UV-B Radiation Affects Photosynthesis-Related Processes of Two Italian Olea europaea (L.) Varieties Differently
Abstract
1. Introduction
2. Results
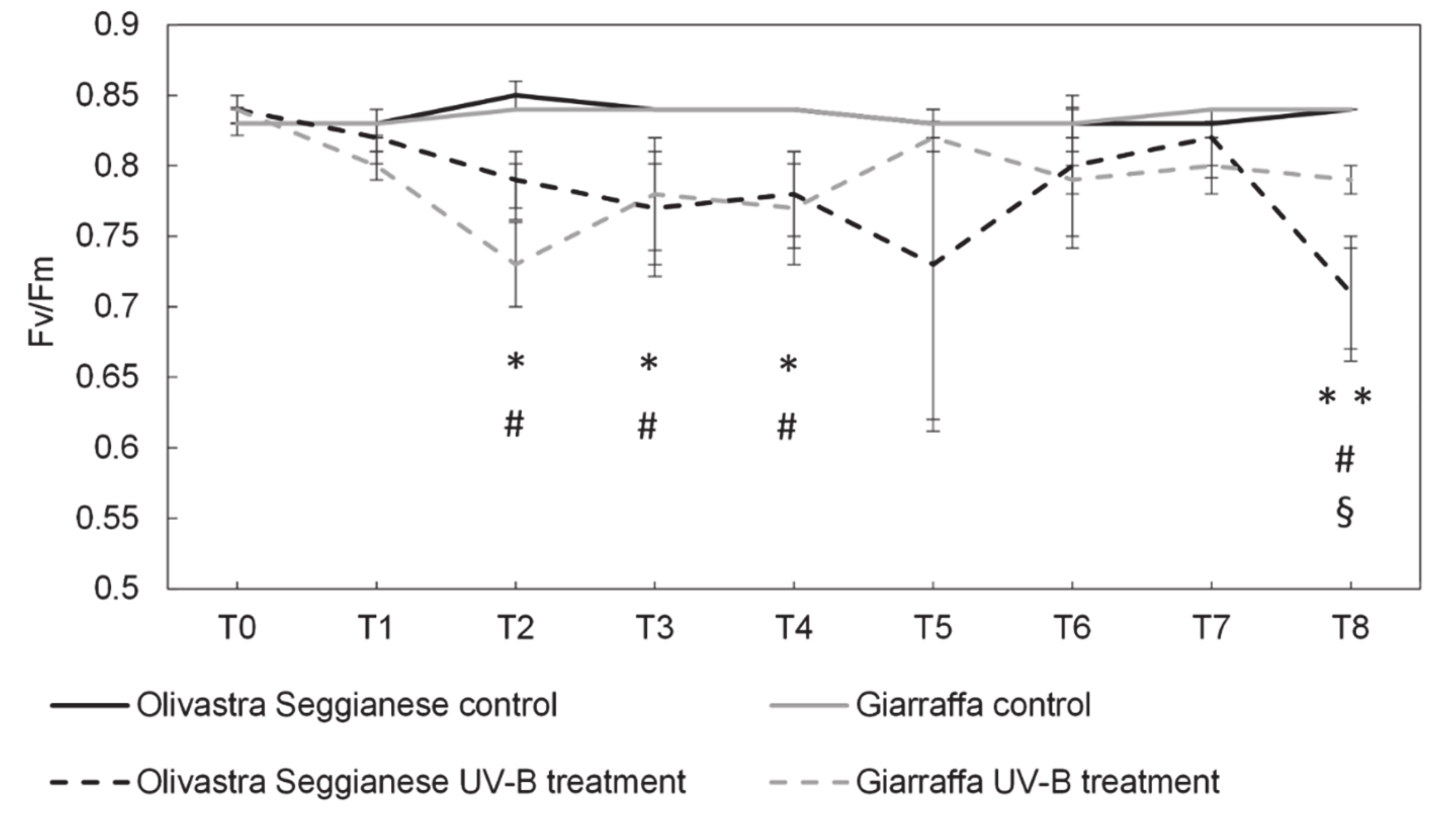
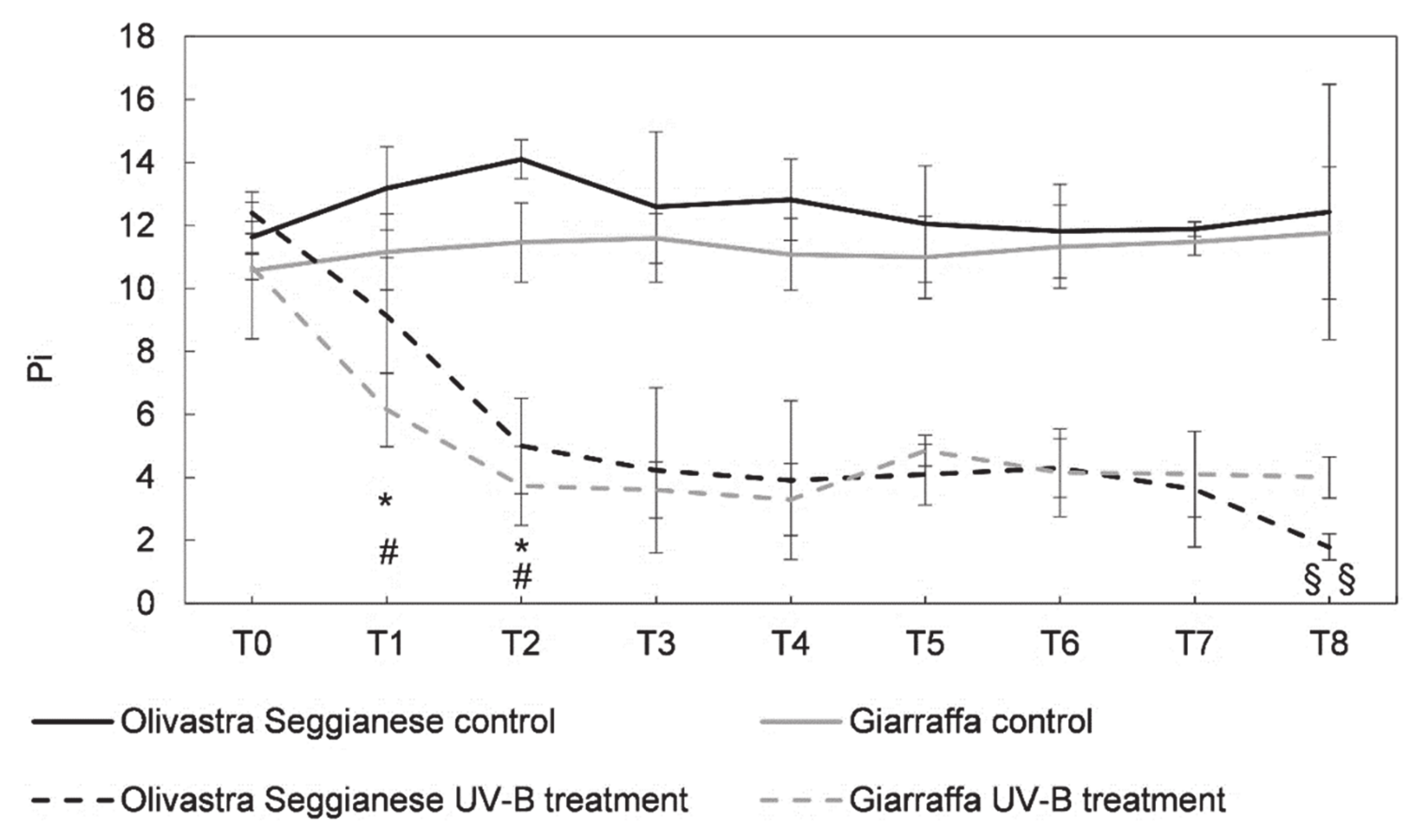
2.1. Photosynthetic Efficiency
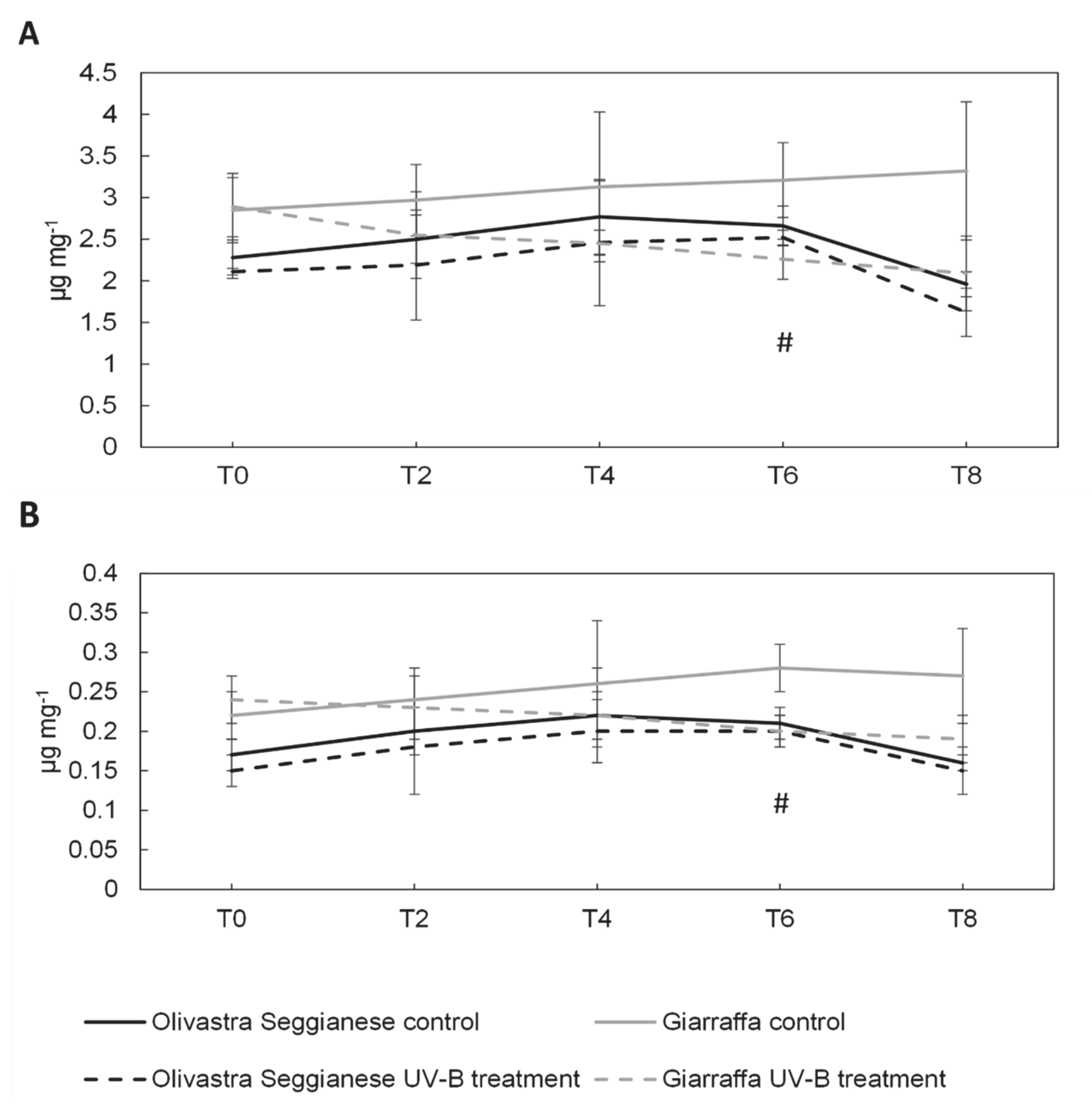
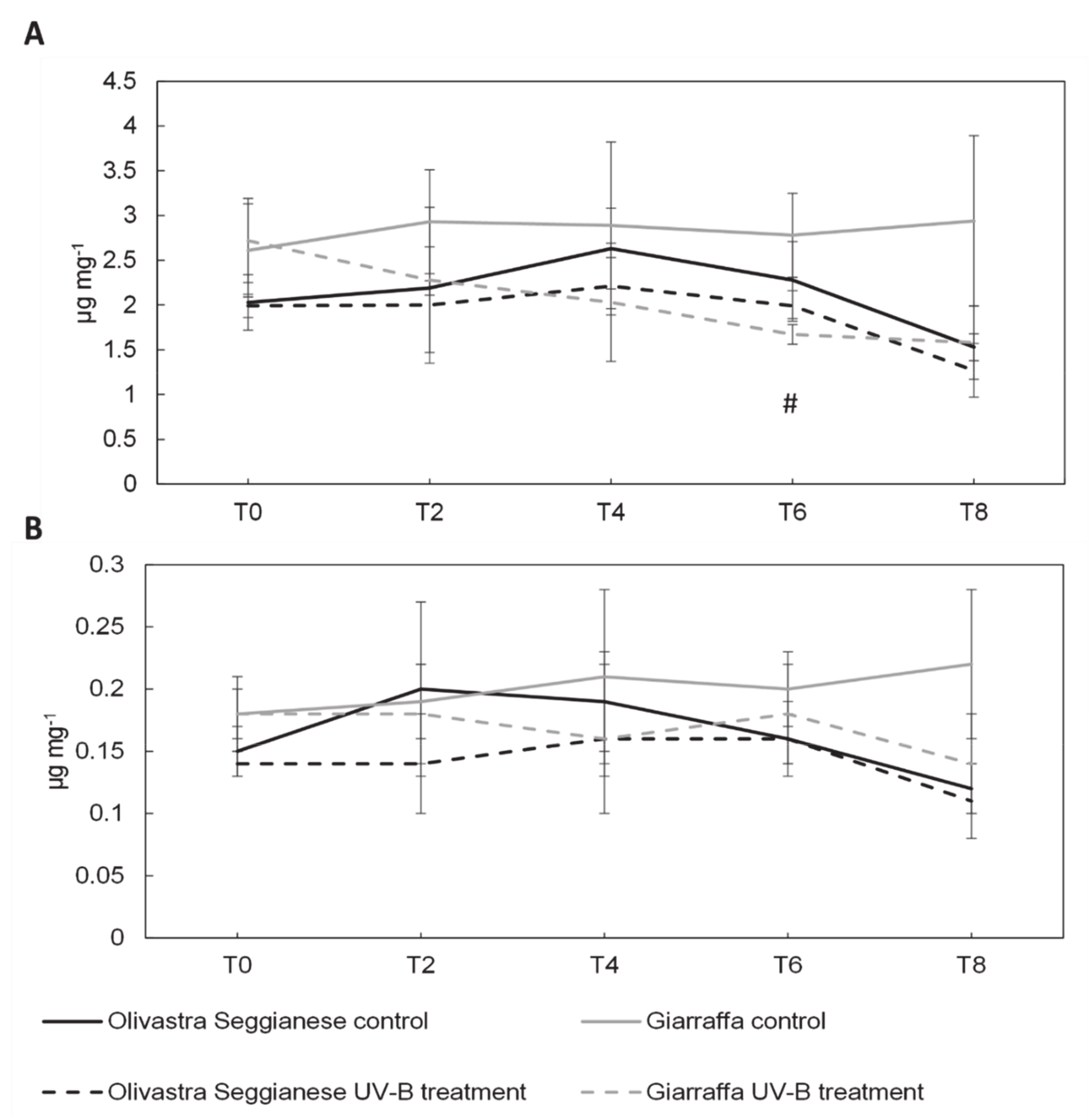
2.2. Photosynthetic Pigments
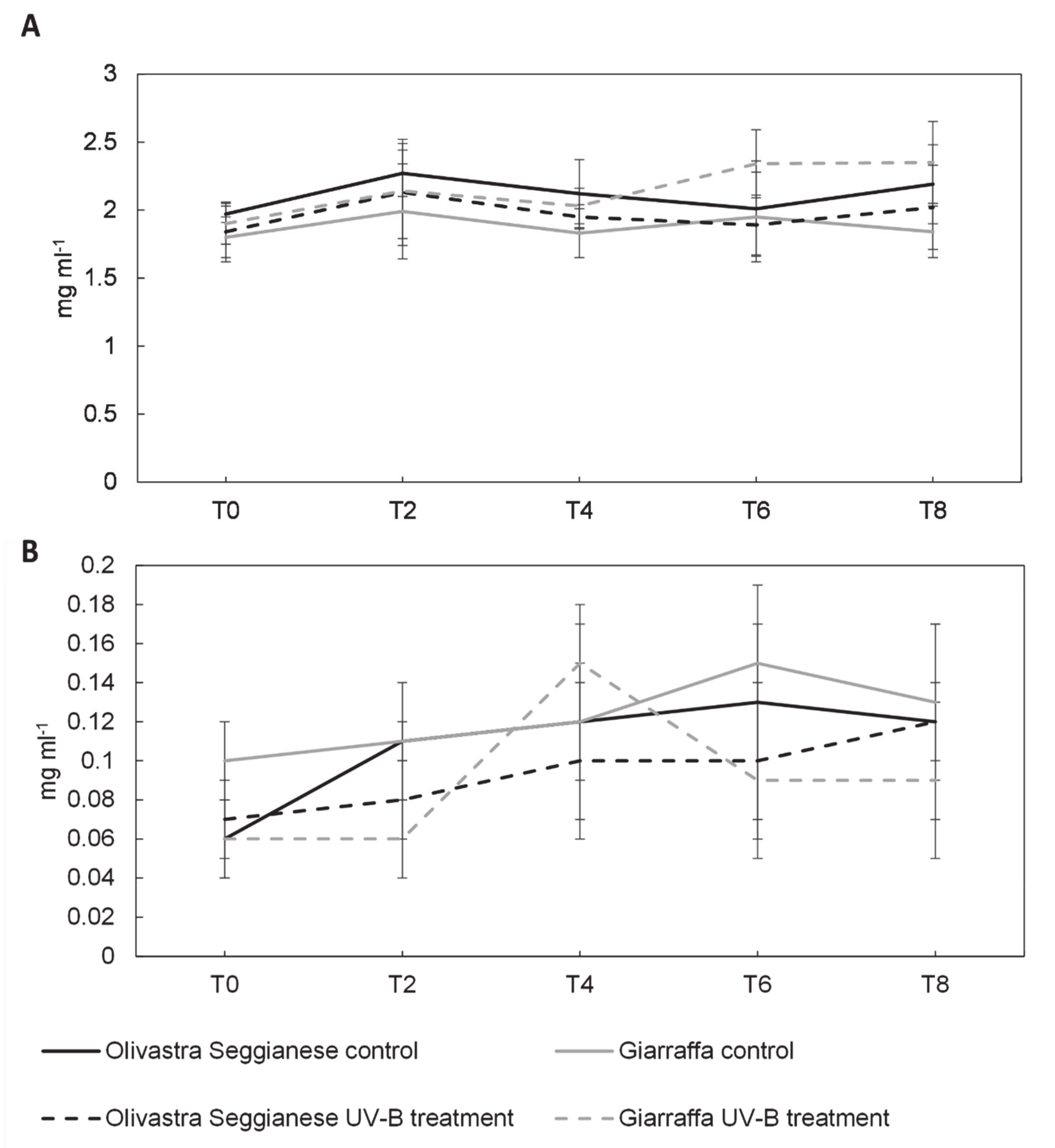
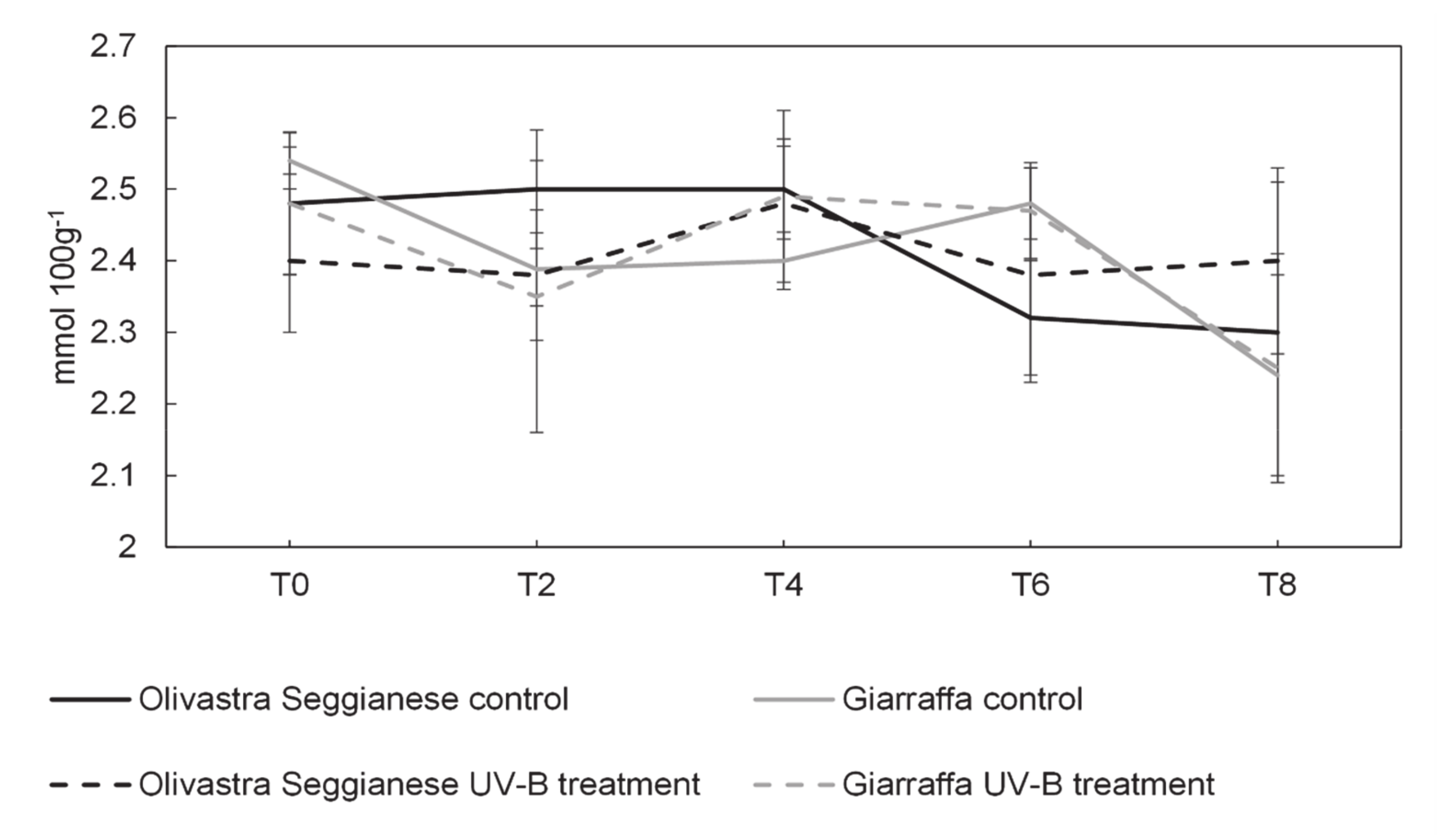
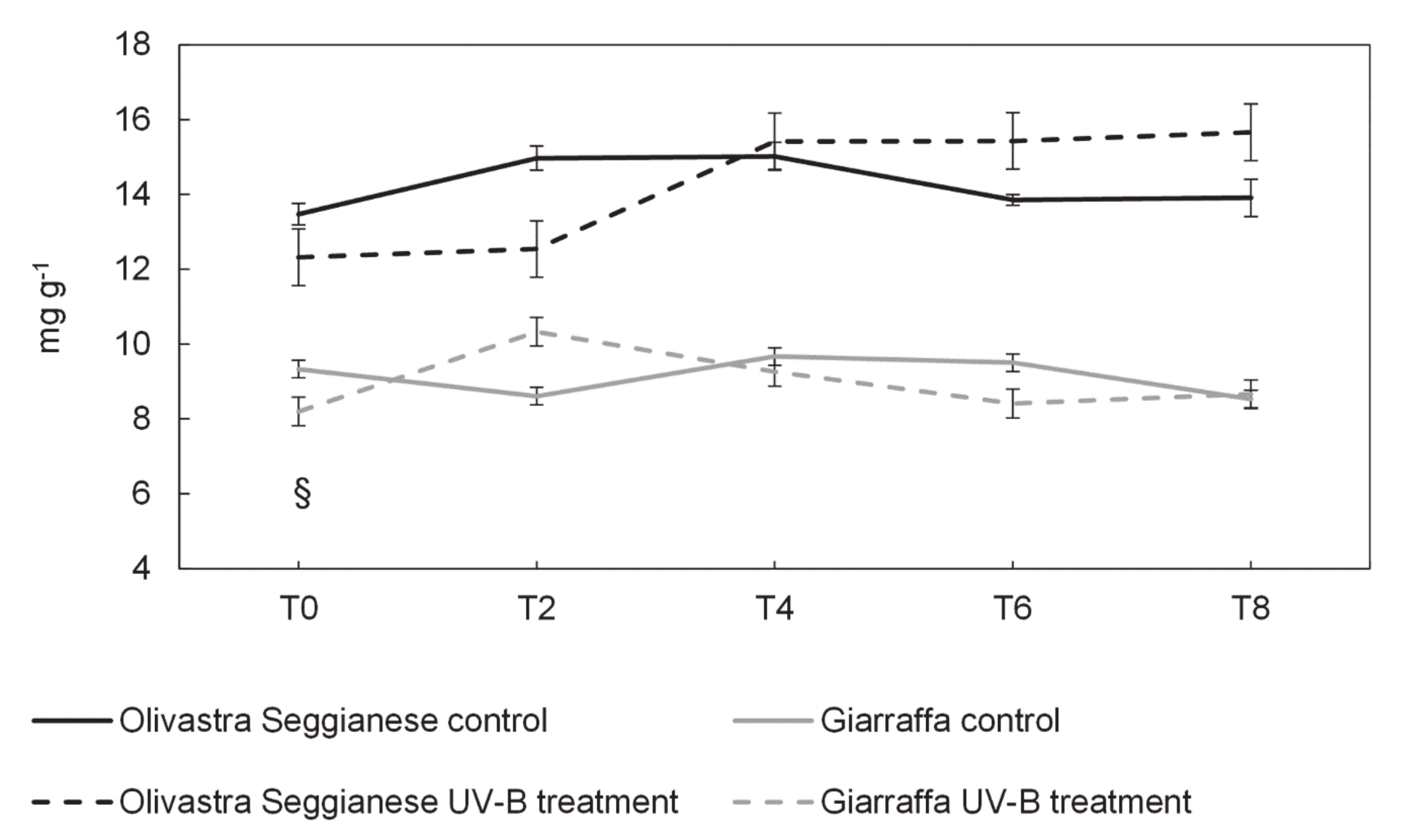
2.3. Sugars
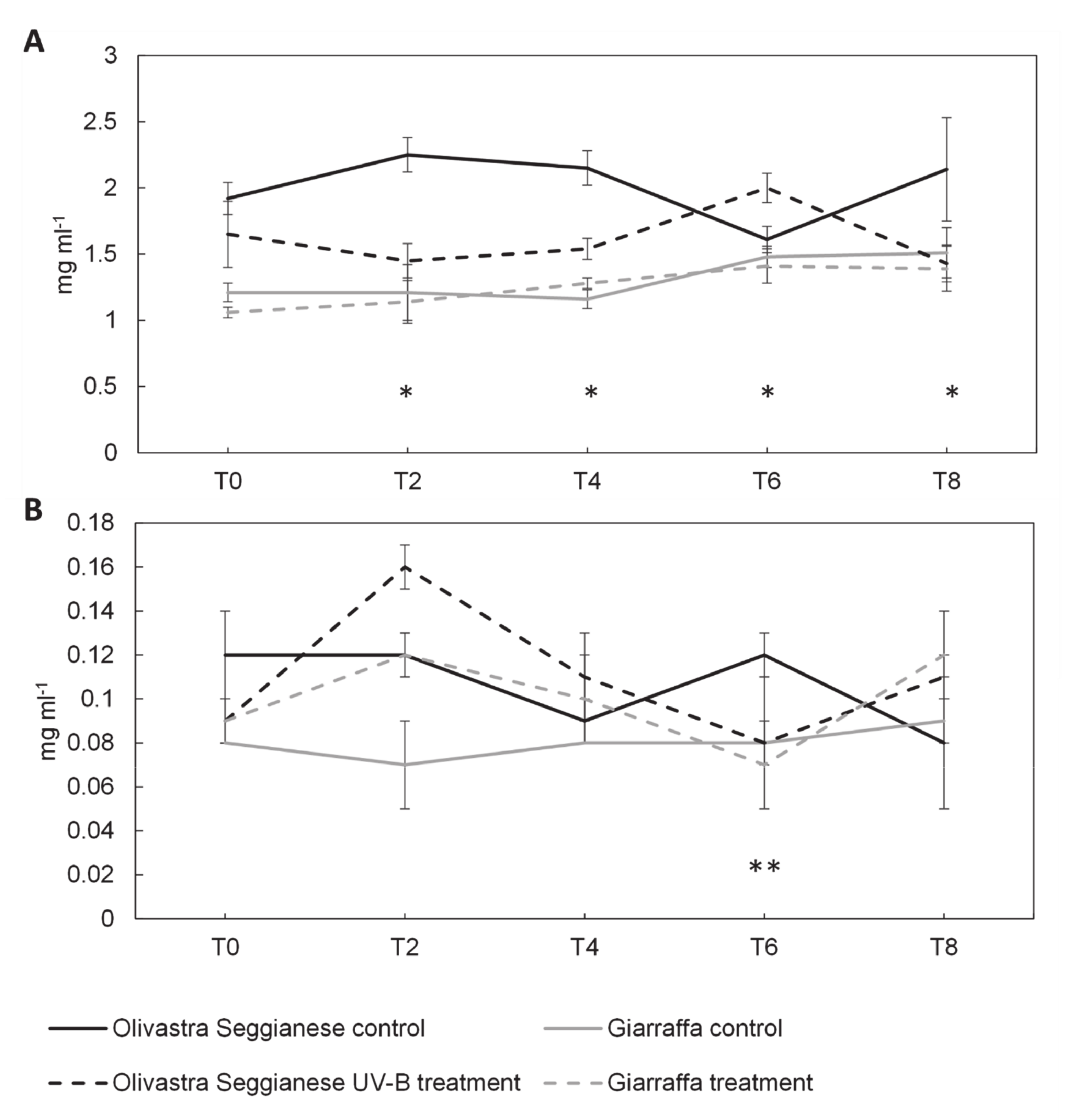
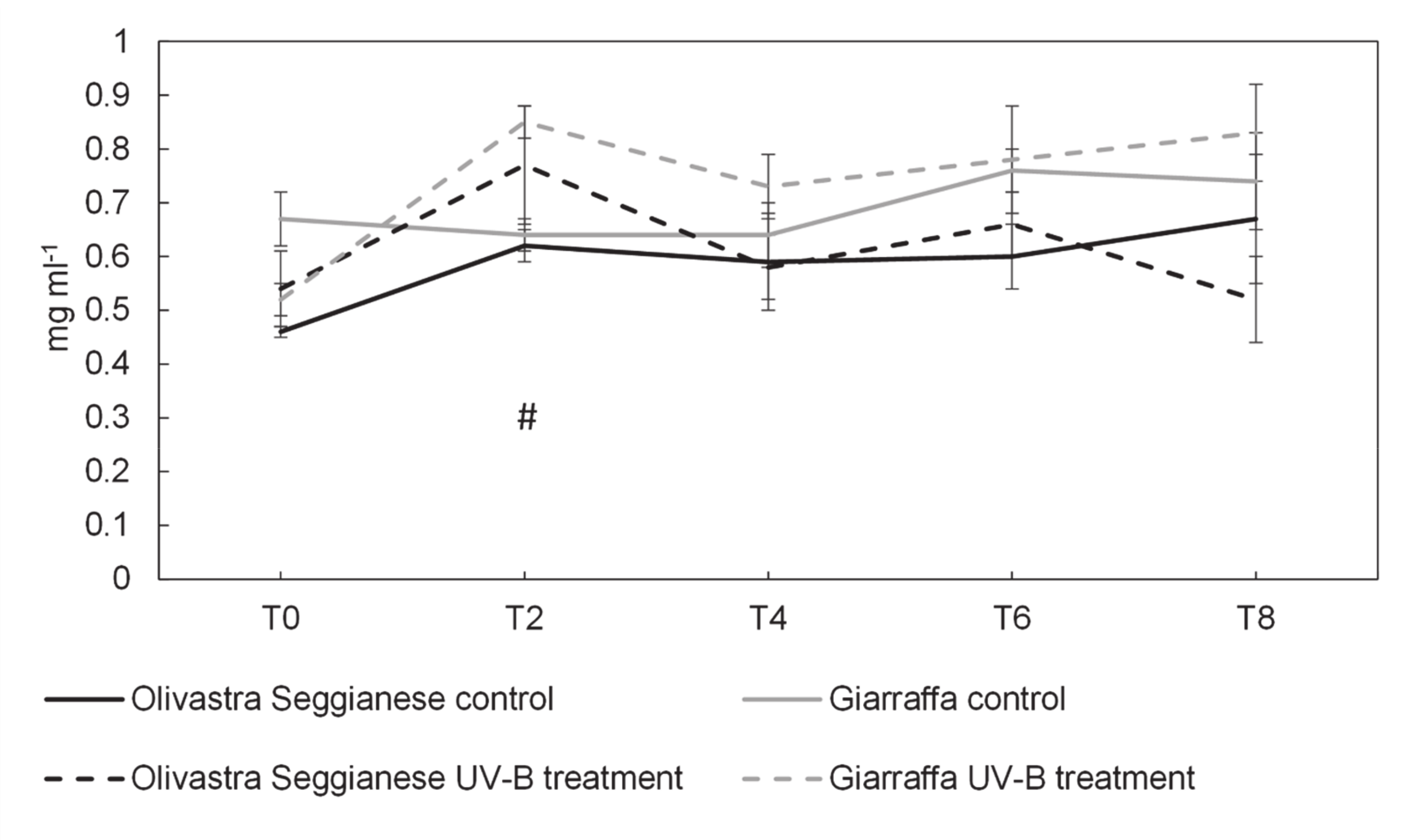
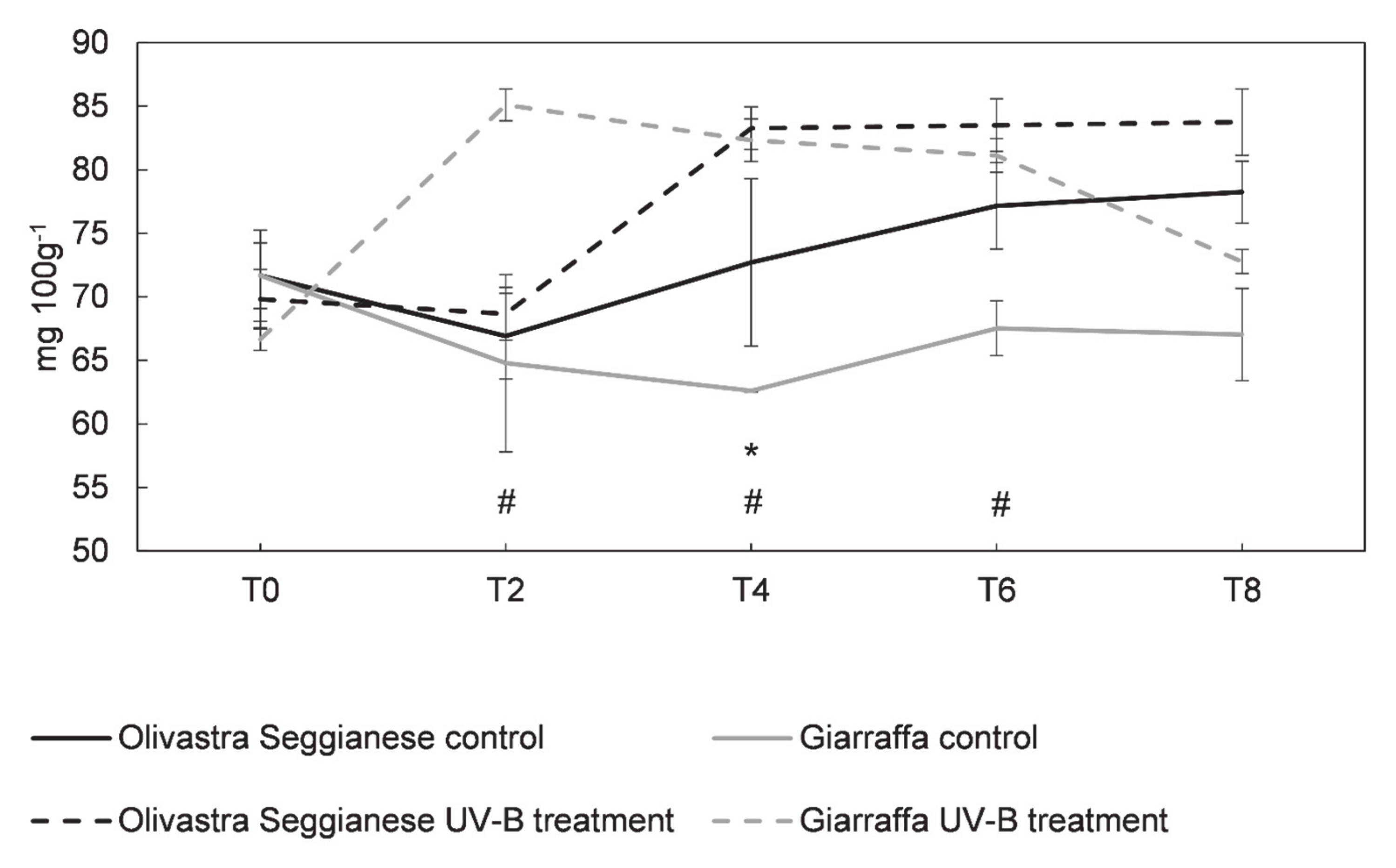
2.4. Antioxidant Capacity, Polyphenols and Flavonoids
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Application of UV-B Treatment
4.3. Determination of Photosynthetic Defficiency
4.4. Analysis of Photosynthetic Pigments
4.5. Analysis of Sugars
4.6. Determination of the Antioxidant Capacity, Polyphenols and Flavonoids
4.6.1. Ferric Ion Reducing Antioxidant Power—FRAP
4.6.2. Folin-Ciocalteu Method for the Determination of Total Polyphenols
4.6.3. Aluminum Chloride Method for the Determination of Total Flavonoids
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Miyamura, Y.; Bowen, M.P.; Correa, G.; Ono, Y.; Goodarzi, H. Light, including ultraviolet. J. Autoimmun. 2010, 34, J247–J257. [Google Scholar] [CrossRef] [PubMed]
- Fernanda Pessoa, M. Algae and aquatic macrophytes responses to cope to ultraviolet radiation—A Review. Emir. J. Food Agric. 2012, 24. [Google Scholar] [CrossRef]
- Abbasi, S.A.; Abbasi, T. Impacts of Ozone Hole. In Ozone Hole; Springer: Berlin, Germany, 2017; pp. 51–99. [Google Scholar] [CrossRef]
- Forster, P.M.; Thompson, D.W.J.; Baldwin, M.P.; Chipperfield, M.P.; Dameris, M.; Haigh, J.D.; Karoly, D.J.; Kushner, P.J.; Randel, W.J.; Rosenlof, K.H.; et al. Stratospheric Changes and Climate. Scientific Assessment of Ozone Depletion, Global Ozone Research and Monitoring Project-Report No 52.; WMO: Geneva, Switzerland; United Nations Environment Programme: Nairobi, Kenya, 2011. [Google Scholar]
- Kakani, V.G. Effects of Ultraviolet-B Radiation on Cotton (Gossypium hirsutum L.) Morphology and Anatomy. Ann. Bot. 2003, 91, 817–826. [Google Scholar] [CrossRef]
- Rácz, A.; Hideg, É.; Czégény, G. Selective responses of class III plant peroxidase isoforms to environmentally relevant UV-B doses. J. Plant Physiol. 2018, 221, 101–106. [Google Scholar] [CrossRef]
- Britt, A.B. DNA damage and repair in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 75–100. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N.; Ahuja, S.; Singh, B. Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. J. Photochem. Photobiol. B Biol. 2013, 127, 140–152. [Google Scholar] [CrossRef]
- Dotto, M.; Casati, P. Developmental reprogramming by UV-B radiation in plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef]
- Koubouris, G.C.; Kavroulakis, N.; Metzidakis, I.T.; Vasilakakis, M.D.; Sofo, A. Ultraviolet-B radiation or heat cause changes in photosynthesis, antioxidant enzyme activities and pollen performance in olive tree. Photosynthetica 2015, 53, 279–287. [Google Scholar] [CrossRef]
- Zlatev, Z.S.; Lidon, F.J.C.; Kaimakanova, M. Plant physiological responses to UV-B radiation. Emir. J. Food Agric. 2012, 24. [Google Scholar] [CrossRef]
- Sebastian, A.; Kumari, R.; Kiran, B.R.; Prasad, M.N.V. Ultraviolet B induced bioactive changes of enzymatic and non-enzymatic antioxidants and lipids in Trigonella foenum-graecum L. (Fenugreek). Eurobiotech J. 2018, 2, 64–71. [Google Scholar] [CrossRef]
- Yu, G.H.; Li, W.; Yuan, Z.Y.; Cui, H.Y.; Lv, C.G.; Gao, Z.P.; Han, B.; Gong, Y.Z.; Chen, G.X. The effects of enhanced UV-B radiation on photosynthetic and biochemical activities in super-high-yield hybrid rice Liangyoupeijiu at the reproductive stage. Photosynthetica 2013, 51, 33–44. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Correia, C.; Moutinho-Pereira, J.; Oliveira, H.; Freitas, H.; Silva, A.M.S.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Strid, Å.; Chow, W.S.; Anderson, J.M. UV-B damage and protection at the molecular level in plants. Photosynth. Res. 1994, 39, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Fonini, A.M.; Barufi, J.B.; Schmidt, E.C.; Rodrigues, A.C.; Randi, A.M. Leaf anatomy and photosynthetic efficiency of Acrostichum danaeifolium after UV radiation. Photosynthetica 2017, 55, 401–410. [Google Scholar] [CrossRef]
- Zuk-Golaszewska, K.; Upadhyaya, M.K.; Golaszewski, J. The effect of UV-B radiation on plant growth and development. Plant Soil Environ. 2011, 49, 135–140. [Google Scholar] [CrossRef]
- Yao, X.; Liu, Q. The effects of enhanced ultraviolet-B and nitrogen supply on growth, photosynthesis and nutrient status of Abies faxoniana seedlings. Acta Physiol. Plant. 2009, 31, 523–529. [Google Scholar] [CrossRef]
- Feng, H.; An, L.; Tan, L.; Hou, Z.; Wang, X. Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 taxa in vitro. Environ. Exp. Bot. 2000, 43, 45–53. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.K.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Freitas, H.; Santos, C.; Silva, A.M.S. The antioxidant system in Olea europaea to enhanced UV-B radiation also depends on flavonoids and secoiridoids. Phytochemistry 2020, 170, 112199. [Google Scholar] [CrossRef] [PubMed]
- Nogues, S. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J. Exp. Bot. 2000, 51, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Morales, F.; Delrot, S.; Sánchez-Díaz, M.; Gomès, E.; Aguirreolea, J.; Pascual, I. Characterization of the adaptive response of grapevine (cv. Tempranillo) to UV-B radiation under water deficit conditions. Plant Sci. 2015, 232, 13–22. [Google Scholar] [CrossRef]
- Sanchez-Lorenzo, A.; Enriquez-Alonso, A.; Calbó, J.; González, J.-A.; Wild, M.; Folini, D.; Norris, J.R.; Vicente-Serrano, S.M. Fewer clouds in the Mediterranean: Consistency of observations and climate simulations. Sci. Rep. 2017, 7, 41475. [Google Scholar] [CrossRef]
- Díaz-Guerra, L.; Verdaguer, D.; Gispert, M.; Pardini, G.; Font, J.; González, J.A.; Peruzzi, E.; Masciandaro, G.; Llorens, L. Effects of UV radiation and rainfall reduction on leaf and soil parameters related to C and N cycles of a Mediterranean shrubland before and after a controlled fire. Plant Soil 2018, 424, 503–524. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Dias, M.C.; Figueiredo, C.; Pinto, D.C.G.A.; Freitas, H.; Santos, C.; Silva, A.M.S. Heat shock and UV-B episodes modulate olive leaves lipophilic and phenolic metabolite profiles. Ind. Crop. Prod. 2019, 133, 269–275. [Google Scholar] [CrossRef]
- Silva, S.; Santos, C.; Serodio, J.; Silva, A.M.S.; Dias, M.C. Physiological performance of drought-stressed olive plants when exposed to a combined heat–UV-B shock and after stress relief. Funct. Plant Biol. 2018, 45, 1233. [Google Scholar] [CrossRef]
- Sullivan, J.H.; Gitz, D.C.; Peek, M.S.; McElrone, A.J. Response of three eastern tree species to supplemental UV-B radiation: Leaf chemistry and gas exchange. Agric. Meteorol. 2003, 120, 219–228. [Google Scholar] [CrossRef]
- Johnson, G.N.; Young, A.J.; Scholes, J.D.; Horton, P. The dissipation of excess excitation energy in British plant species. Plant Cell Environ. 1993, 16, 673–679. [Google Scholar] [CrossRef]
- Demmig, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 1987, 171, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H.; Arndal, M.F.; Michelsen, A. Ambient UV-B radiation reduces PSII performance and net photosynthesis in high Arctic Salix arctica. Environ. Exp. Bot. 2011, 73, 10–18. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazar, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Springer: New Delhi, India, 2013; pp. 87–131. ISBN 9788132208075. [Google Scholar]
- Shine, M.B.; Guruprasad, K.N. Oxyradicals and PSII activity in maize leaves in the absence of UV components of solar spectrum. J. Biosci. 2012, 37, 703–712. [Google Scholar] [CrossRef]
- Meteo Aeronautica Militare. Available online: http://www.meteoam.it/ (accessed on 30 October 2020).
- Mirecki, R.M.; Teramura, A.H. Effects of Ultraviolet-B Irradiance on Soybean. Plant Physiol. 1984, 74, 475–480. [Google Scholar] [CrossRef]
- He, J.; Huang, L.; Chow, W.; Whitecross, M.; Anderson, J. Effects of Supplementary Ultraviolet-B Radiation on Rice and Pea Plants. Funct. Plant Biol. 1993, 20, 129. [Google Scholar] [CrossRef]
- Pal, M.; Sengupta, U.K.; Srivastava, A.C.; Jain, V.; Meena, R. Changes in growth and photosynthesis of mungbean induced by UV-B radiation. Indian J. Plant Physiol. 1999, 4, 79–84. [Google Scholar]
- Tevini, M.; Iwanzik, W.; Thoma, U. Some effects of enhanced UV-B irradiation on the growth and composition of plants. Planta 1981, 153, 388–394. [Google Scholar] [CrossRef]
- Kakani, V.; Reddy, K.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Agric. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Siefermann-Harms, D. Carotenoids in photosynthesis. I. Location in photosynthetic membranes and light-harvesting function. Biochim. Biophys. Acta Rev. Bioenerg. 1985, 811, 325–355. [Google Scholar] [CrossRef]
- Siefermann-Harms, D. Chlorophyll, carotenoids and the activity of the xanthophyll cycle. Environ. Pollut. 1990, 68, 293–303. [Google Scholar] [CrossRef]
- Vasques, A.R.; Pinto, G.; Dias, M.C.; Correia, C.M.; Moutinho-Pereira, J.M.; Vallejo, V.R.; Santos, C.; Keizer, J.J. Physiological response to drought in seedlings of Pistacia lentiscus (mastic tree). New For. 2016, 47, 119–130. [Google Scholar] [CrossRef]
- Lidon, F.C.; Ramalho, J.C. Impact of UV-B irradiation on photosynthetic performance and chloroplast membrane components in Oryza sativa L. J. Photochem. Photobiol. B Biol. 2011, 104, 457–466. [Google Scholar] [CrossRef]
- Ranjbarfordoei, A.; Samson, R.; Damme, P. Photosynthesis performance in sweet almond [Prunus dulcis (Mill) D. Webb] exposed to supplemental UV-B radiation. Photosynthetica 2011, 49. [Google Scholar] [CrossRef]
- Hui, R.; Li, X.; Chen, C.; Zhao, X.; Jia, R.; Liu, L.; Wei, Y. Responses of photosynthetic properties and chloroplast ultrastructure of Bryum argenteum from a desert biological soil crust to elevated ultraviolet-B radiation. Physiol. Plant. 2013, 147, 489–501. [Google Scholar] [CrossRef]
- Machado, F.; Dias, M.C.; de Pinho, P.G.; Araújo, A.M.; Pinto, D.; Silva, A.; Correia, C.; Moutinho-Pereira, J.; Santos, C. Photosynthetic performance and volatile organic compounds profile in Eucalyptus globulus after UVB radiation. Environ. Exp. Bot. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Stoop, J.; Williamson, J.; Masonpharr, D. Mannitol metabolism in plants: A method for coping with stress. Trends Plant Sci. 1996, 1, 139–144. [Google Scholar] [CrossRef]
- Vanlerberghe, G. Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Araújo, M.; Santos, C.; Costa, M.; Moutinho-Pereira, J.; Correia, C.; Dias, M.C. Plasticity of young Moringa oleifera L. plants to face water deficit and UVB radiation challenges. J. Photochem. Photobiol. B Biol. 2016, 162, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.; Pollock, C.; Gallagher, J. Sucrose and the integration of metabolism in vascular plants. Plant Sci. 2000, 154, 1–11. [Google Scholar] [CrossRef]
- Salerno, G. Origin of sucrose metabolism in higher plants: When, how and why? Trends Plant Sci. 2003, 8, 63–69. [Google Scholar] [CrossRef]
- Roitsch, T.; González, M.-C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Rinnan, R.; Nerg, A.M.; Ahtoniemi, P.; Suokanerva, H.; Holopainen, T.; Kyrö, E.; Bååth, E. Plant-mediated effects of elevated ultraviolet-B radiation on peat microbial communities of a subarctic mire. Glob. Chang. Biol. 2008, 14, 925–937. [Google Scholar] [CrossRef]
- Flora, L.; Madore, M. Stachyose and mannitol transport in olive (Olea europaea L.). Planta 1993, 189. [Google Scholar] [CrossRef]
- Loescher, W.H.; Everard, J.D.; Cantini, C.; Grumet, R. Sugar Alcohol Metabolism in Source Leaves; American Society of Plant Physiologists: Rockville, MA, USA, 1995. [Google Scholar]
- Everard, J.D.; Cantini, C.; Grumet, R.; Plummer, J.; Loescher, W.H. Molecular Cloning of Mannose-6-Phosphate Reductase and Its Developmental Expression in Celery. Plant Physiol. 1997, 113, 1427–1435. [Google Scholar] [CrossRef]
- Pharr, D.M.; Stoop, J.M.H.; Williamson, J.D.; Feusi, M.E.S.; Massel, M.O.; Conkling, M.A. The Dual Role of Mannitol as Osmoprotectant and Photoassimilate in Celery. HortScience 1995, 30, 1182–1188. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar alcohols as carbohydrate reserves in some higher plants. In Carbohydrate Reserves in Plants-Synthesis and Regulation; Elsevier: Amsterdam, The Netherlands, 2000; p. 337. [Google Scholar]
- Loescher, W.H.; Everard, J.D. Regulation of Sugar Alcohol Biosynthesis. In Photosynthesis Physiology and Metabolism; Springer: Berlin, Germany, 2000; pp. 275–299. [Google Scholar]
- Dichio, B.; Xiloyannis, C.; Angelopoulos, K.; Nuzzo, V.; Bufo, S.A.; Celano, G. Drought-induced variations of water relations parameters in Olea europaea. Plant Soil 2003, 257, 381–389. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Agasse, A.; Lemoine, R.; Delrot, S.; Tavares, R.; Geros, H. Utilization and Transport of Mannitol in Olea europaea and Implications for Salt Stress Tolerance. Plant Cell Physiol. 2006, 48, 42–53. [Google Scholar] [CrossRef]
- Ennajeh, M.; Vadel, A.M.; Khemira, H. Osmoregulation and osmoprotection in the leaf cells of two olive cultivars subjected to severe water deficit. Acta Physiol. Plant. 2009, 31, 711–721. [Google Scholar] [CrossRef]
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of Mannitol-Accumulating Transgenic Wheat to Water Stress and Salinity. Plant Physiol. 2003, 131, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Mannitol Protects against Oxidation by Hydroxyl Radicals. Plant Physiol. 1997, 115, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A.K. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Bilger, W.; Veit, M.; Schreiber, L.; Schreiber, U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol. Plant. 1997, 101, 754–763. [Google Scholar] [CrossRef]
- Luengo Escobar, A.; Alberdi, M.; Acevedo, P.; Machado, M.; Nunes-Nesi, A.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Distinct physiological and metabolic reprogramming by highbush blueberry (Vaccinium corymbosum) cultivars revealed during long-term UV-B radiation. Physiol. Plant. 2017, 160, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K. Mathematical Contributions to the Theory of Evolution. III. Regression, Heredity and Panmixia. Philos. Trans. R. Soc. Lond. Ser. A 1896, 253–318. [Google Scholar] [CrossRef]
- Allen, D.J.; Mckee, I.F.; Farage, P.K.; Baker, N.R. Analysis of limitations to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Environ. 1997, 20, 633–640. [Google Scholar] [CrossRef]
- Correia, C.M.; Coutinho, J.F.; Bacelar, E.A.; Gonçalves, B.M.; Björn, L.O.; Moutinho Pereira, J. Ultraviolet-B Radiation and Nitrogen Affect Nutrient Concentrations and the Amount of Nutrients Acquired by Above-Ground Organs of Maize. Sci. World J. 2012, 2012, 608954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sriastava, A.; Strasser, R.J.; Govindjee, G. Greening of Peas: Parallel Measurements of 77 K Emission Spectra, OJIP Chlorophyll a Fluorescence Transient, Period Four Oscillation of the Initial Fluorescence Level, Delayed Light Emission, and P700. Photosynthetica 1999, 37. [Google Scholar] [CrossRef]
- Suzuki, R.; Takahashi, M.; Furuya, K.; Ishimaru, T. Simplified technique for the rapid determination of phytoplankton pigments by reverse-phase high-performance liquid chromatography. J. Oceanogr. 1993, 49, 571–580. [Google Scholar] [CrossRef]
| Time (min.) | % A | % B | % C |
|---|---|---|---|
| Initial | 75 | 25 | 0 |
| 4 | 75 | 25 | 0 |
| 5 | 100 | 0 | 0 |
| 11 | 80 | 0 | 20 |
| 20 | 65 | 0 | 35 |
| 30 | 75 | 25 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccini, C.; Cai, G.; Dias, M.C.; Romi, M.; Longo, R.; Cantini, C. UV-B Radiation Affects Photosynthesis-Related Processes of Two Italian Olea europaea (L.) Varieties Differently. Plants 2020, 9, 1712. https://doi.org/10.3390/plants9121712
Piccini C, Cai G, Dias MC, Romi M, Longo R, Cantini C. UV-B Radiation Affects Photosynthesis-Related Processes of Two Italian Olea europaea (L.) Varieties Differently. Plants. 2020; 9(12):1712. https://doi.org/10.3390/plants9121712
Chicago/Turabian StylePiccini, Chiara, Giampiero Cai, Maria Celeste Dias, Marco Romi, Roberta Longo, and Claudio Cantini. 2020. "UV-B Radiation Affects Photosynthesis-Related Processes of Two Italian Olea europaea (L.) Varieties Differently" Plants 9, no. 12: 1712. https://doi.org/10.3390/plants9121712
APA StylePiccini, C., Cai, G., Dias, M. C., Romi, M., Longo, R., & Cantini, C. (2020). UV-B Radiation Affects Photosynthesis-Related Processes of Two Italian Olea europaea (L.) Varieties Differently. Plants, 9(12), 1712. https://doi.org/10.3390/plants9121712






