Recent Development in Micropropagation Techniques for Rare Plant Species
Abstract
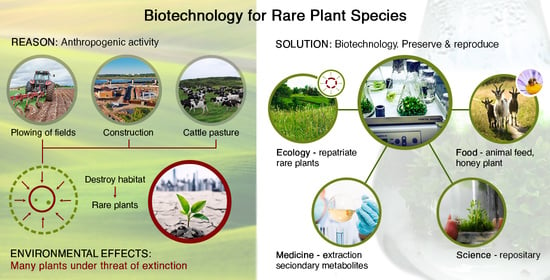
1. Introduction
2. Red-List Plant Species
3. Established Regeneration Techniques for Some of the Rare Plants
3.1. Examples of Micropropagation Protocols Used for Some of the Rare Plant Species
3.1.1. Sterilization of Artemisia hololeuca
3.1.2. Multiplication of Artemisia hololeuca
3.1.3. Rhizogenesis of Artemisia hololeuca
3.1.4. Sterilization of Hyssopus angustifolius
3.1.5. Multiplication of Hyssopus angustifolius
3.1.6. Rhyzogenesis of Hyssopus angustifolius
4. Emerging and Updated Micropropagation Techniques for Rare Plant Species
5. Future Perspectives
- conservation of the gene pool of rare plant species (cryopreservation);
- study of the resistance of “red list” plant species to various adverse abiotic and biotic environmental factors;
- repatriation of rare and endangered plant species to plant community to restore disturbed vegetation cover;
- genetic improvement of endangered plant species;
- creating hybrid forms of plants;
- design of cells through the introduction of various cellular organelles;
- synthesis of new unusual compounds;
- obtaining secondary synthesis substances that are valuable for medicine, perfumes, cosmetics, and other industries;
- a storehouse of a variety of gene pool for various breeder preferred and consumers preferred traits; especially in these rapidly changing climatic conditions.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corlett, R.T. A Bigger Toolbox: Biotechnology in Biodiversity Conservation. Trends Biotechnol. 2017, 35, 55–65. [Google Scholar] [CrossRef]
- Molkanova, O.I.; Vasilyeva, O.G.; Konovalova, L.N. The scientific basis for conservation and sustainable reproduction of plant genofond in culture in vitro. Bull. Udsu. Biol. Earth Sci. 2015, 25, 95–100. (In Russian) [Google Scholar]
- Red List of the Rostov Region. Ministry of Natural Resources and Ecology of the Rostov Region, 2nd ed.; Ministry of Natural Resources of the Rostov Region: Rostov-on-don, Russia, 2014; Volume 2. (In Russian)
- Marchese, C. Biodiversity hotspots: A shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Andreev, L.N.; Gorbunov, Y.N. Conservation of rare and endangered plants ex situ: Achievements and problems. In Study and Protection of the Divers of Fauna, Flora and Major Ecosystems of Eurasia, Proceedings of the International Conference. M., Moscow, Russia, 21–23 April 1999; Pavlov, D.S., Shatunovsky, M.I., Eds.; Moscow, Russia, 2000; pp. 19–23. ISBN 5-866695-005-7. (In Russian) [Google Scholar]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. Vitr. Cell. Dev. Biol. Plant. 2019, 12. [Google Scholar] [CrossRef]
- González-Benito, M.E.; Martín, C. In Vitro Preservation of Spanish Biodiversity. Vitr. Cell. Dev. Biol. Plant. 2011, 417, 46–54. [Google Scholar] [CrossRef]
- Vinogradova, Y.K.; Gorbunov, Y.N.; Makridin, A.I.; Molkanova, O.I. Development of principles of conservation and reproduction of genetic phytoresources. In Basic Grounds of Biological Resources Management; Rysin, L.P., Striganova, B.R., Shatunovskiy, M.I., Petrosyan, V.G., Eds.; Fellowship of Scientific Publications KMK: Moscow, Russia, 2005; pp. 343–351. (In Russian) [Google Scholar]
- Vetchinkina, E.M.; Shirnina, I.V.; Shenin, S.Y.; Molkanova, O.I. Conservation of rare plant species in genetic collections in vitro. Bull. IKBFU 2012, 7, 109–118. (In Russian) [Google Scholar]
- Novikova, T.I.; Nabieva, A.Y.; Poluboyarova, T.V. Rare and useful plants’ conservation in the in vitro collection of central Siberian botanical garden. Bull. ARSGaB 2008, 12, 564–572. (In Russian) [Google Scholar]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools. Vitr. Cell. Dev. Biol. Plant. 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Federal Law No. 16-FL of February 17, 1995 «On Ratification of the Convention on Biological Diversity», Russia. 1995.
- Convention on Biological Diversity: Text Adj, NEP/CBD/COP/8/12:38. Curitiba, Brazil, 20–31 March 2006.
- Global Strategy. Plant Conservation; 2002; Available online: www.bgci.org.uk/files/7/0/global_strategy.pdf (accessed on 28 July 2020).
- International Program of Botanical Gardens for Plant Protection. In International Council of Botanical Gardens for Plant Protection; Smirnov, I., Tikhonova, V.L., Eds.; Botanic Gardens Conservation International: Moscow, Russia, 2000; p. 57. (In Russian) [Google Scholar]
- Gorbunov, Y.N. Strategy of Botanic Gardens of Russia for the Conservation of Plant Biological Diversity; Krasnaya Zvezda: Moscow, Russia, 2003; p. 32. (In Russian) [Google Scholar]
- On Approval of the Strategy for the Conservation of Rare and Endangered Species of Animals, Plants and Fungi: Order of the Ministry of Natural Resources of the Russian Federation No. 323 of 6 April 2004. Available online: https://legalacts.ru/doc/prikaz-mpr-rf-ot-06042004-n-323/ (accessed on 28 July 2020). (In Russian).
- Red List of the Russian Federation (Plants and Fungi); Ministry of Natural Resources and Ecology of the Russian Federation; Federal Service for Supervision of Natural Resources; RAS; Russian Botanical Society; Moscow State University M.V. Lomonosov; Chief editorial board: Trutnev, Y.P. et al.; Compiled by Kamelin, R.V.; Gizatulin, R.R., Mitvol, O.L., Amirkhanov, A.M., Bardunov, L.V., Orlov, V.A., Stepanitsky, V.B., Belanovich, D.M., Varlygina, T.I., Belyakova, G.A. et al.; Fellowship of Scientific Publications KMK: Moscow, Russia, 2008; p. 855. ISBN 958-5-87317-476-8. (In Russian)
- Salas Pascual, M.; Quintana Vega, G.; Hernández Negrín, E. Isoplexis isabelliana. In Atlas y Libro Rojo de la Flora Vascular Amenazada de España; Bañares, A., Blanca, G., Güemes, J., Moreno, J.C., Ortiz, S., Eds.; Dirección General de la Conservación de la Naturaleza: Madrid, Spain, 2004; pp. 724–725. [Google Scholar]
- Bachiri, L.; Labazi, N.; Daoudi, A.; Ibijbijen, J.; Nassiri, L.; Echchegadda, G.; Mokhtari, F. Etude ethnobotanique de quelques lavandes marocaines spontanées. Int. J. Biol. Chem. Sci. 2015, 9, 1308–1318. [Google Scholar] [CrossRef]
- Fertig, W. Rare Vascular Plant Species in the Wyoming Portion of the Utah-Wyoming Rocky Mountains Ecoregion; Wyoming Nature Conservancy by the Wyoming Natural Diversity Database: Laramie, WY, USA, 2000. [Google Scholar]
- Prosperi, J.; Auricht, G.; Génier, G.; Johnson, R. Medics (Medicago L.). In Plant Genetic Resources of Legumes in the Mediterranean; Maxted, N., Bennett, S.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 99–114. [Google Scholar]
- Dransfield, J.; Beentje, H. The Palms of Madagascar; Royal Botanic Gardens Kew and The International Palm Society: Richmond, VA, USA, 1995. [Google Scholar]
- Yuzammi, T.K.N.; Handayani, T. The peculiar petiole calluses growth of Amorphophallus titanum (Becc.) Becc. ex Arcang and its implications for ex situ conservation efforts. Biotropia 2018, 25, 56–63. [Google Scholar]
- Brar, D.S. Broadening the gene pool of rice through introgression from wild species. In Rice is Life: Scientific Perspectives for the 21st Century, Proceedings of the World Rice Resolution Conference, held in Tokyo and Tsukuba, Japan, 4–7 November 2004; Toriyama, K., Heong, K.L., Hardy, B., Eds.; International Rice Research Institute (IRRI): Tsukuba, Japan, 2004; pp. 157–159. ISBN 971-22-0204-6. [Google Scholar]
- Rivero-Guerra, A.O. Cytogenetics, geographical distribution, pollen stainability and fecundity of five diploid taxa of Santolina rosmarinifolia L. aggregate (Asteraceae: Anthemideae). Plant Syst. Evol. 2009, 281, 17–34. [Google Scholar] [CrossRef]
- Omar, K. Bufonia multiceps. The IUCN Red List of Threatened Species 2017, Adama. e.T84119945A84119949. [CrossRef]
- Danova, K.; Bertoli, A.; Pistelli, L.; Dimitrov, D.; Pistelli, L. In vitro culture of Balkan endemic and rare Pulsatilla species for conservational purposes and secondary metabolites production. Bot. Serbica. 2009, 33, 157–162. [Google Scholar]
- Barkalov, V.Y.; Eremenko, N.A. Flora of the Kurilsky Nature Reserve and the Small Kurils Nature Reserve (Sakhalin Region); Dalnauka: Vladivostok, Russia, 2003; p. 285. (In Russian) [Google Scholar]
- Mikheev, A.D. Some issues of protection of Botanical objects of the Caucasian Mineral Waters region. Bull. Moip. Biol. 1979, 84, 101–110. (In Russian) [Google Scholar]
- Skripchinsky, V.V. Sternbergia Zimovnikotsvetkovaya—Sternbergia Colchiciflora Waldst. et Kit. Save for Posterity; Book Publishing House: Stavropol, Russia, 1984; Volume 65, p. 159. (In Russian) [Google Scholar]
- Kljuykov, E.V.; Tikhomirov, V.N. Aralievs—Araliaceae. Vascular plants of the Soviet Far East. Harkevich, S.S., Ed.; Nauka: Leningrad, Russia, 1987; Volume 2, pp. 195–203. (In Russian) [Google Scholar]
- Tsareva, V.T. Biological Flora of the Murmansk Region; Publishing House of the Kola Scientific Center of the RAS: Apatites, Russia, 1993; pp. 93–121. (In Russian) [Google Scholar]
- Smirnov, S.V. On the hybrid origin of Brachanthemum baranovii (Krasch. et Poljak) Krasch. (Asteraceae). In Proceedings of the VII Youth Conference of Botanists in St. Petersburg, Buslay, Germany, 15–19 May 2000; p. 38. (In Russian). [Google Scholar]
- Popov, K.P. European Hop-Hornbeam. Ostrya Carpinifolia Scop. Red List of the RSFSR (Plants). Rosagropromizdat: Moscow, Russia, 1988; pp. 90–91. (In Russian) [Google Scholar]
- Valova, Z.G.; Kurentsova, G.E. Relic Lianas in the Southwestern Primorye; Komarov Readings: Vladivostok, Russia, 1974; Volume 21, pp. 43–50. (In Russian) [Google Scholar]
- Rubtsova, T.A. Flora of Maly Khingan; Dalnauka: Vladivostok, Russia, 2002; p. 194. (In Russian) [Google Scholar]
- Melnikova, A.B.; Batalov, A.S. On the Distribution of Nuphar Japonica DC. (Nymphaceae) in the Khabarovsk Territory; MOIP Bull. Department of Biology, Publishing House of Moscow University: Moscow, Russia, 1998; Volume 103, p. 63. (In Russian) [Google Scholar]
- Red List of Ukraine. Plant World; Didukh, Y.P., Ed.; Globalconsulting: Kiev, Ukraine, 2009; p. 438. (In Ukranian) [Google Scholar]
- Shmaraeva, A.N.; Shishlova, Z.N.; Fedyaeva, V.V.; Kuzmenko, I.P. Experience of introduction of a rare species of the Rostov region Astragalus ponticus Pall.) in the Botanical garden of the southern Federal University. In Mission of Youth in Science: Collection of Abstracts of the Regional Scientific and Practical Conference-Rostov/on/D; Publishing House of the Southern Federal University: Rostov Oblast, Russia, 2013; pp. 41–45. (In Russian) [Google Scholar]
- Kozlovsky, B.L.; Fedorinova, O.I. Prospects of introduction to the culture of Calophaca wolgarica (L. fil.) Fisch. In Botanical Gardens. Problems of Introduction: Proc. Tomsk state University. Ser. Biological; Tomsk University Publishing House: Tomsk, Rostov-on-don, Russia, 2010; Volume 274, pp. 202–204. (In Russian) [Google Scholar]
- Adamec, L. How to grow Aldrovanda vesiculosa outdoors. Carniv. Pl. Newslett. 1997, 26, 85–88. [Google Scholar]
- Tsvelev, N.N. Genus Kupena—Polygonatum Mill. Flora of the European part of the USSR. L. Nauka. 1979, 4, 258–260. (In Russian) [Google Scholar]
- Lukonina, A.V.; Volodina, N.G.; Sagalaev, V.A. Nagolovatka melovaya. Red List Volgogr. Region. Volgogr. Plants Fungi 2006, 2, 62. (In Russian) [Google Scholar]
- Sluginova, I.S. Seed productivity of some rare plant species in the Cretaceous outcrops of the basin ‘Polnoy’. News Univ. North Cauc. Reg. Nat. Sci. 2008, 4, 75–79. (In Russian) [Google Scholar]
- Shmaraeva, A.N.; SHishlova, Z.H.N.; Fedyaeva, V.V. State of populations of Hyacinthella pallasiana (Stev.) Losinsk in the Rostov region. In Steppes of Northern Eurasia: Proceedings of the IV International Symp; Publishing House “IPK Gazprompechat”: Orenburg, Russia, 2006; pp. 795–799. (In Russian) [Google Scholar]
- Shmaraeva, A.N.; Shishlova, Z.H.N. State of populations of Eremurus remarkable (Eremurus spectabilis Bieb.) in the Rostov region. Conservation of Plant Biodivers. In Nature and during Introduction: Proceedings of the International Science Conference Dedicated to the 165th Anniversary of the Sukhum Botanical Garden and the 110th Anniversary of the Sukhum Subtropical Arboretum of the Institute of Botany of the ANA, Sukhum, Abkhazia, 15–20 October 2006; Publishing House of the Institute of Botany of the ANA: Sukhum, Abkhazia, 2006; pp. 666–670. (In Russian) [Google Scholar]
- Demina, O.N. Hyssopus angustifolius Bieb. on the Donets Ridge. Zhivye Biokosnye Sist. 2014, 6, 21. Available online: http://www.jbks.ru/archive/issue-6/article-12 (accessed on 28 July 2020). (In Russian).
- Demina, O.; Bragina, T. Fundamental basis for the conservation of biodiversity of the Black Sea-Kazakh steppes. Hacquetia 2014, 13, 215–228. (In Russian) [Google Scholar] [CrossRef]
- Fedchenko, B.A. Wormwood—Artemisia. In Flora of the USSR; Publishing House of the USSR Academy of Sciences: Leningrad, Russia, 1948; Volume 26, pp. 301–318. (In Russian) [Google Scholar]
- Zemlyanuhina, O.A.; Kalaev, V.N.; Lepeshkina, L.A.; Karpechenko, K.A.; Veprintsev, V.N.; Serikova, V.I. Physiological and biochemical characteristics of some species of Artemisia in the culture of Botanical garden of Voronezh state university. Fundam. Study 2012, 5, 143–147. (In Russian) [Google Scholar]
- Mamontov, A.K. Cultivation of calciphilic species outside native area and new method of creation of rockeries as ecotrons. Sci. Rep. Ser. Nat. Sci. 2015, 3, 23–29. (In Russian) [Google Scholar]
- Sidorova, L.A. Heterogeneity of the structural organization of coenopopulations of Artemisia hololeuca BIEB. EX BESS. (ASTERACEAE) in time and space. Bull. VSPU. Ser. 11 2011, 1, 30–35. (In Russian) [Google Scholar]
- Mamontov, A.K.; Ryabchenko, A.S. Features of morphology of seeds and fruits of some rare calcifilous species of European Russia. Bull. VSU Ser. Chem. Biol. Pharm. 2017, 4, 56–61. (In Russian) [Google Scholar]
- Fedchenko, B.A. Hyssop—Hyssopus. Flora Ussr. Publ. House Ussr. Acad. Sci. Mosc. L. 1948, 20, 451–452. (In Russian) [Google Scholar]
- Hatipoglu, G.; Sökmen, M.; Bektaş, E.; Daferera, D.; Sökmen, A.; Demir, E.; Şahin, H. Automated and standard extraction of antioxidant phenolic compounds of Hyssopus officinalis L. ssp. angustifolius. Ind. Crops Prod. 2013, 43, 427–433. [Google Scholar] [CrossRef]
- Aghaei, K.; Pirbalouti, A.G.; Mousavi, A.; Badi, H.N.; Mehnatkesh, A. Effects of foliar spraying of l-phenylalanine and application of bio-fertilizers on growth, yield, and essential oil of hyssop [Hyssopus officinalis L. subsp. Angustifolius (Bieb.)]. Biocatal. Agric. Biotechnol. 2019, 21, 10. [Google Scholar] [CrossRef]
- Waller, S.B.; Cleff, M.B.; Serra, E.F.; Silva, A.L.; dos Gomes, A.R.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Plants from Lamiaceae Family as Source of Antifungal Molecules in Humane and Veterinary Medicine. Microb. Pathog. 2017, 104, 232–237. [Google Scholar] [CrossRef]
- Murakami, Y.; Omoto, T.; Asai, I.; Shimomura, K.; Yoshihira, K.; Ishimaru, K. Rosmarinic acid and related phenolics in transformed root cultures of Hyssopus officinalis. Plant Cell Tissue Organ Cult. 1998, 53, 75–78. [Google Scholar] [CrossRef]
- Hilal, S.H.; El Alfy, T.S.; El Sherei, M.M. Investigation of the volatile oil of Hyssopus officinalis. Egypt. J. Pharm. Sci. 1978, 19, 177–184. [Google Scholar]
- Nanova, Z.; Slavova, Y.; Nenkova, D.; Ivanova, I. Microclonal propagation of hyssop (Hyssopus officinalis L.). Bulg. J. Agric. Sci. 2007, 13, 213–219. [Google Scholar]
- Stankovic’, N.; Mihajilov-Krstev, T.; Zlatkovic’, B.; Stankov-Jovanovic´, V.; Mitic´, V.; Jovic´, J.; Comi, L.; Kocic´, B.; Bernsteine, N. Antibacterial and Antioxidant Activity of Traditional Medicinal Plants from the Balkan Peninsula. Njas Wagening J. Life Sci. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Alinezhad, H.; Baharfar, R.; Zare, M.; Azimi, R.; Nabavi, S.F.; Nabavi, S.M. Biochemical activities of acetone extracts of Hyssopus angustifolius. Acta Pol. Pharm. Drug Res. 2012, 69, 617–622. [Google Scholar]
- Nabavi, S.F.; Nabavi, S.M.; Hellio, C.; Alinezhad, H.; Zare, M.; Azimi, R.; Baharfar, R. Antioxidant and antihemolytic activities of methanol extract of Hyssopus angustifolius. J. Appl. Bot. Food Qual. 2012, 85, 4. [Google Scholar]
- Kotyuk, L.A. Biochemical features of Hyssopus angustifolius under the conditions of introduction in Polesie of Ukraine. Optim. Prot. Ecosyst. 2014, 10, 94–98. [Google Scholar]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. (Eds.) PDR for Herbal Medicines; Medical Economics Co.: Montvale, NJ, USA, 2000; pp. 414–415. ISBN 1-56363-361-2. [Google Scholar]
- Gollapudi, S.; Sharma, H.A.; Aggarwal, S.; Byers, L.D.; Ensly, H.E.; Gupta, S. Isolation of a previously unidentified polysaccharide (MAR-10) from Hyssop officinalis that exhibits strong activity against human immunodeficiency virus type 1. Biochem. Biophys. Res. Commun. 1995, 210, 145–151. [Google Scholar] [CrossRef]
- Maxted, N.; Ford-Lloyd, B.V.; Hawkes, J.G. Complementary conservation strategies. In Plant Genetic Conservation. The In Situ Approach; Maxted, N., Ford-Lloyd, B.V., Hawkes, J.G., Eds.; Chapman: London, UK, 1997; pp. 15–41. [Google Scholar]
- Laslo, V.; Zăpвrţan, M.; Agud, E. In vitro conservation of certain endangered and rare species of romanian spontaneous flora. An. Univ. Oradea Fasc. Protecţia Mediu. 2011, XVI, 252–261. [Google Scholar]
- Pence, V.C.; Finke, L.R.; Chaiken, M.F. Tools for the ex situ conservation of the threatened species, Cycladenia Humilis Var. Jonesii. Conserv. Physiol. 2017, 5, cox053. [Google Scholar] [CrossRef]
- Leung, D.W.M. Plant biotechnology helps quest for sustainability: With an emphasis on climate change and endangered plants. In Climate Change and Sustainable Development; Reck, R., Ed.; Linton Atlantic Books: Louisville, KY, USA, 2010; pp. 247–250. [Google Scholar]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol. Plant. 2011, 47, 5–16. [Google Scholar] [CrossRef]
- González-Benito, M.E.; Clavero-Ramírez, I.; López-Aranda, J.M. Review. The use of cryopreservation for germplasm conservation of vegetatively propagated crops. Span. J. Agric. Res. 2004, 2, 341–351. [Google Scholar] [CrossRef]
- Matsumoto, T. Cryopreservation of plant genetic resources: Conventional and new methods. Rev. Agric. Sci. 2017, 5, 13–20. [Google Scholar] [CrossRef]
- Rani, V.; Raina, S.N. Genetic fidelity of organized meristem derived micropropagated plants: A critical reappraisal. Vitr. Cell Dev. Biol. Plant. 2000, 36, 319–330. [Google Scholar] [CrossRef]
- Cordeiro, S.Z.; Simas, N.K.; Henriques, A.B.; Sato, A. In vitro conservation of Mandevilla moricandiana (Apocynaceae): Short-term storage and encapsulation–dehydration of nodal segments. Vitr. Cell Dev. Biol. Plant. 2014, 50, 326–336. [Google Scholar] [CrossRef]
- Molkanova, O.I.; Korotkov, O.I.; Vetchinkina, E.M.; Mamayeva, N.A.; Vasilieva, O.G. Genetic banks of plants: Problems of formation, conservation and use. Bull. UdSU 2010, 3, 33–39. (In Russian) [Google Scholar]
- Sujatha, G.; Ranjitha Kumari, B.D. Micropropagation, encapsulation and growth of Artemisia vulgaris node explants for germplasm preservation. S. Afr. J. 2008, 74, 93–100. [Google Scholar] [CrossRef]
- Mathur, A.K.; Kumar, S. Micropropagation of Artemisia annua via the Inflorescence. J. Herbs Spices Med. Plants. 1996, 4, 61–71. [Google Scholar] [CrossRef]
- Shinde, S.; Sebastian, J.K.; Jain, J.R.; Hanamanthagouda, M.S.; Murthy, H.N. Efficient in vitro propagation of Artemisia nilagirica var. nilagirica (Indian wormwood) and assessment of genetic fidelity of micropropagated plants. Physiol. Mol. Biol. Plants 2016, 22, 595–603. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Pietrosiuk, A. Artemisia species in vitro cultures for production of biologically active secondary metabolites. Biotechnology 2012, 93, 371–380. [Google Scholar] [CrossRef]
- Cruz-Cruz, C.A.; Gonzбlez-Arnao, M.T.; Engelmann, F. Biotechnology and conservation of plant biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Fahy, G.M.; MacFarlane, D.R.; Angell, C.A.; Meryman, H.T. Vitrification as an approach to cryopreservation. Cryobiology 1984, 21, 407–426. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 437–497. [Google Scholar] [CrossRef]
- Skirvin, R.M.; Chu, M.C.; Mann, M.L.; Young, H.; Sullivan, J.; Fermanian, T. Stability of tissue culture medium pH as a function of autoclaving, time, and cultured plant material. Plant Cell Rep. 1986, 5, 292–294. [Google Scholar] [CrossRef]
- Lloyd, G.B.; McCown, B.H. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int. Plant. Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Cerovi, R.; Runic, J. Micropropagation of sour cherry (Prunus cerasus L.) cv. Sumadinka. Plant Cell Tissue Organ Cult. 1987, 51, 157. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Quorin, M.; Lepivre, P.; Bonus, P. Un premier bilan de 10 annees de recherches sur les “cultures” de meristems et la multiplication in vitro de fruitiers ligneux. In C.R. des Recherches 1976–1977. Station des Cultures Fruitières et Maraîchères; 1977; pp. 93–117. [Google Scholar]
- Sereda, M.M.; Kozlovsky, B.L.; Lutsenko, E.V. Promising technology of propagation of Spanish sycamore for green construction in the South-West of the Rostov region. Engineering Bull. Don. 2014, 31, 1–9. (In Russian) [Google Scholar]
- Fay, M.F. Conservation of rare and endangered plants using in vitro methods. Vitr. Cell Dev. Biol. Plant. 1992, 28, 1–4. [Google Scholar] [CrossRef]
- Singh, R.K.; Anandhan, S.; García-Pérez, L.M.; Ruiz-May, E.; Pérez, E.N.; Quiroz-Figueroa, F.R. An efficient protocol for in vitro propagation of the wild legume Cicer microphyllum Benth, a crop wild relative of chickpea (Cicer arietinum L.). Vitr. Cell Dev. Biol. Plant. 2019, 55, 9–14. [Google Scholar] [CrossRef]
- Singh, R.K.; Anandhan, S.; Singh, S.; Patade, V.Y.; Ahmed, Z.; Pande, V. Metallothionein-like gene from Cicer microphyllum is regulated by multiple abiotic stresses. Protoplasma 2011, 248, 839–847. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, S.; Pandey, P.; Anandhan, S.; Goyary, D.; Pande, V.; Ahmed, Z. Construction of cold induced subtracted cDNA library from Cicer microphyllum and transcript characterization of identified novel wound induced gene. Protoplasma 2013, 250, 459–469. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, S.; Anandhan, S.; Shannon, L.M.; Quiroz-Figueroa, F.R.; Ruiz-May, E. First insights into the biochemical and molecular response to cold stress in Cicer microphyllum, a crop wild relative of chickpea (Cicer arietinum). Russ. J. Plant. Physiol. 2017, 64, 758–765. [Google Scholar] [CrossRef]
- Singh, R.K.; Bohra, N.; Sharma, L.; Anandhan, S.; Ruiz-May, E.; Quiroz-Figueroa, F.R. Inspection of Crop Wild Relative (Cicer microphyllum) as Potential Genetic Resource in Transgenic Development. In Advances in Plant Transgenics: Methods and Applications; Sathishkumar, R., Kumar, S., Hema, J., Baskar, V., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef]
- Yoon, Y.-J.; Murthy, H.N.; Hahn, E.J.; Paek, K.Y. Biomass production of Anoectochilus formosanus hayata in a bioreactor system. J. Plant Biol. 2007, 50, 573–576. [Google Scholar] [CrossRef]
- Arano-Avalosa, S.; Gómez-Merinoa, F.C.; Mancilla-Álvarezb, E.; Sánchez-Páeza, R.; Bello-Belloc, J.J. An efficient protocol for commercial micropropagation of malanga (Colocasia esculenta L. Schott) using temporary immersion. Sci. Hortic. Amst. 2020, 261, 6. [Google Scholar] [CrossRef]
- Debnath, S.C. Thidiazuron in micropropagation of small fruits. In Thidiazuron: Urea Deriv. Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Bayraktar, M. Micropropagation of Stevia rebaudiana Bertoni Using RITA® Bioreactor. Hortic. Sci. 2019, 54, 725–731. [Google Scholar] [CrossRef]
- Almusawi, A.H.A.; Sayegh, A.J.; Alshanaw, A.M.S.; Griffis, J.L., Jr. Plantform Bioreactor for Mass Micropropagation of Date Palm. Methods Mol. Biol. 2017, 1637, 251–265. [Google Scholar]
- Shandil, A.S.; Tuia, V.S. Micropropagation of breadfruit (A. altilis) enhanced using a bioreactor system. Acta Hortic. 2015, 1101, 159–164. [Google Scholar] [CrossRef]
- Benson, E.E. Do free radicals have a role in plant tissue culture recalcitrance? Vitr. Cell Dev. Biol. Plant. 2000, 36, 163–170. [Google Scholar] [CrossRef]
- Nguyen, H. In vitro physiology of recalcitrant tissue cultured plants in the Nymphaeaceae, Alismataceae, and Orchidaceae. In A Dissertation Presented to the Graduate School of The University of Florida in Partial fulfillment of the Requirements for the Degree of Doctor of Philosophy; University of Florida: Gainesville, FL, USA, 2016. [Google Scholar]
- Jenks, M.; Kane, M.; Marousky, F.; McConnell, D.; Sheehan, T. In vitro establishment and epiphyllous planet regeneration of Nymphaea ‘Daubeniana’. Hortic. Sci. 1990, 25, 1664. [Google Scholar]
- Harrap, A.; Harrap, S. Orchids of Britain and Ireland A Field and Site Guide; A&C Black Publishers Ltd.: London, UK, 2005. [Google Scholar]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. Amst. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Yaseen, M.; Tajuddin, K. Effect of plant growth regulators on yield, oil composition and artemisinina of Artemisia annua under temperate conditions. J. Med. Aromat. Plant Sci. 1998, 1, 113–116. [Google Scholar]
- Moorthy, P.; Kathiresan, K. Physiological responses of a mangrove seedling to tricontanol. Biol. Plant. 1993, 35, 577–581. [Google Scholar] [CrossRef]
- Balyan, S.S.; Pal, S.; Dutt, P. Triacontanol effect in growth and yield parameters of CKP-25 variety lemongrass w. Indian Perfum. 1994, 36, 60–64. [Google Scholar]
- Malabadi, R.B.; Mulgund, G.S.; Kallappa, N. Micropropagation of Dendrobium nobile from shoot tip sections. J. Plant Physiol. 2005, 162, 473–478. [Google Scholar] [CrossRef]
- Mitrofanova, I.; Moroz, L. Development of the protocol for protoplast isolation from lavender and lavandin plants cultured In Vitro. J. Biotechnol. 2018, 280, 83. [Google Scholar] [CrossRef]
- Padilla, I.M.G.; Encina, C.L. The use of consecutive micrografting improves micropropagation of cherimoya (Annona cherimola Mill.) ciltivars. Sci. Hortic. Amst. 2011, 129, 167–169. [Google Scholar] [CrossRef]
- Bayraktar, M.; Hayta-Smedley, S.; Unal, S.; Varol, N.; Gurel, A. Micropropagation and prevention of hyperhydricity in olive (Olea europaea L.) cultivar ‘Gemlik’. S. Afr. J. 2020, 128, 264–273. [Google Scholar] [CrossRef]
- Herman, E.B. Beneficial effects of bacteria and fungi on plant tissue cultures. Agricell Rep. 1996, 27, 26–27. [Google Scholar]
- Kanani, P.; Modi, A.; Kumar, A. Biotization of Endophytes in Micropropagation; A Helpful Enemy; Series in Food Science, Technology and Nutrition; Woodhead Publishing: Elsevier, The Netherlands, 2020; pp. 357–379. [Google Scholar] [CrossRef]
- Alonzo, M.; Bookhagen, B.; Roberts, D.A. Urban tree species mapping using hyperspectral and lidar data fusion. Remote Sens. Environ. 2014, 148, 70–83. [Google Scholar] [CrossRef]
- Ortiz, S.M.; Breidenbach, J.; Knuth, R.; Kändler, G. The influence of DEM quality on mapping accuracy of coniferous- and deciduous-dominated forest using TerraSAR-X images. Remote Sens. 2012, 4, 661–681. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Latifi, H.; Sterenczak, K.; Modzelewska, A.; Lefsky, M.; Waser, L.T.; Straub, C.; Ghosh, A. Review of studies on tree species classification from remotely sensed data. Remote Sens. Environ. 2016, 186, 64–87. [Google Scholar] [CrossRef]
- Marques, P.; Pádua, L.; Adão, T.; Hruška, J.; Peres, E.; Sousa, A.; Sousa, J.J. UAV-Based Automatic Detectionand Monitoring of Chestnut Trees. Remote Sens. 2019, 11, 855. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Neumann, C.; Förster, M.; Buddenbaum, H.; Ghosh, A.; Clasen, A.; Joshi, P.K.; Koch, B. Comparison of feature reduction algorithms for classifying tree species with hyperspectral data on threecentral European test sites. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2547–2561. [Google Scholar] [CrossRef]
- Bareth, G.; Aasen, H.; Bendig, J.; Gnyp, M.L.; Bolten, A.; Jung, A.; Michels, R.; Soukkamäki, J. Low-weight and UAV-based hyperspectral full-frame cameras for monitoring crops: Spectral comparison with portable spectroradiometer measurements. Photogr. Fernerkund. Geoinf. 2015, 1, 69–79. [Google Scholar] [CrossRef]
- Aasen, H.; Burkart, A.; Bolten, A.; Bareth, G. Generating 3D hyperspectral information with lightweight UAV snapshot cameras for vegetation monitoring: From camera calibration to quality assurance. ISPRS J. Photogramm. Remote Sens. 2015, 108, 245–259. [Google Scholar] [CrossRef]

| Species | Family | IUCN Red List Category and Criteria or Status of Rare Plants in Russia | Threats | Use and Trade | Citation |
|---|---|---|---|---|---|
| Isoplexis isabelliana | Scrophulariaceae | Endangered B2ab(iii); C2a(i) | Housing and urban areas, livestock farming and ranching, invasive non-native/alien species/diseases | Medicine—human and veterinary, food—animal | [21] |
| Lavandula maroccana | Lamiaceae | Vulnerable B2ab(ii,iii,iv) | Tourism and recreation areas, annual and perennial nontimber crops, gathering terrestrial plants, work and other activities, habitat shifting and alteration. | Medicine—human and veterinary, food—human, food—animal | [22] |
| Cypripedium montanum | Orchidaceae | Vulnerable B2ab(ii,iii,iv,v) | Tourism and recreation areas, annual and perennial nontimber crops, wood and pulp plantations, livestock farming and ranching, agricultural and forestry effluents | Medicine—human and veterinary, pets/display animals, horticulture | [23] |
| Medicago sativa | Fabaceae | Least concern | Hybridization/introgression between the crop and wild populations | Medicine—human and veterinary, food—human, food—animal | [24] |
| Voanioala gerardii | Arecaceae | Critically endangered D | Annual and perennial nontimber crops, gathering terrestrial plants, logging and wood harvesting | Pets/display animals, horticulture, food—human | [25] |
| Amorphophallus titanum | Araceae | Endangered A2ac; C2a(i); D | Annual and perennial nontimber crops, gathering terrestrial plants logging and wood harvesting, fire and fire suppression | Fire and fire suppression, medicine—human and veterinary, food—human | [26] |
| Oryza officinalis | Poaceae | Least concern | Commercial and industrial areas, renewable energy, dams and water management/use, storms and flooding | Food—human | [27] |
| Santolina oblongifolia | Asteraceae | Near threatened | Livestock farming and ranching, gathering terrestrial plants, fire and fire suppression, problematic species/disease of unknown origin | Food—animal, medicine—human and veterinary | [28] |
| Bufonia multiceps | Caryophyllaceae | Endangered B1ab(i,ii,iii,v) + 2ab(i,ii,iii,v); C2a(i) | Livestock farming and ranching, gathering terrestrial plants, dams and water management/use, recreational activities, work and other activities, invasive nonnative/alien species/diseases | Food—animal, medicine—human and veterinary | [29] |
| Anemone halleri | Ranunculaceae | Least concern | Gathering terrestrial plants, recreational activities | Medicine—human and veterinary, pets/display animals, horticulture, establishing ex situ production | [30] |
| Acer japonicum | Sapindaceae | 1—Species under threat of extinction | Small number of individuals in the population, lack of seed renewal | Ornamental plant. | [31] |
| Galanthus caucasicus (Baker) Grossh. | Amaryllidaceae | 3 d—Rare species with a limited range, part of which is located on the territory of Russia | Suffers from mass harvesting of flowering plants and the digging of bulbs for commercial purposes. Habitat disturbance, deforestation. Low seed productivity. Weak frost resistance. | Decorative, honey-bearing, poisonous, medicinal plant. | [32] |
| Sternbergia colhiciflora Waldst. et Kit. | Amaryllidaceae | 1—Species under threat of extinction | Destruction of natural habitats during economic activities (plowing, construction, etc.). Weak seed renewal. Suffers from collecting and digging bulbs. | Ornamental plant. | [33] |
| Kalopanax septemlobus (Thunb.) Koidz. | Araliaceae | 3 g—A rare subtropical species on the northern border of distribution, with a significant gap from the main range | Felling for valuable wood, forest fires, irregular seed maturation, poor vegetative reproduction, reduced ability to reproduce seeds. | Medicinal, food plant, wood is used for making musical instruments. | [34] |
| Arnica alpina (L.) Olin | Asteraceae | 2 a—Species that is declining in number as a result of changing living conditions | Low vitality due to weakened seed renewal, narrow ecological amplitude. Industrial development of mountain ranges, increasing recreational loads. | Ornamental plant. | [35] |
| Brachanthemum baranovii (Krasch. et Pol.) Krasch. excl. typo | Asteraceae | 1—Species under threat of extinction | Narrow ecological range of the species, weak seed renewal. Mining and road works, grazing. | Ornamental plant. Endemic to the Altai Mountains. | [36] |
| Ostrya carpinifolia Scop. | Betulaceae | 2 a—Species that is declining in number as a result of changing living conditions | Deforestation, fires, and uncontrolled grazing. Strict adherence to carbonate rocks, weak seed renewal, low germination, late entry into fruiting, low competitiveness. | Source of timber. | [37] |
| Pueraria lobata (Willd.) Ohwi | Fabaceae | 3 g—Rare subtropical species on the northern border of distribution, with a significant gap from the main range | Instability to frost. Small and disjointed populations, low seed productivity, economic activity, fires and grazing | Ornamental plant, and soil-strengthening plants. | [38] |
| Adlumia asiatica Ohwi. | Papaveraceae | 2 a—Species that is declining in number as a result of changing living conditions | Difficult seed reproduction. Weak competitive ability of the species. Human economic activity (deforestation, plowing of land), recreation, territory development. | Ornamental plant | [39] |
| Nuphar japonica DC. | Nymphaeaceae | 1—Species under threat of extinction | Natural—limited range, sensitivity to water pollution, changes in the water regime, poor seed productivity. Anthropogenic impact. | Ornamental and medicinal plants. | [40] |
| Astragalus dasyanthus Pall. | Fabaceae | 4—Undefined by status | Seed productivity is low, and seeds are often damaged by pests and diseases | A valuable medicinal, honey-bearing, ornamental plant. | [41] |
| Astragalus ponticus Pall. | Fabaceae | 3 e—Rare species having a limited range, part of which is located on the territory of the Rostov region | Seed productivity is low. The coefficient of semnificatia—13% | Ornamental plant | [42] |
| Calophaca wolgarica (L. f.) DC. | Fabaceae | 2—Declining in number. Taxa with steadily decreasing numbers, which, under the further influence of factors that reduce the number, may in a short time fall into the category of endangered: 2 a—taxa whose number is reduced as a result of changes in the conditions of existence or destruction of habitats | Seed productivity is low, field germination of seeds is 25–60% | Decorative, honey-bearing, fibrous, antierosion plant; promising for introduction to gardening | [43] |
| Aldrovanda vesiculosa L. | Droseraceae | 1 b—Taxa and populations that are at high risk of loss due to extremely low numbers and/or narrow range or extremely limited number of locations. | Seed productivity is low due to the need for high water temperature for normal development (23–30 °C), at 17 °C growth is inhibited | Ornamental, forage, and aquarium. | [44] |
| Polygonatum multiflorum (L.) All. | Convallariaceae | 3 d—Having a significant range, but located in the Rostov region on the border of distribution | Seed productivity is low | Medicinal, ornamental plant, cosmetic, poisonous plant | [45] |
| Jurinea cretacea Bunge (incl. J. talievii Klok.) | Asteraceae | 3 b (3). Rare species with a narrow ecological amplitude associated with a specific substrate for production | Seed productivity is low. The coefficient of semnificatia—33.5% | Ornamental plant | [46] |
| Erysimum cretaceum (Rupr.) Schmalh. (E. ucranicum auct. non J. Gay) | Brassicaceae | 3 c, d—Rare species with a narrow ecological amplitude, associated with a specific substrate for growth and having a limited range, part of which is located on the territory of the Rostov region; Pliocene relic | Seed productivity is low. The coefficient of semnificatia from 10.5 to 46.5% | Ornamental plant, antierosion plant | [47] |
| Iris pumila L. [I. pumila L. subsp. taurica (Llod.) Rodion. & Shewcz., I. taurica Llod.] | Iridaceae | 2 a—Declining in number. Taxa whose number is reduced as a result of changes in the conditions of existence or destruction of habitats | Seed productivity is low. The coefficient of semnificatia 33.3% | Ornamental plant | [47] |
| Hyacinthella pallasiana (Stev.), Losinsk. | Hyacinthaceae | 3 c, d—Rare species with a narrow ecological amplitude, associated with a specific substrate for growth and having a limited range, part of which is located on the territory of the Rostov region; Pliocene relic | Seed productivity is low. The coefficient of semnificatia 55–60% | Ornamental plant | [48] |
| Eremurus spectabilis Bieb. | Asphodelaceae | 2 a—Declining in number. Taxa whose number is reduced as a result of changes in the conditions of existence or destruction of habitats | Seed productivity is low. The coefficient of semnificatia 16.5–60% | Medicinal, ornamental plant, | [49] |
| MS | MS + 1 mg/L BAP | MS + Vitamins B5 + Sucrose 3% + 1 mg/L BAP | ½ MS | 1/4 MS + 2 mg/l BAP | ½ MS + 0.5 mg/L BAP + 0.1 mg/L IBA |
|---|---|---|---|---|---|
| 3.4 ± 0.31 | 3.8 ± 0.9 | 8.3 ± 1.6 | 4.0 ± 0.63 | 3.4 ± 0.62 | 17.0 ± 1.0 |
| MS + 2 mg/L IBA | MS + 2 mg/L IAA | MS + 5 g/L IBA | MS + 0.05 mg/L BAP + 0.5 mg/L NAA | MS + 0.025 mg/L NAA | MS |
|---|---|---|---|---|---|
| 20% | 10% | 0% | 10% | 10% | 20% |
| QL | QL + 0.2 mg/L BAP | QL + 0.5 mg/L BAP | MS | MS + 0.2 mg/L BAP | MS + 0.5 mg/L BAP |
|---|---|---|---|---|---|
| 4.5 ± 0.69 | 6.5 ± 1.11 | 7.3 ± 0.75 | 4.0 ± 0.71 | 2.2 ± 0.59 | 1.0 ± 0.80 |
| Medium | Percentage of Rooting |
|---|---|
| ½ QL | 30% |
| QL | 20% |
| ½ QL + 1 mg/L NAA | 10% |
| QL + 1 mg/L NAA | 5% |
| ½ MS | 40% |
| MS | 40% |
| ½ MS + 1 mg/L NAA | 30% |
| MS + 1 mg/L NAA | 20% |
| ½ WPM | 20% |
| WPM | 30% |
| ½ WPM + 1 mg/L NAA | 30% |
| WPM + 1 mg/L NAA | 20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. https://doi.org/10.3390/plants9121733
Chokheli VA, Dmitriev PA, Rajput VD, Bakulin SD, Azarov AS, Varduni TV, Stepanenko VV, Tarigholizadeh S, Singh RK, Verma KK, et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants. 2020; 9(12):1733. https://doi.org/10.3390/plants9121733
Chicago/Turabian StyleChokheli, Vasiliy A., Pavel A. Dmitriev, Vishnu D. Rajput, Semyon D. Bakulin, Anatoly S. Azarov, Tatiana V. Varduni, Victoria V. Stepanenko, Sarieh Tarigholizadeh, Rupesh Kumar Singh, Krishan K. Verma, and et al. 2020. "Recent Development in Micropropagation Techniques for Rare Plant Species" Plants 9, no. 12: 1733. https://doi.org/10.3390/plants9121733
APA StyleChokheli, V. A., Dmitriev, P. A., Rajput, V. D., Bakulin, S. D., Azarov, A. S., Varduni, T. V., Stepanenko, V. V., Tarigholizadeh, S., Singh, R. K., Verma, K. K., & Minkina, T. M. (2020). Recent Development in Micropropagation Techniques for Rare Plant Species. Plants, 9(12), 1733. https://doi.org/10.3390/plants9121733