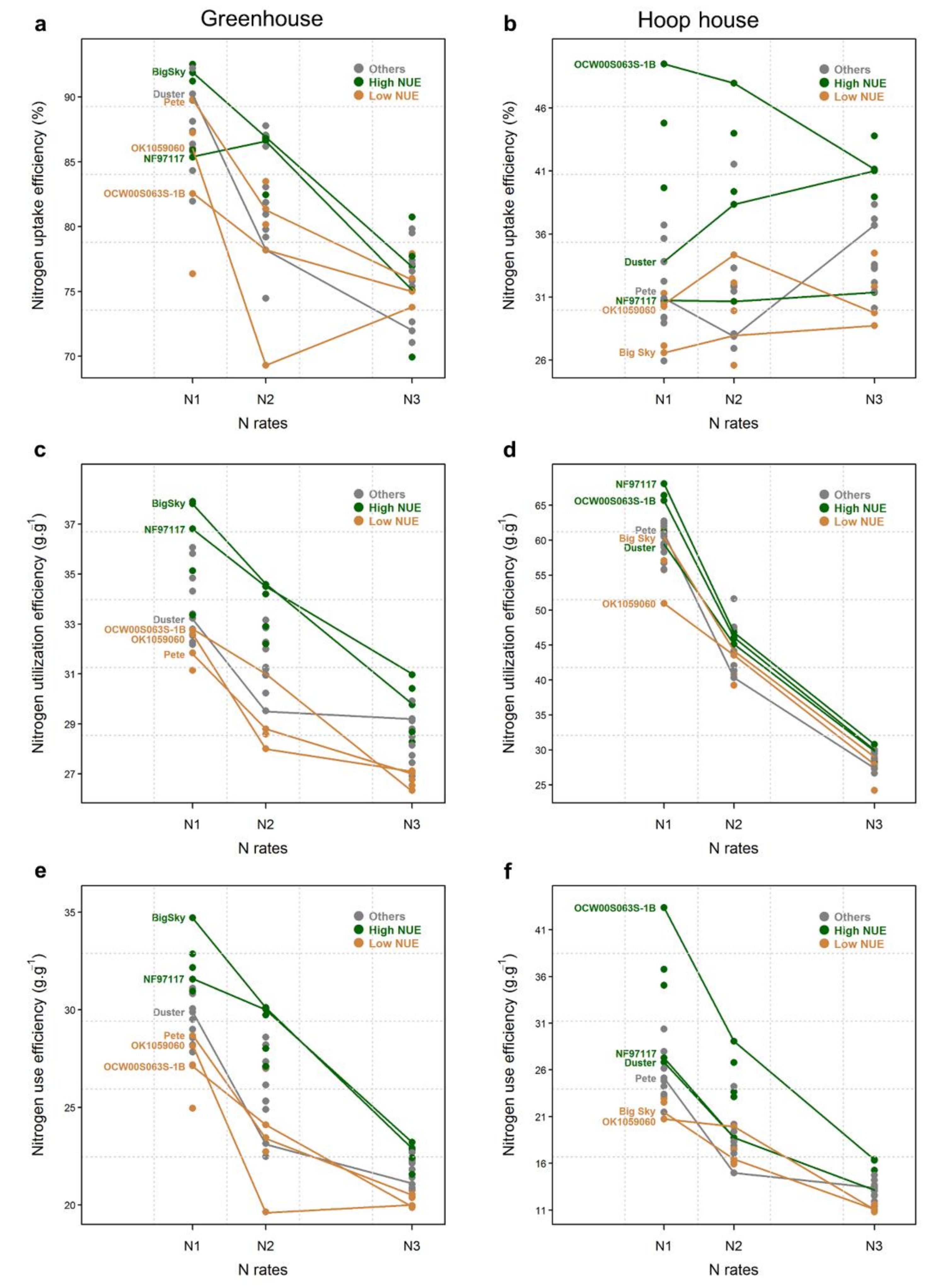
2.4. Nitrogen Uptake Efficiency
2.5. Nitrogen Utilization Efficiency
2.6. Nitrogen Use Efficiency
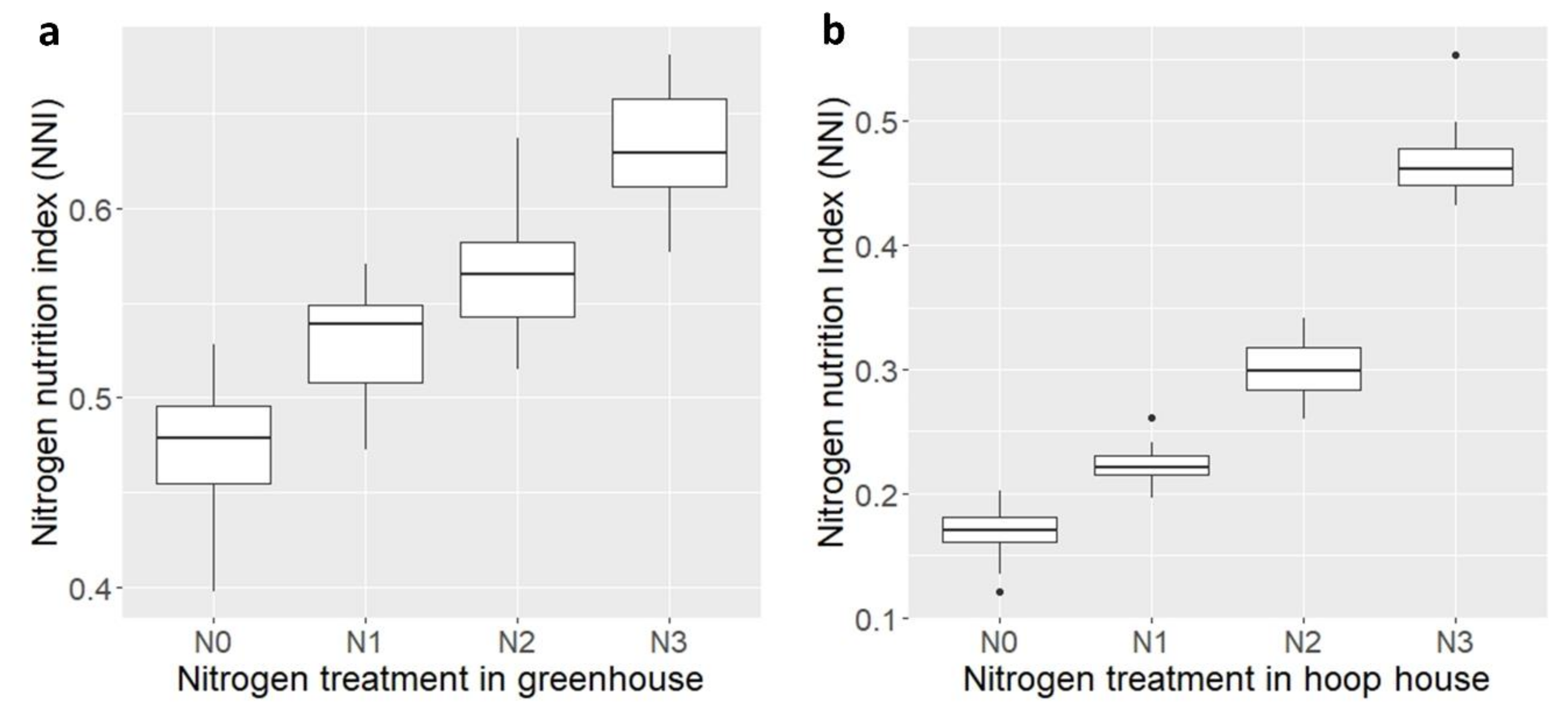
2.7. Nitrogen Nutrition Index
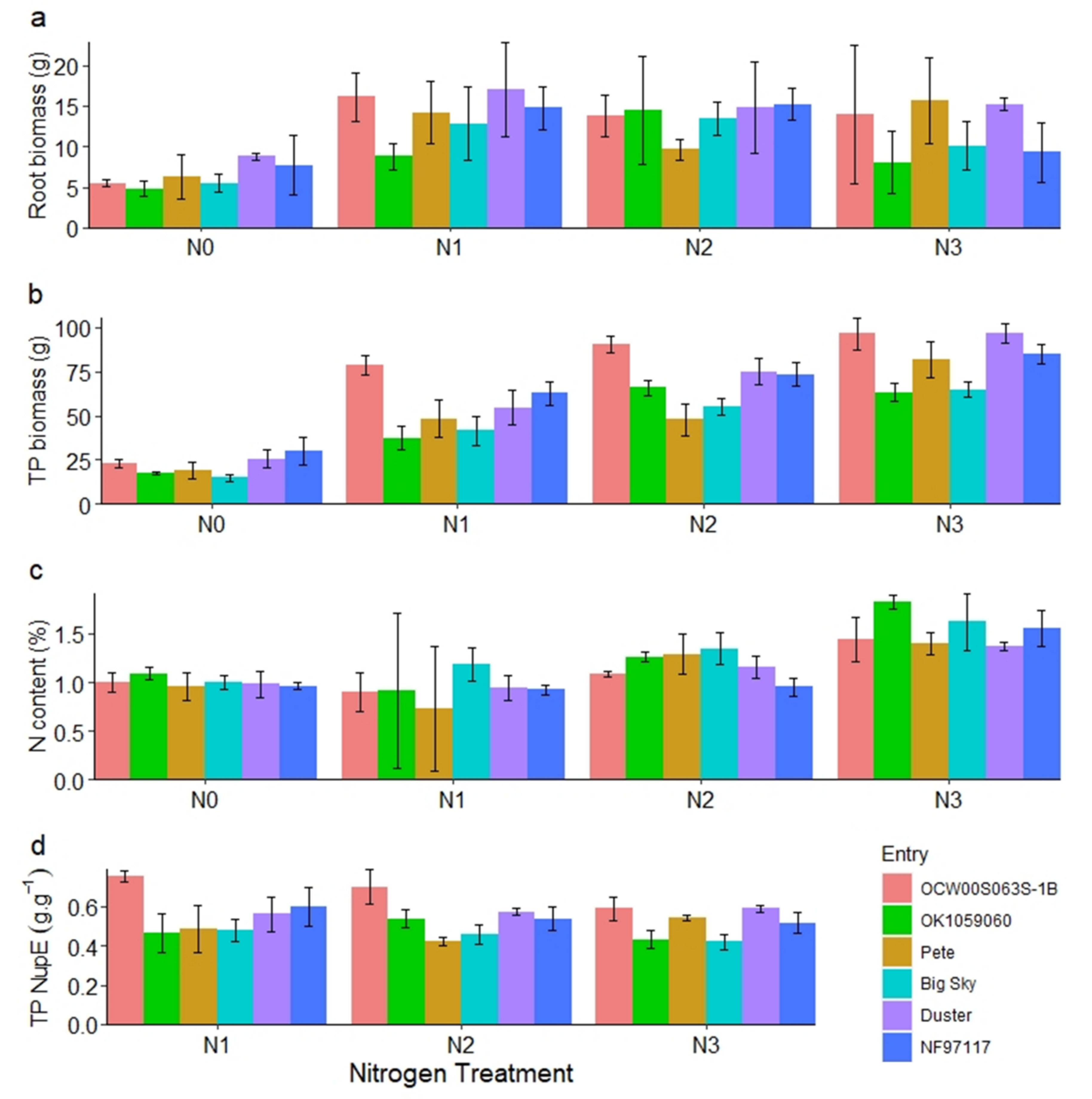
2.8. Root Evaluation in Hoop House
2.9. Relationship among Traits
3. Materials and Methods
3.1. Plant Materials and Soil
3.2. Greenhouse and Hoop House Evaluations
3.3. Calculating NUE and NNI
NUE (g.g−1) = biomass (g)/Nav (g)
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| N | nitrogen |
| NUE | N use efficiency |
| NNI | N nutrition index |
| NUpE | N uptake efficiency |
| NUtE | N utilization efficiency |
| CP | crude protein |
| TP | total plant |
| Na | actual plant N concentration |
| Nc | critical N concentration |
References
- Krenzer, E.G. Wheat for Pasture; Oklahoma Cooperative Extension Service and Oklahoma Agricultural Experimentation Station, F-2586; Oklahoma State University: Stillwater, OK, USA, 1994. [Google Scholar]
- Kim, K.S.; Anderson, J.D. Forage yield and nutritive value of winter wheat varieties in the southern Great Plains. Euphytica 2015, 202, 445–457. [Google Scholar] [CrossRef]
- True, R.R.; Epplin, F.M.; Krenzer, E.G.; Horn, G.W. A Survey of Wheat Production and Wheat Forage Use Practices in Oklahoma; B-815; Oklahoma Cooperative Extension Service: Stillwater, OK, USA, 2001. [Google Scholar]
- Hossain, I.; Epplin, F.M.; Krenzer, E.G. Planting date influence on dual-purpose winter wheat forage yield, grain yield, and test weight. Agron. J. 2003, 95, 1179–1188. [Google Scholar] [CrossRef]
- Krenzer, E.G. Wheat management in Oklahoma. In Wheat Management in Oklahoma: A Handbook for Oklahoma’s Wheat Industry; Royer, T.A., Krenzer, E.G., Eds.; Oklahoma Cooperative Extension Service and Oklahoma Agriccultural Experimentation Station, E-831: Stillwater, OK, USA, 2000; pp. 27–30. [Google Scholar]
- Redfearn, D. Fertilizing cool-season forages. In Oklahoma Forage and Pasture Fertility Guide; Arnall, B., Redfearn, D., Eds.; Oklahoma Cooperative Extension Service, E-1021: Stillwater, OK, USA, 2018. [Google Scholar]
- Pan, J.; Zhu, Y.; Jiang, D.; Dai, T.; Li, Y.; Cao, W. Modeling plant nitrogen uptake and grain nitrogen accumulation in wheat. Field Crops Res. 2006, 97, 322–336. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Udvardi, M.K.; Brodie, E.L.; Riley, W.J.; Kaeppler, S.M.; Lynch, J.P. Impacts of agricultural nitrogen on the environment and strategies to reduce these impacts. Procedia Environ. Sci. 2015, 29, 303. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Pan, B.; Lam, S.K.; Mosier, A.R.; Luo, Y.; Chen, D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agric. Ecosyst. Environ. 2016, 232, 283–289. [Google Scholar] [CrossRef]
- Altom, W.; Rogers, J.L.; Ram, W.R.; Johnson, G.V.; Taylor, S.L. Long-term rye-wheat-ryegrass forage yields as affected by rate and date of applied nitrogen. J. Prod. Agric. 1996, 9, 510–516. [Google Scholar] [CrossRef]
- Sanz-Cobeña, A.; Sánchez-Martín, L.; García-Torres, L.; Vallejo, A. Gaseous emissions of N2O and NO and NO3− leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agric. Ecosyst. Environ. 2012, 149, 64–73. [Google Scholar] [CrossRef]
- Lemaire, G. Diagnosis of the Nitrogen Status in Crops; Springer: Berlin, Germany, 1997. [Google Scholar]
- Cassman, K.G.; Dobermann, A.R.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Hirel, B.; Gouis, J.L.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Expt. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef]
- Glass, A.D. Nitrogen use efficiency of crop plants: Physiological constraints upon nitrogen absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar] [CrossRef]
- Dawson, J.C.; Huggins, D.R.; Jones, S.S. Characterizing nitrogen use efficiency in natural and agricultural ecosystems to improve the performance of cereal crops in low input and organic agricultural systems. Field Crops Res. 2008, 107, 89–101. [Google Scholar] [CrossRef]
- Hawkesford, M.J. An overview of nutrient use efficiency and strategies for crop improvement. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Hawkesford, M.J., Barraclough, P.B., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 3–19. [Google Scholar]
- Anbessa, Y.; Juskiw, P. Strategies to increase nitrogen use efficiency of spring barley. Can. J. Plant. Sci. 2012, 92, 617–625. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, R.; Sayre, K.; Rajaram, S.; McMahon, M. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 1997, 37, 898–904. [Google Scholar] [CrossRef]
- Todeschini, M.H.; Milioli, A.S.; Trevizan, D.M.; Bornhofen, E.; Finatto, T.; Storck, L.; Benin, G. Nitrogen use efficiency in modern wheat cultivars. Bragantia 2016, 75, 351–361. [Google Scholar] [CrossRef][Green Version]
- Gauer, L.E.; Grant, C.A.; Bailey, L.D.; Gehl, D.T. Effects of nitrogen fertilization on grain protein content, nitrogen uptake, and nitrogen use efficiency of six spring wheat (Triticum aestivum L.) cultivars, in relation to estimated moisture supply. Can. J. Plant Sci. 1992, 72, 235–241. [Google Scholar] [CrossRef]
- Duncan, E.G.; O’Sullivan, C.; Roper, M.; Palta, J.; Whisson, K.; Peoples, M. Yield and nitrogen use efficiency of wheat increased with root length and biomass due to nitrogen, phosphorus, and potassium interactions. J. Plant Nutr. Soil Sci. 2018, 181, 364–373. [Google Scholar] [CrossRef]
- Yin, L.; Dai, X.; He, M. Delayed sowing improves nitrogen utilization efficiency in winter wheat without impacting yield. Field Crops Res. 2018, 221, 90–97. [Google Scholar] [CrossRef]
- Thomason, W.E.; Raun, W.R.; Johnson, G.V. Winter wheat fertilizer nitrogen use efficiency in grain and forage production systems. J. Plant Nutr. 2000, 23, 1505–1516. [Google Scholar] [CrossRef]
- Schneider-Canny, R.; Chekhovskiy, K.; Muñoz, P.; Kwon, S.; Saha, M.C. Characterization of bermudagrass (Cynodon dactylon L.) germplasm for nitrogen use efficiency. Euphytica 2019, 2153, 40. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Haile, D.; Nigussie, D.; Ayana, A. Nitrogen use efficiency of bread wheat: Effects of nitrogen rate and time of application. J. Soil Sci. Plant Nutr. 2012, 12, 389–410. [Google Scholar]
- Gill, K.S.; Omokanye, A.T.; Pettyjohn, J.P.; Elsen, M. Evaluation of forage type barley varieties for forage yield and nutritive value in the Peace Region of Alberta. J. Agric. Sci. 2013, 5, 24–36. [Google Scholar] [CrossRef]
- Brentrup, F.; Pallière, C. Nitrogen use efficiency as an agro-environmental indicator. In Proceedings of the OECD Workshop on Agrienvironmental Indicators, Leysin, Switzerland, 23–26 March 2010. [Google Scholar]
- Limón-Ortega, A.; Sayre, K.D.; Francis, C. Wheat nitrogen use efficiency in a bed planting system in northwest Mexico. Agron. J. 2000, 92, 303–308. [Google Scholar] [CrossRef]
- Gallais, A.; Hirel, B. An approach to the genetics of nitrogen use efficiency in maize. J. Expt. Bot. 2004, 55, 295–306. [Google Scholar] [CrossRef]
- Singh, U.; Ladha, J.K.; Castillo, E.G.; Punzalan, G.; Tirol-Padre, A.; Duqueza, M. Genotypic variation in nitrogen use efficiency in medium-and long-duration rice. Field Crops Res. 1998, 58, 35–53. [Google Scholar] [CrossRef]
- Wu, P.; Tao, Q.N. Genotypic response and selection pressure on nitrogen-use efficiency in rice under different nitrogen regimes. J. Plant Nutr. 1995, 18, 487–500. [Google Scholar] [CrossRef]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Cambouris, A.N.; Tremblay, N.; Nolin, M.C.; Claessens, A.; Parent, L.E. Critical nitrogen curve and nitrogen nutrition index for corn in eastern Canada. Agron. J. 2008, 100, 271–276. [Google Scholar] [CrossRef]
- Justes, E.; Meynard, J.M.; Mary, B.; Plénet, D. Diagnosis using stem base extract: JUBIL method. In Diagnosis of the Nitrogen Status in Crops; Lemaire, G., Ed.; Springer: Berlin, Germany, 1997; pp. 163–187. [Google Scholar]
- Rasmussen, I.S.; Dresbøll, D.B.; Thorup-Kristensen, K. Winter wheat cultivars and nitrogen (N) fertilization—Effects on root growth, N uptake efficiency and N use efficiency. Eur. J. Agron. 2015, 68, 38–49. [Google Scholar] [CrossRef]
- Dordas, C.A. Nitrogen nutrition index and its relationship to N use efficiency in linseed. Eur. J. Agron. 2011, 34, 124–132. [Google Scholar] [CrossRef]
- Hu, D.W.; Sun, Z.P.; Li, T.L.; Yan, H.Z.; Zhang, H. Nitrogen nutrition index and its relationship with N use efficiency, tuber yield, radiation use efficiency, and leaf parameters in potatoes. J. Integer. Agric. 2014, 13, 1008–1016. [Google Scholar] [CrossRef]
- Dordas, C.A. Nitrogen nutrition index and leaf chlorophyll concentration and its relationship with nitrogen use efficiency in barley (Hordeum vulgare L.). J. Plant Nutr. 2017, 40, 1190–1203. [Google Scholar] [CrossRef]
- Van Sanford, D.A.; MacKown, C.T. Variation in nitrogen use efficiency among soft red winter wheat lines. Theor. Appl. Genet. 1986, 72, 158–163. [Google Scholar] [CrossRef]
- Martin, T.J.; Fritz, A.K.; Seifers, D.; Shroyer, J.P. ‘RonL’ Hard White Wheat; Agricultural Experiment Station and Cooperative Extension Service; Kansas State University: Manhattan, KS, USA, 2007. [Google Scholar]
- Graybosch, R.A.; Peterson, C.J.; Baenziger, P.S.; Baltensperger, D.; Nelson, L.A.; Jin, Y.; Kolmer, J.A.; Seabourn, B.W.; French, R.C.; Hein, G.L.; et al. Registration of ‘Mace’ hard red winter wheat. J. Plant Regist. 2009, 3, 51–56. [Google Scholar] [CrossRef]
- Raupp, W.J. Annual Wheat Newsletter; Kansas State University: Manhattan, KS, USA, 2010; Volume 55. [Google Scholar]
- Baenziger, P.S.; Graybosch, R.A.; Regassa, T.; Nelson, L.A.; Klein, R.N.; Santra, D.K.; Jin, Y. Registration of ‘NI04421′ hard red winter wheat. J. Plant Regist. 2012, 6, 54–59. [Google Scholar] [CrossRef]
- Carver, B.F.; Smith, E.L.; Hunger, R.M.; Klatt, A.R.; Edwards, J.T.; Porter, D.R.; Verchot-Lubicz, J.; Rayas-Duarte, P.; Bai, G.; Martin, B.; et al. Registration of ‘Endurance’ wheat. Crop Sci. 2006, 46, 1816–1817. [Google Scholar] [CrossRef]
- Sparks, P. Small Grains Breeding at Noble Research Institute; Noble Research Institute: Ardmore, OK, USA, 2017; Available online: https://www.noble.org/blog/small-grains-breeding (accessed on 7 July 2020).
- Carver, B.F.; Khalil, I.; Krenzer, E.G.; MacKown, C.T. Breeding winter wheat for a dual-purpose management system. Euphytica 2001, 119, 231–234. [Google Scholar] [CrossRef]
- Carver, B.F.; Hunger, R.M.; Smith, E.L.; Klatt, A.R.; Edwards, J.T.; Porter, D.R.; Rayas-Duarte, P.; Verchot-Lubicz, J.; Martin, B.; Krenzer, E.G.; et al. Registration of ‘Deliver’ wheat. Crop Sci. 2006, 46, 1819–1820. [Google Scholar] [CrossRef]
- Edwards, J.T.; Hunger, R.M.; Smith, E.L.; Horn, G.W.; Chen, M.; Yan, L.; Bai, G.; Bowden, R.L.; Klatt, A.R.; Rayas-Duarte, P.; et al. ‘Duster’ wheat: A durable, dual-purpose cultivar adapted to the southern Great Plains of the USA. J. Plant Regist. 2012, 6, 37–48. [Google Scholar] [CrossRef]
- Scanlan, C. Critical Tissue Nitrogen Concentrations for Diagnosis of Nitrogen Deficiency in Wheat; Department of Primary Industries and Regional Development: Perth, Australia, 2017. Available online: https://www.agric.wa.gov.au/wheat/critical-tissue-nitrogen-concentrations-diagnosis-nitrogen-deficiency-wheat (accessed on 30 July 2020).
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research; Northwestern University: Evanston, IL, USA, 2018. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]

| Variables | N Rate | Line | N Rate × Lines |
|---|---|---|---|
| All Lines in Greenhouse (p > F) | |||
| Biomass (all N rates) | 0.001 | <0.001 | 0.965 |
| Crude protein (all N rates) | <0.001 | <0.001 | 0.168 |
| Nitrogen nutrition index (all N rates) | <0.001 | <0.001 | 0.267 |
| Biomass (N+) | 0.008 | <0.001 | 0.934 |
| Crude protein (N+) | <0.001 | <0.001 | 0.089 |
| Nitrogen uptake efficiency | 0.005 | 0.458 | 0.978 |
| Nitrogen utilization efficiency | 0.005 | <0.001 | 0.050 |
| Nitrogen use efficiency | 0.009 | <0.001 | 0.854 |
| Nitrogen nutrition index (N+) | <0.001 | <0.001 | 0.099 |
| All lines in hoop house (p > F) | |||
| Biomass (all N rates) | <0.001 | <0.001 | <0.001 |
| Crude protein (all N rates) | <0.001 | <0.001 | 0.229 |
| Nitrogen nutrition index (all N rates) | <0.001 | <0.001 | 0.330 |
| Biomass (N+) | 0.0005 | <0.001 | 0.005 |
| Crude protein (N+) | <0.001 | <0.001 | 0.449 |
| Nitrogen uptake efficiency | 0.577 | <0.001 | <0.001 |
| Nitrogen utilization efficiency | <0.001 | <0.001 | 0.241 |
| Nitrogen use efficiency | 0.023 | <0.001 | <0.001 |
| Nitrogen nutrition index (N+) | <0.001 | <0.001 | 0.489 |
| Six lines in hoop house (p > F) | |||
| Root biomass | 0.002 | 0.013 | 0.188 |
| Root N | 0.002 | 0.161 | 0.806 |
| TP biomass | <0.001 | <0.001 | <0.001 |
| TP nitrogen uptake efficiency | 0.229 | <0.001 | 0.042 |
| Variables | Greenhouse | Hoop House |
|---|---|---|
| Biomass (all N rates) | 94.9 | 96.5 |
| Crude protein (all N rates) | 94.9 | 96.7 |
| Nitrogen nutrition index (all N rates) | 94.3 | 96.4 |
| Biomass (N+) | 88.1 | 92.0 |
| Crude protein (N+) | 94.1 | 95.9 |
| Nitrogen uptake efficiency | 53.7 | 75.0 |
| Nitrogen utilization efficiency | 94.2 | 95.1 |
| Nitrogen use efficiency | 82.4 | 90.4 |
| Nitrogen nutrition index (N+) | 93.8 | 95.6 |
| Six lines in hoop house | ||
| Root biomass | 76.3 | |
| Root N | 69.7 | |
| TP biomass | 97.3 | |
| TP nitrogen uptake efficiency | 81.0 | |
| N Rate | Shoot N (%) | Root Biomass | Root N (%) | TP Biomass | TP NUpE | NUtE | NUpE | NUE | |
|---|---|---|---|---|---|---|---|---|---|
| N0 | 0.64 * | 0.55 * | 0.34 | 0.96 * | - | - | - | - | |
| Shoot biomass | N1 | −0.67 * | 0.53 * | −0.17 | 0.97 * | 0.90 * | 0.67 * | 0.96 * | 1.00 * |
| N2 | −0.50 * | 0.20 | −0.51 * | 0.97 * | 0.89 * | 0.50 * | 0.92 * | 1.00 * | |
| N3 | −0.52 * | 0.38 | −0.55 * | 0.95 * | 0.86 * | 0.52 * | 0.92 * | 1.00 * | |
| N0 | −0.32 | −0.38 | −0.60 * | - | - | - | - | ||
| Shoot N (%) | N1 | −0.65 * | 0.10 | −0.73 * | −0.51 * | −0.99 * | −0.41 | −0.64 * | |
| N2 | −0.25 | 0.42 | −0.52 * | −0.16 | −0.98 * | −0.12 | −0.51 * | ||
| N3 | 0.18 | 0.25 | −0.36 | −0.06 | −1.00 * | −0.14 | −0.51 * | ||
| N0 | 0.25 | 0.76 * | - | - | - | - | |||
| Root biomass | N1 | −0.02 | 0.71 * | 0.59 * | 0.63 * | 0.44 | 0.56 * | ||
| N2 | −0.34 | 0.43 | 0.35 | 0.22 | 0.14 | 0.19 | |||
| N3 | −0.47 * | 0.65 * | 0.70 * | −0.19 | 0.54 * | 0.40 | |||
| N0 | 0.35 | - | - | - | - | ||||
| Root N (%) | N1 | −0.15 * | 0.16 | −0.10 | −0.19 | −0.19 | |||
| N2 | −0.55 * | −0.38 | −0.39 | −0.41 | −0.48 * | ||||
| N3 | −0.61 * | −0.50 * | −0.26 | −0.59 * | −0.62 * | ||||
| N0 | - | - | - | - | |||||
| TP biomass | N1 | 0.90 * | 0.72 * | 0.91 * | 0.97 * | ||||
| N2 | 0.91 * | 0.52 * | 0.89 * | 0.97 * | |||||
| N3 | 0.95 * | 0.36 | 0.94 * | 0.96 * | |||||
| TP NUpE | N1 | 0.50 * | 0.91 * | 0.89 * | |||||
| N2 | 0.14 | 0.97 * | 0.89 * | ||||||
| N3 | 0.06 | 0.98 * | 0.87 ** | ||||||
| NUtE | N1 | 0.40 | 0.64 * | ||||||
| N2 | 0.11 | 0.51 * | |||||||
| N3 | 0.14 | 0.51 * | |||||||
| NUpE | N1 | 0.96 * | |||||||
| N2 | 0.91 * | ||||||||
| N3 | 0.92 * |
| Wheat Lines | GRIN Accession | Improvement Status | Trait |
|---|---|---|---|
| 2174 | - | Cultivar | Dual-purpose |
| Big Sky | PI 619166 | Cultivar | Grain production |
| Deliver | PI 639232 | Cultivar | Dual-purpose |
| Duster | PI 644016 | Cultivar | Dual-purpose |
| Endurance | PI 639233 | Cultivar | Grazing-tolerant, disease-resistant |
| Mace | PI 651043 | Cultivar | Disease-resistant |
| NF00108 | - | Experimental line | |
| NF101 | - | Cultivar | Forage |
| NF97117 | - | Experimental line | |
| OCW00S063S-1B | - | Experimental line | |
| OK05511-Rhf2 | - | Experimental line | |
| OK08328 | - | Experimental line | |
| OK09520 | - | Experimental line | |
| OK0986050 | - | Experimental line | |
| OK1059060 | - | Experimental line | |
| Pete | PI 656844 | Cultivar | Dual-purpose |
| Robidoux | PI 659690 | Cultivar | Disease-resistant |
| Ron-L | - | Cultivar | Disease-resistant |
| Scout 66 | CItr 13996 | Cultivar | Disease-resistant |
| Triumph 64 | CItr 13679 | Cultivar | Disease-resistant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Schneider-Canny, R.; Chekhovskiy, K.; Kwon, S.; Saha, M.C. Opportunities for Increased Nitrogen Use Efficiency in Wheat for Forage Use. Plants 2020, 9, 1738. https://doi.org/10.3390/plants9121738
Sharma N, Schneider-Canny R, Chekhovskiy K, Kwon S, Saha MC. Opportunities for Increased Nitrogen Use Efficiency in Wheat for Forage Use. Plants. 2020; 9(12):1738. https://doi.org/10.3390/plants9121738
Chicago/Turabian StyleSharma, Nirmal, Raquel Schneider-Canny, Konstantin Chekhovskiy, Soonil Kwon, and Malay C. Saha. 2020. "Opportunities for Increased Nitrogen Use Efficiency in Wheat for Forage Use" Plants 9, no. 12: 1738. https://doi.org/10.3390/plants9121738
APA StyleSharma, N., Schneider-Canny, R., Chekhovskiy, K., Kwon, S., & Saha, M. C. (2020). Opportunities for Increased Nitrogen Use Efficiency in Wheat for Forage Use. Plants, 9(12), 1738. https://doi.org/10.3390/plants9121738





