Effects of Phosphate Shortage on Root Growth and Hormone Content of Barley Depend on Capacity of the Roots to Accumulate ABA
Abstract
1. Introduction
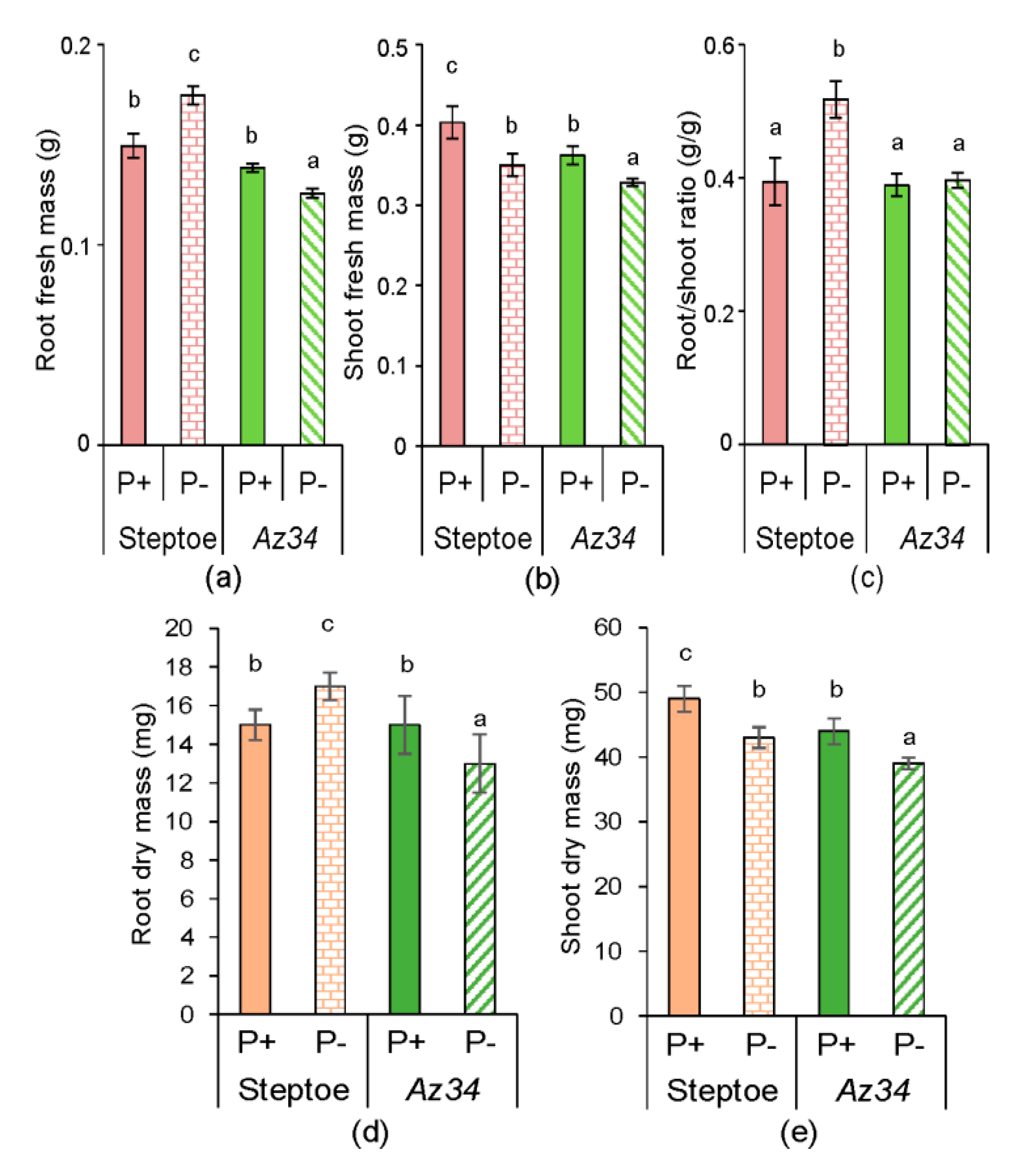
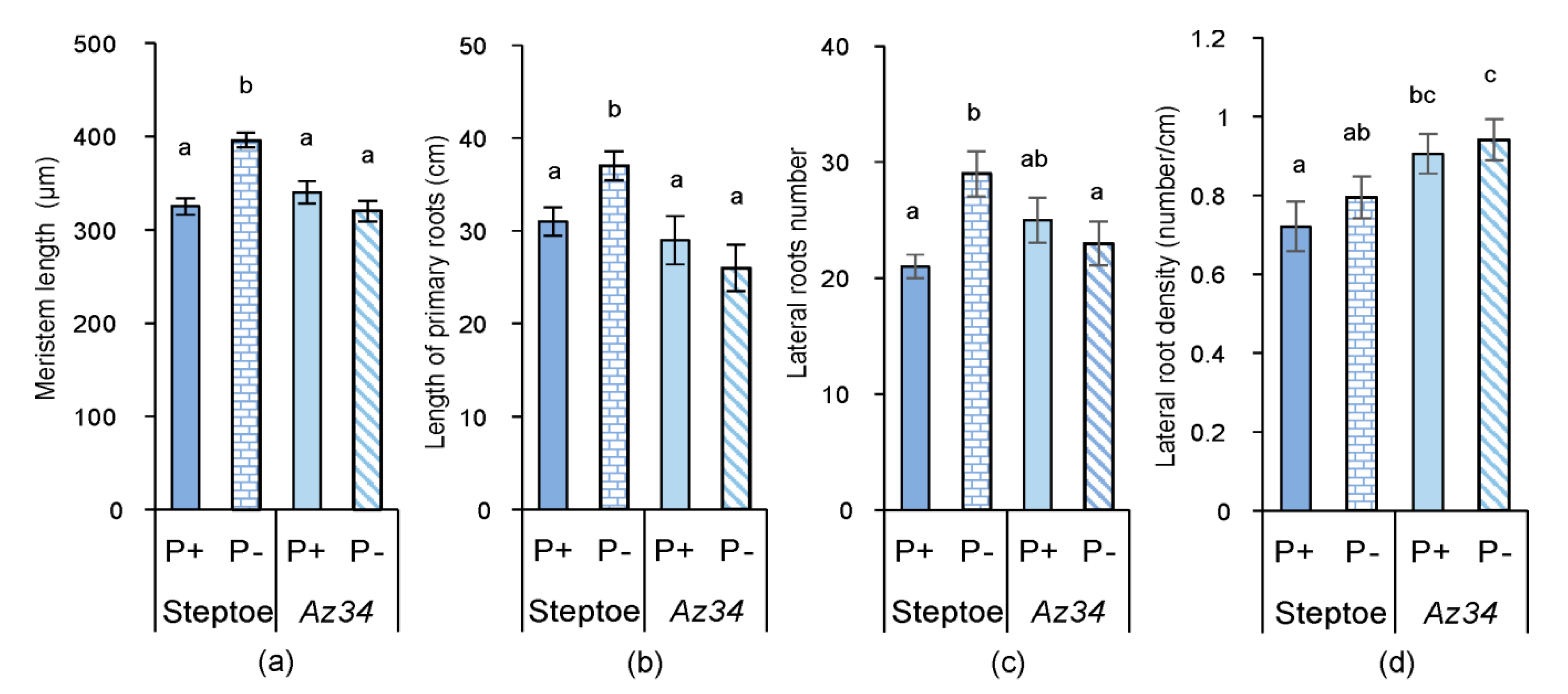
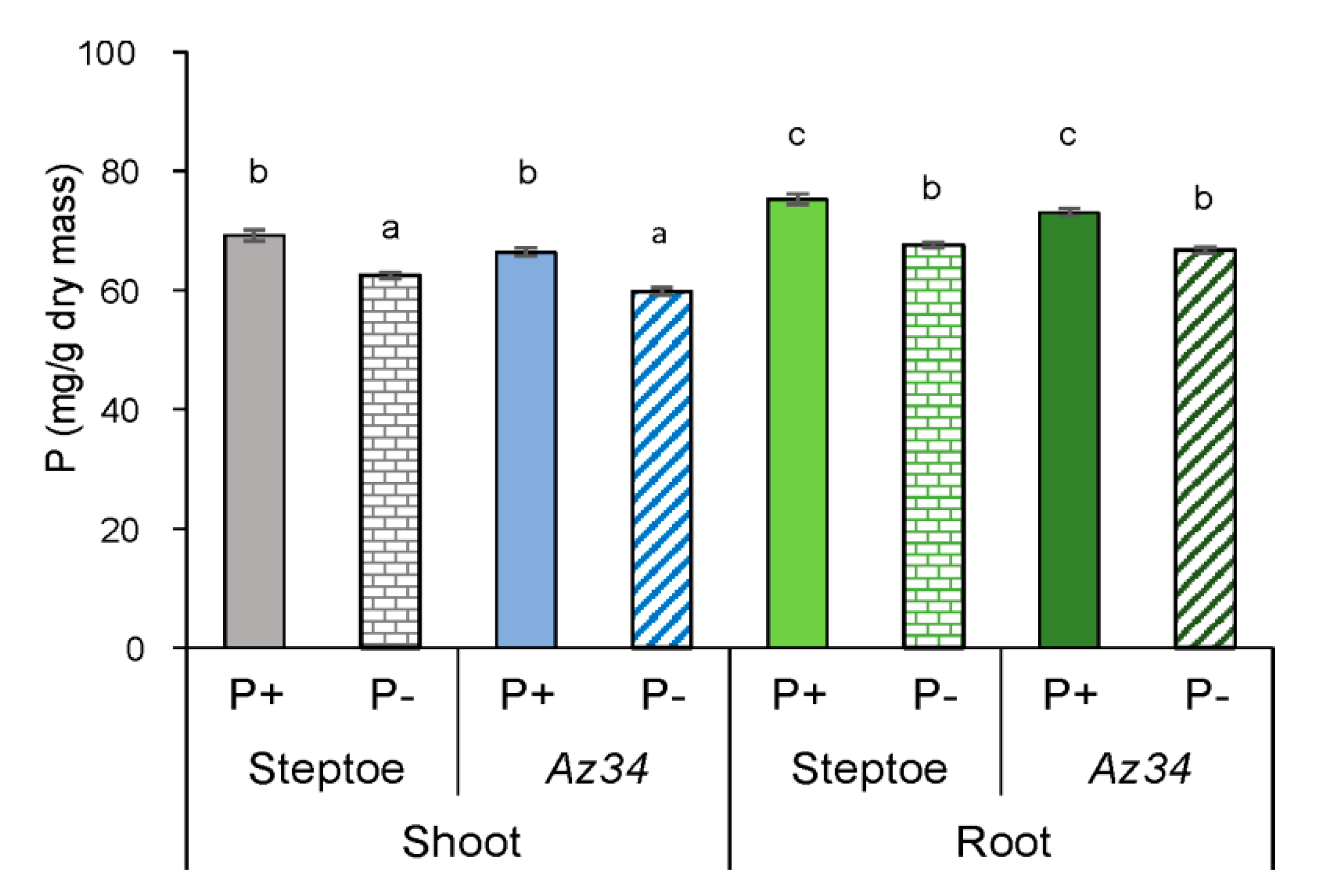
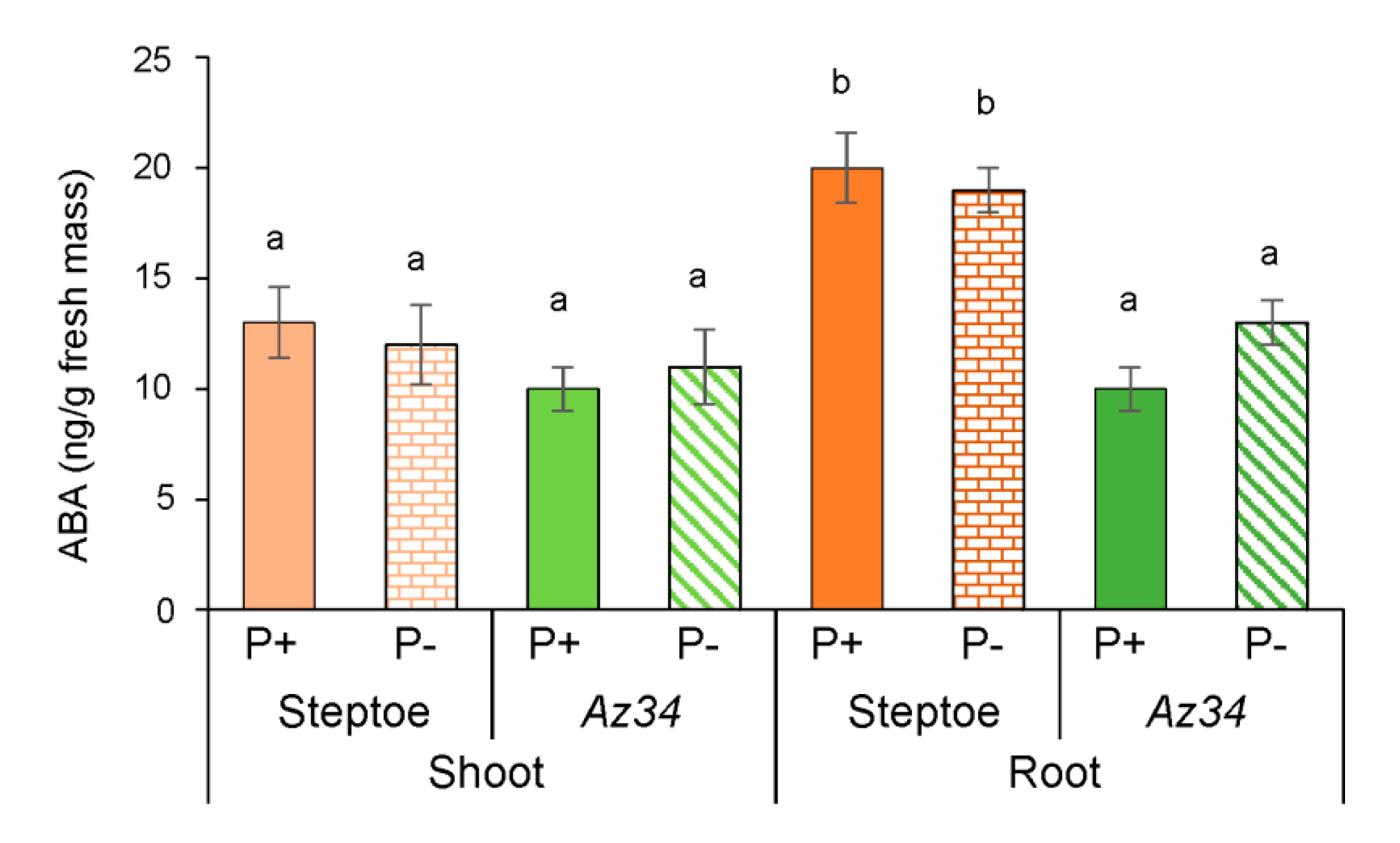
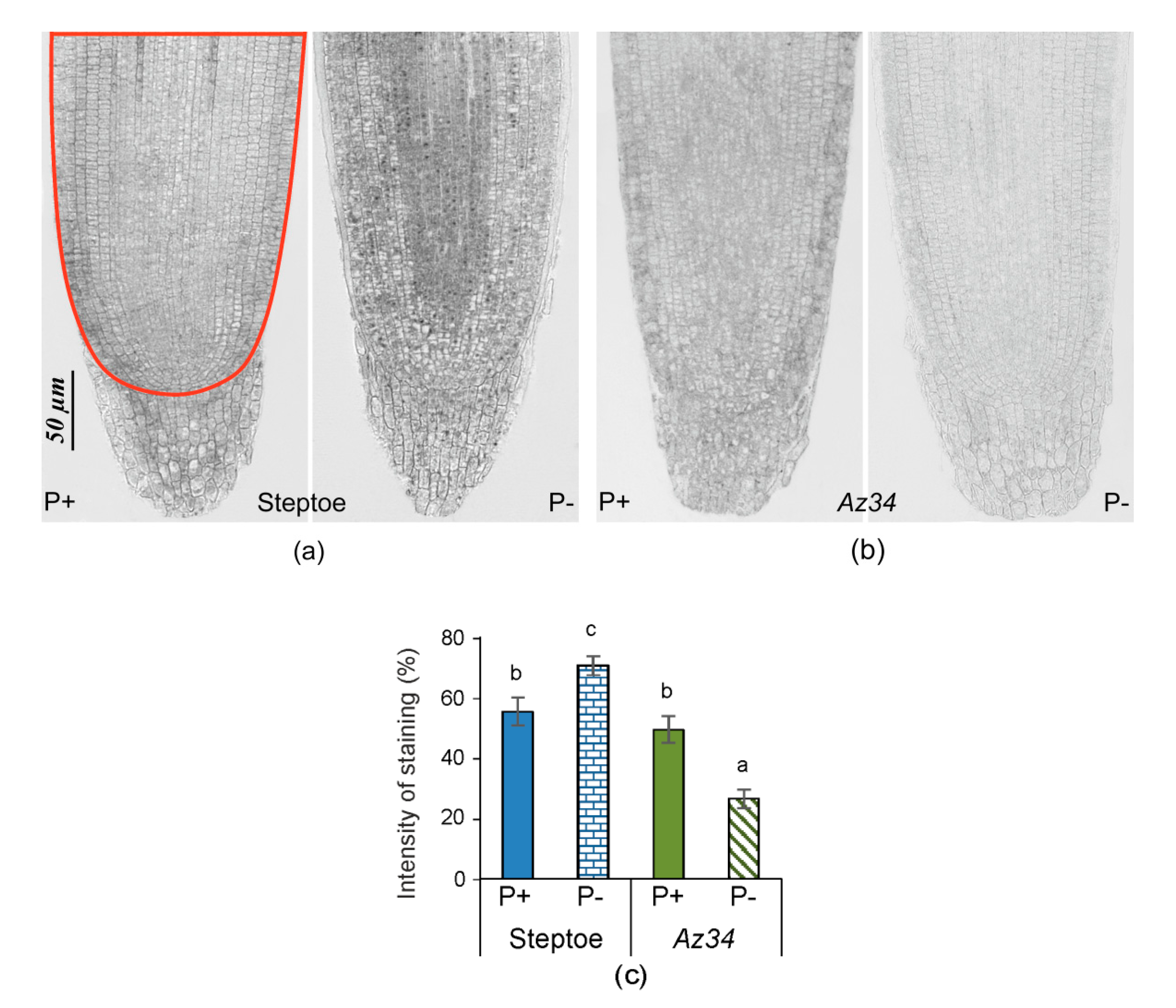
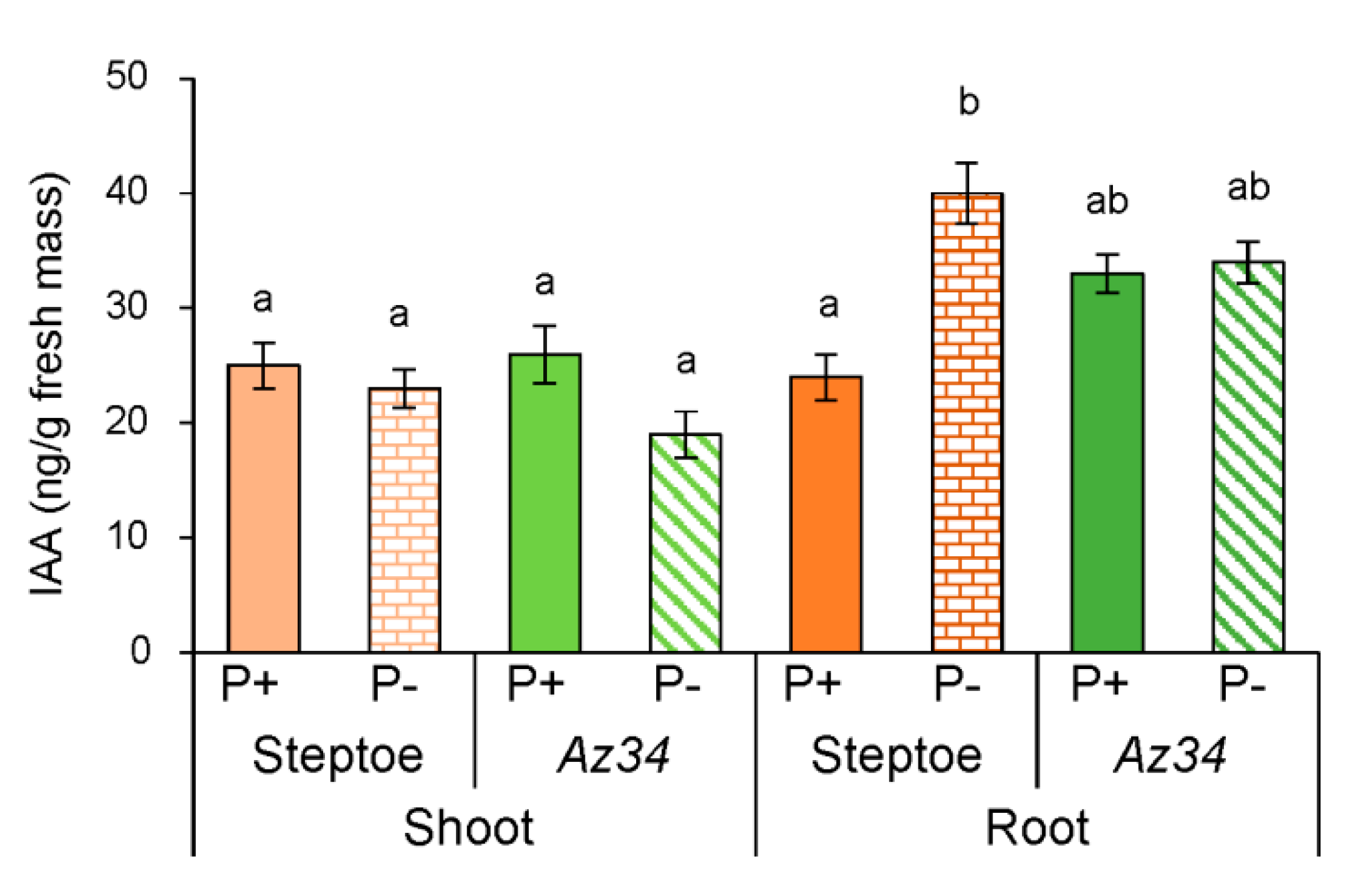
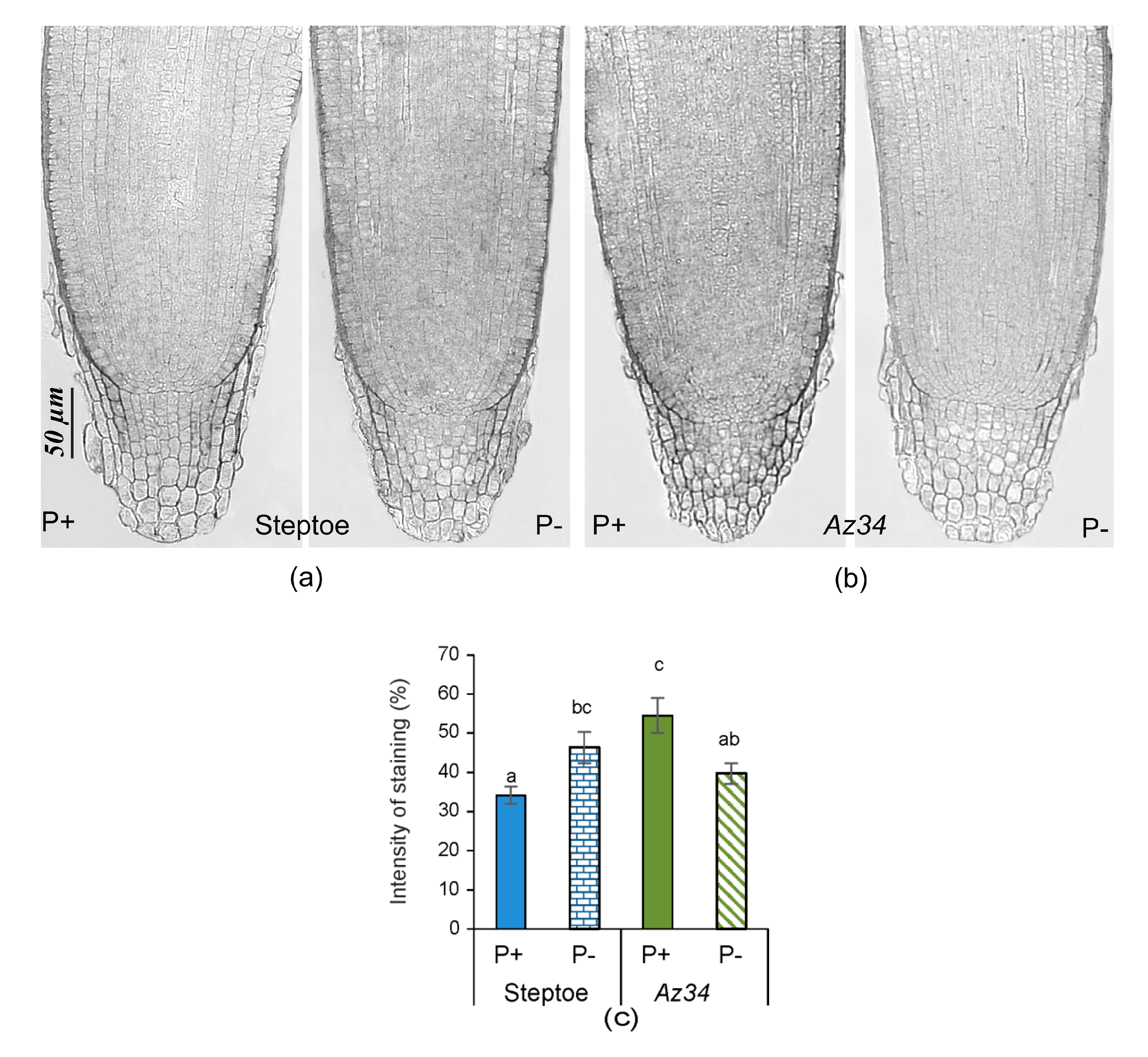
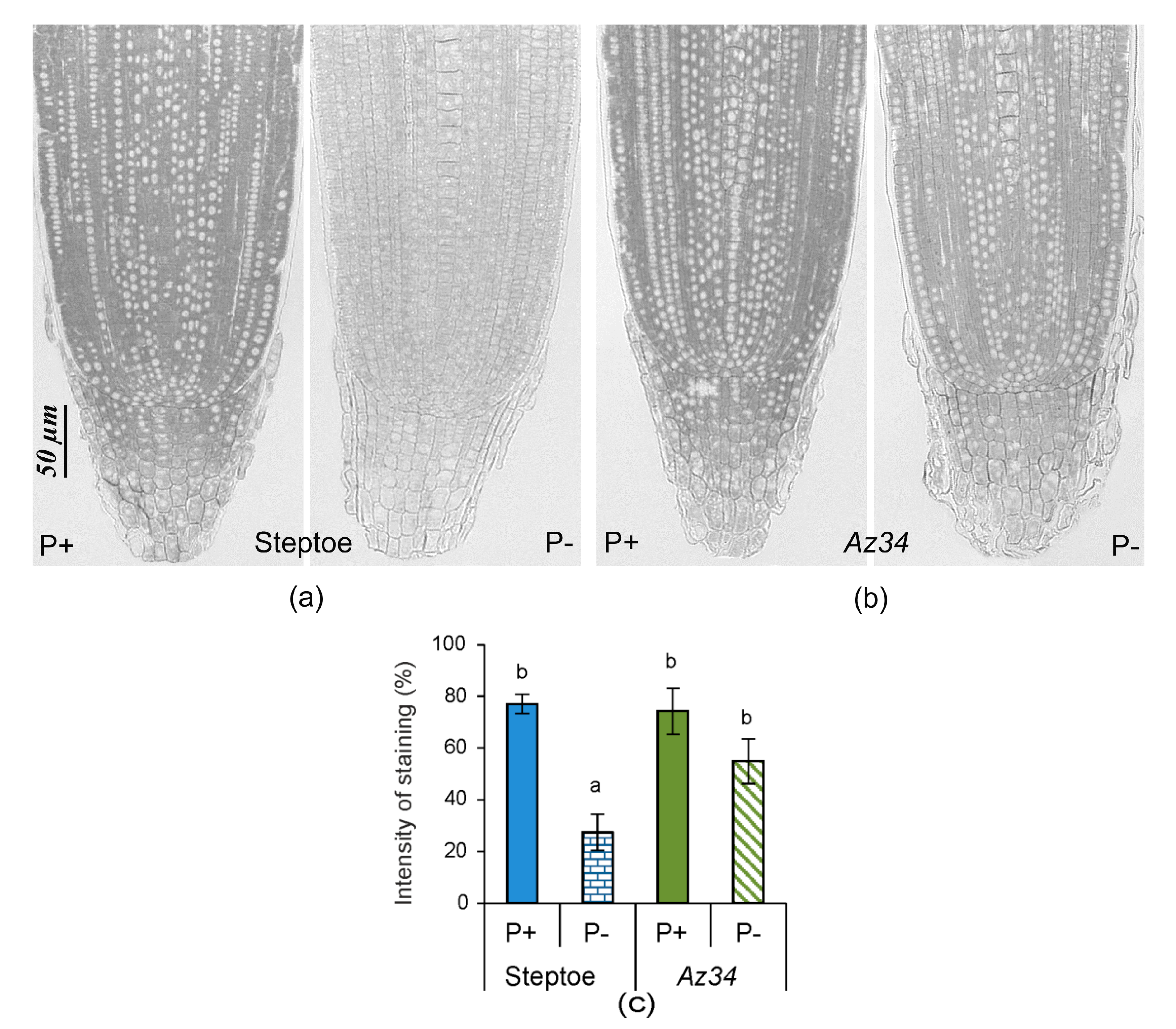
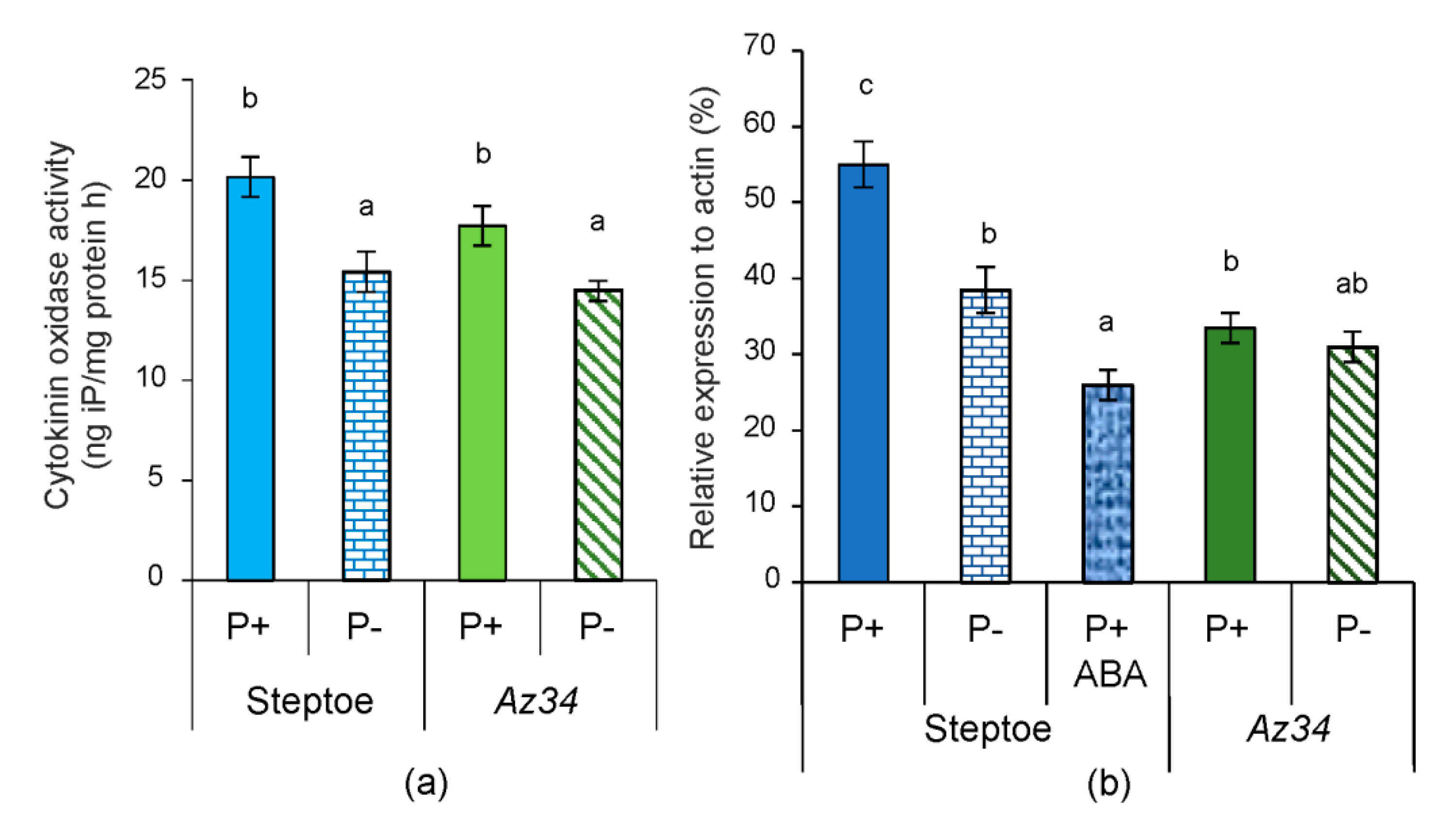
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Treatments
4.2. Hormone Analyses and Immunolocalization
4.3. RNA Extraction and Analysis of Abundance of HvIPT1 mRNA
4.4. Determination of Phosphorus in Roots and Leaves of Barley Plants
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 56, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A. Roots: The acquisition of water and nutrients from the heterogeneous soil environment. Prog. Bot. 2010, 71, 307–337. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.R.; Dodd, I.C.; Veselov, D.S.; Rothwell, S.A.; Veselov, S.Y. Common and specific responses to availability of mineral nutrients and water. J. Exp. Bot. 2015, 66, 2133–2144. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.M. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef]
- Pérez-Torres, C.A.; López-Bucio, J.; Cruz-Ramírez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, B. The plasticity of root systems in response to external phosphate. Int. J. Mol. Sci. 2020, 21, 5955. [Google Scholar] [CrossRef]
- Mollier, A.; Pellerin, S. Maize root system growth and development as influenced by phosphorus deficiency. J. Exp. Bot. 1999, 50, 487–497. [Google Scholar] [CrossRef]
- Ciereszko, I.; Balwicka, H.; Żebrowska, E. Acid phosphatases activity and growth of barley, oat, rye and wheat plants as affected by Pi deficiency. Open Plant Sci. J. 2017, 10, 110–122. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Wu, Z.; Jia, Y.; Wang, H.; Sun, S.; Mao, C.; Wang, X. Abscisic acid regulates auxin homeostasis in Rice root tips to promote root hair elongation. Front. Plant Sci. 2017, 8, 1121. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sergeeva, L.L.; Vreugdenhil, D. Quantitative trait loci analysis of hormone levels in Arabidopsis roots. PLoS ONE 2019, 14, e0219008. [Google Scholar] [CrossRef] [PubMed]
- Rubio, V.; Bustos, R.; Irigoyen, M.L.; Cardona-López, X.; Rojas-Triana, M.; Paz-Ares, J. Plant hormones and nutrient signaling. Plant Mol. Biol. 2009, 69, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Korobova, A.V.; Veselov, S.Y.; Dodd, I.C.; Kudoyarova, G.R. ABA mediation of shoot cytokinin oxidase activity: Assessing its impacts on cytokinin status and biomass allocation of nutrient deprived durum wheat. Funct. Plant Biol. 2009, 36, 66–72. [Google Scholar] [CrossRef]
- Dello, I.R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef]
- Cerutti, T.; Delatorre, C.A. Nitrogen and phosphorus interaction and cytokinin: Responses of the primary root of Arabidopsis thaliana and the pdr1 mutant. Plant Sci. 2013, 198, 91–97. [Google Scholar] [CrossRef]
- Takei, K.; Takahashi, T.; Sugiyama, T.; Yamaya, T.; Sakakibara, H. Multiple routes communicating nitrogen availability from roots to shoots: A signal transduction pathway mediated by cytokinin. J. Exp. Bot. 2002, 53, 971–977. [Google Scholar] [CrossRef]
- Landrein, B.; Formosa-Jordan, P.; Malivert, A.; Schuster, C.; Melnyk, C.W.; Yang, W.; Turnbull, C.; Meyerowitz, E.M.; Locke, J.C.W.; Jönsson, H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. USA 2018, 115, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Korobova, A.V.; Akhiyarova, G.R.; Fedyaev, V.V.; Farkhutdinov, R.G.; Veselov, S.Y.; Kudoyarova, G.R. Participation of nitrate sensor NRT1.1 in the control of cytokinin level and root elongation under normal conditions and nitrogen deficit. Moscow Univ. Biol. Sci. Bull. 2019, 74, 221–226. [Google Scholar] [CrossRef]
- Sato, A.; Miura, K. Root architecture remodeling induced by phosphate starvation. Plant Signal. Behav. 2011, 6, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J.; Lin, S.I. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 2011, 62, 185–206. [Google Scholar] [CrossRef]
- Ha, S.; Tran, L.S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef]
- Baek, D.; Chun, H.J.; Yun, D.J.; Kim, M.C. Cross-talk between phosphate starvation and other environmental stress signaling pathways in plants. Mol. Cells 2017, 40, 697–705. [Google Scholar] [CrossRef]
- Lambers, H.; Martinoia, E.; Renton, M. Plant adaptations to severely phosphorus-impoverished soils. Curr. Opin. Plant Biol. 2015, 25, 23–31. [Google Scholar] [CrossRef]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef]
- Salama, A.M.S.E.D.A.; Wareing, P.F. Effects of mineral nutrition on endogenous cytokinins in plants of sunflower (Helianthus annuus L.). J. Exp. Bot. 1979, 30, 971–981. [Google Scholar] [CrossRef]
- Horgan, J.M.; Wareing, P.F. Cytokinins and the growth responses of seedlings of Betula pendula Roth. and Acer pseudoplatanus L. to nitrogen and phosphorus deficiency. J. Exp. Bot. 1980, 31, 525–532. [Google Scholar] [CrossRef]
- Kuiper, D.; Schuit, J.; Kuiper, P.J.C. Effects of internal and external cytokinin concentrations on root growth and shoot to root ratio of Plantago major ssp. pleiosperma at different nutrient conditions. Plant Soil 1988, 111, 231–236. [Google Scholar] [CrossRef]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization, and translocation. J. Exp. Bot. 2008, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.B.; Trekozova, A.W.; Kudoyarova, G.R. Effect of phosphorus starvation on hormone content and growth of barley plants. Acta Physiol. Plant. 2016, 38, 108. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Ruiz-Lozano, J.M.; Dodd, I.C.; Albacete, A.; Pérez-Alfocea, F. Hormonal and nutritional features in contrasting rootstock-mediated tomato growth under low-phosphorus nutrition. Front. Plant Sci. 2017, 11, 533. [Google Scholar] [CrossRef]
- Prerostova, S.; Kramna, B.; Dobrev, P.; Gaudinova, A.; Marsik, P.; Fiala, R.; Knirsch, V.; Vanek, T.; Kuresova, G.; Vankova, R. Organ–specific hormonal cross-talk in phosphate deficiency. Environ. Exp. Bot. 2018, 153, 198–208. [Google Scholar] [CrossRef]
- Li, W.; de Ollas, C.; Dodd, I.C. Long-distance ABA transport can mediate distal tissue responses by affecting local ABA concentrations. J. Integr. Plant Biol. 2018, 60, 16–33. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhao, X.S.; Wu, Q.; Shen, R.F. Abscisic acid is involved in root cell wall phosphorus remobilization independent of nitric oxide and ethylene in rice (Oryza sativa). Ann. Bot. 2018, 121, 1361–1368. [Google Scholar] [CrossRef]
- Jeschke, W.D.; Andreas, D.; Peuke, A.D.; John, S.; Pate, J.S.; Hartung, W. Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. J. Exp. Bot. 1997, 48, 1737–1747. [Google Scholar] [CrossRef]
- Eshraghi, L.; Anderson, J.P.; Aryamanesh, N.; McComb, J.A.; Shearer, B.; Hardy, G.S. Suppression of the auxin response pathway enhances susceptibility to Phytophthora cinnamomi while phosphite-mediated resistance stimulates the auxin signalling pathway. BMC Plant Biol. 2014, 14, 68. [Google Scholar] [CrossRef]
- Trull, M.C.; Guiltinan, M.J.; Lynch, J.P.; Deikman, J. The responses of wild-type and ABA mutant Arabidopsis thaliana plants to phosphorus starvation. Plant Cell Environ. 1997, 20, 85–92. [Google Scholar] [CrossRef]
- Nacry, P.; Canivenc, G.; Muller, B.; Azmi, A.; Onckelen, H.V.; Rossignol, M.; Doumas, P. A role for auxin redistribution in the response of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 2005, 138, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Lee, J.; Gong, Q.; Ma, S.; Jin, J.B.; Yoo, C.Y.; Miura, T.; Sato, A.; Bohnert, H.J.; Hasegawa, P.M. SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation. Plant Physiol. 2011, 155, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Walker-Simmons, M.; Kudrna, D.A.; Warner, R.L. Reduced accumulation of ABA during water stress in a molybdenum cofactor mutant of barley. Plant Physiol. 1989, 90, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, G.; Veselov, D.; Kudoyarova, G.; Fricke, W.; Dodd, I.; Katsuhara, M.; Furuichi, T.; Ivanov, I.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Kollmer, I.; Novak, O.; Strnad, M.; Kramer, U.; Schmulling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef]
- Riou-Khamlichi, C.; Huntley, R.; Jacqmard, A.; Murray, J.A. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 1999, 283, 1541–1544. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Jain, A.; Poling, M.D.; Karthikeyan, A.S.; Blakeslee, J.J.; Peer, W.A.; Titapiwatanakun, B.; Murphy, A.S.; Raghothama, K.G. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol. 2007, 144, 232–247. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Korobova, A.V.; Kudoyarova, G.R. Abscisic acid accumulation in the roots of nutrient-limited plants: Its impact on the differential growth of roots and shoots. J. Plant Physiol. 2008, 165, 1274–1279. [Google Scholar] [CrossRef]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.B.; Filin, A.N. Cytokinins regulate root growth through its action on meristematic cell proliferation but not on the transition to differentiation. Funct. Plant Biol. 2017, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, G.K.; Stah, Y.; Korff, M.; Simon, R. Unique and conserved features of the barley root meristem. Front Plant Sci. 2017, 8, 1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, Y.; Zou, X.; Li, F.; Zhang, J.; Kang, Z.; Li, X.; Yin, C.; Lin, Y. Nitrogen deficiency-induced decrease in cytokinins content promotes rice seminal root growth by promoting root meristem cell proliferation and cell elongation. Cells 2020, 9, 916. [Google Scholar] [CrossRef]
- Takei, K.; Sakakibara, H.; Sugiyama, T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 26405–26410. [Google Scholar] [CrossRef]
- Zeenat, A.; Zulfiqar, A.; Ramzan, A.; Heckathorn, S.A.; Shakeel, S.N. Cytokinin interaction to cope with phosphorous starvation in rice. Int. J. Agric. Biol. 2018, 20, 2446–2454. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef]
- Talboys, P.J.; Healey, J.R.; Withers, P.J.A.; Jones, D.L. Phosphate depletion modulates auxin transport in Triticum aestivium leading to altered root branching. J. Exp. Bot. 2014, 65, 5023–5032. [Google Scholar] [CrossRef]
- Li, G.; Song, H.; Li, B.; Kronzucker, H.J.; Shi, W. Auxin resistant and PIN-FORMED2 protect lateral root formation in Arabidopsis under iron stress. Plant Physiol. 2015, 169, 2608–2623. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Munguía-Rodríguez, A.G.; López-Bucio, J.S.; Ruiz-Herrera, L.F.; Ortiz-Castro, R.; Guevara-García, Á.A.; Marsch-Martínez, N.; Carreón-Abud, Y.; López-Bucio, J.; Martínez-Trujillo, M. YUCCA4 overexpression modulates auxin biosynthesis and transport and influences plant growth and development via crosstalk with abscisic acid in Arabidopsis thaliana. Genet. Mol. Biol. 2020, 43, e20190221. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.-A.; Agbevenou, K.; Herrbach, V.; Gough, C.; Bensmihen, S.S. Abscisic acid promotes pre-emergence stages of lateral root development in Medicago truncatula. Plant Signal Behav. 2015, 10, e977741. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M. Abscisic Acid: Hidden architect of root system structure. Plants 2015, 4, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Dodd, I.C.; Mooney, S.J. Using X-ray Computed Tomography to explore the role of abscisic acid in moderating the impact of soil compaction on root system architecture. Environ. Exp. Bot. 2015, 110, 11–18. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, C.-M. Auxin homeostasis during lateral root development under drought condition. Plant Signal. Behav. 2009, 4, 1002–1004. [Google Scholar] [CrossRef]
- Wang, W.; Ding, G.; White, P.J.; Wang, X.-H.; Jin, K.-M.; Xu, F.-S.; Shi, L. Mapping and cloning of quantitative trait loci for phosphorus efficiency in crops: Opportunities and challenges. Plant Soil 2019, 439, 91–112. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inzé, D.; Sandberg, G.; Casero, P.J.; et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R.; Veselov, S.U. Ability of bacterium bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Korobova, A.V.; Akhiyarova, G.R.; Arkhipova, T.N.; Zaytsev, D.Y.; Prinsen, E.; Egutkin, N.L.; Medvedev, S.S.; Veselov, S.Y. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). J. Exp. Bot. 2014, 65, 2287–2294. [Google Scholar] [CrossRef]
- Veselov, S.U.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.G.; Mustafina, A.R.; Kof, E.K. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole 3-acetic acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Dewitte, W.; Chiappetta, A.; Azmi, A.; Witters, E.; Strnad, M.; Rembur, J.; Noin, M.; Chriqui, D.; Van Onckelen, H. Dynamics of cytokinins in apical shoot meristems of a day neutral tobacco during floral transition and flower formation. Plant Physiol. 1999, 119, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.B.; Akhiyarova, G.R.; Sharipova, G.V.; Dedova, M.A.; Veselov, S.Y.; Zaitsev, D.Y.; Kudoyarova, G.R. The influence of local IPT gene induction in roots on content of cytokinins in cells of tobacco leaves. Cell Tiss. Biol. 2015, 9, 127–132. [Google Scholar] [CrossRef]
- Veselov, S.Y.; Simonyan, M.V. Immunoenzyme analysis of cytokinins as an assay for cytokinin oxidase activity. Russ. J. Plant Physiol. 2004, 51, 266–270. [Google Scholar] [CrossRef]
- Cai, J.; Li, P.; Luo, X.; Chang, T.; Li, J.; Zhao, Y.; Xu, Y. Selection of appropriate reference genes for the detection of rhythmic gene expression via quantitative real-time PCR in Tibetan hulless barley. PLoS ONE 2018, 13, e0190559. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vysotskaya, L.; Akhiyarova, G.; Feoktistova, A.; Akhtyamova, Z.; Korobova, A.; Ivanov, I.; Dodd, I.; Kuluev, B.; Kudoyarova, G. Effects of Phosphate Shortage on Root Growth and Hormone Content of Barley Depend on Capacity of the Roots to Accumulate ABA. Plants 2020, 9, 1722. https://doi.org/10.3390/plants9121722
Vysotskaya L, Akhiyarova G, Feoktistova A, Akhtyamova Z, Korobova A, Ivanov I, Dodd I, Kuluev B, Kudoyarova G. Effects of Phosphate Shortage on Root Growth and Hormone Content of Barley Depend on Capacity of the Roots to Accumulate ABA. Plants. 2020; 9(12):1722. https://doi.org/10.3390/plants9121722
Chicago/Turabian StyleVysotskaya, Lidiya, Guzel Akhiyarova, Arina Feoktistova, Zarina Akhtyamova, Alla Korobova, Igor Ivanov, Ian Dodd, Bulat Kuluev, and Guzel Kudoyarova. 2020. "Effects of Phosphate Shortage on Root Growth and Hormone Content of Barley Depend on Capacity of the Roots to Accumulate ABA" Plants 9, no. 12: 1722. https://doi.org/10.3390/plants9121722
APA StyleVysotskaya, L., Akhiyarova, G., Feoktistova, A., Akhtyamova, Z., Korobova, A., Ivanov, I., Dodd, I., Kuluev, B., & Kudoyarova, G. (2020). Effects of Phosphate Shortage on Root Growth and Hormone Content of Barley Depend on Capacity of the Roots to Accumulate ABA. Plants, 9(12), 1722. https://doi.org/10.3390/plants9121722





