Abscisic Acid and Sulfate Offer a Possible Explanation for Differences in Physiological Drought Response of Two Maize Near-Isolines
Abstract
1. Introduction
2. Results
2.1. Long Exposure to Chemicals
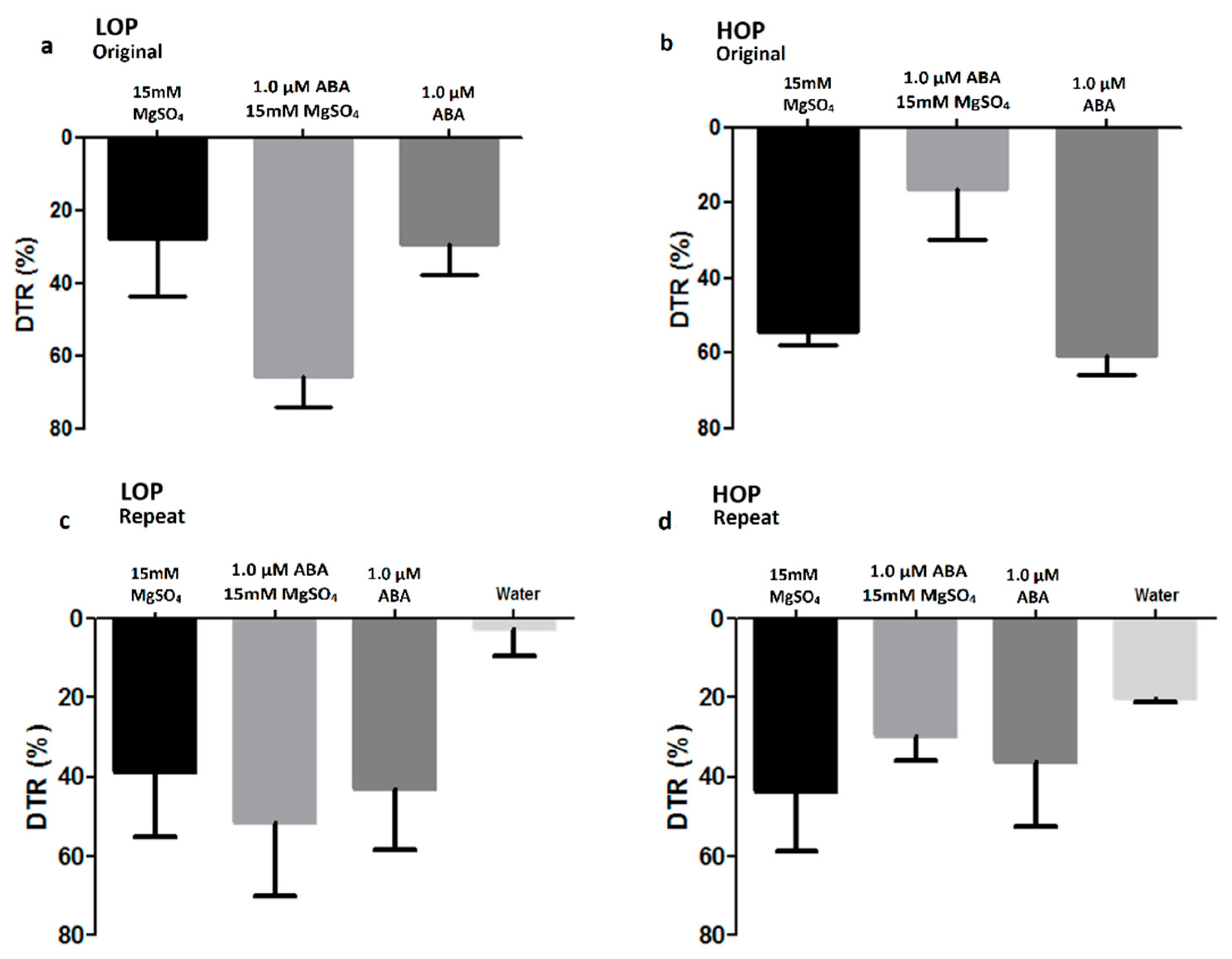
2.1.1. Intact Plants Grown on Soil
2.1.2. Intact Plants Grown Hydroponically
2.2. Short Exposure to Chemicals
2.2.1. Intact Plants
2.2.2. Detached Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Long Exposure to Chemicals
4.2.1. Intact Plants Grown on Soil
4.2.2. Intact Plants Grown Hydroponically
4.3. Short Exposure to Chemicals
4.3.1. Intact Plants
4.3.2. Detached Leaves
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| VPD | Vapor pressure deficit |
| OP | Osmotic potential |
| LOP | Low osmotic potential |
| HOP | High osmotic potential |
| TR | Transpiration rate |
| DTR | Decreases in transpiration rate |
| FTSW | Fraction of transpirable soil water |
| NTR | Normalized transpiration rate |
References
- Devi, M.J.; Reddy, V.R. Transpiration Response of Cotton to Vapor Pressure Deficit and Its Relationship with Stomatal Traits. Front. Plant Sci. 2018, 9, 1572. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.; Domec, J.C.; Oren, R.; Way, D.A.; Moshelion, M. Growth and physiological responses of isohydric and anisohydric poplars to drought. J. Exp. Bot. 2015, 66, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.L.; Sinclair, T.R.; Allen, L.H., Jr. Transpiration responses to vapor pressure deficit in well-watered “slow-wilting” and commercial soybean. Environ. Exp. Bot. 2007, 61, 145–151. [Google Scholar] [CrossRef]
- Devi, M.J.; Sinclair, T.R.; Vadez, V. Genotypic variation in peanut for transpiration response to vapor pressure deficit. Crop Sci. 2010, 50, 191–196. [Google Scholar] [CrossRef]
- Kholová, J.; Hash, C.T.; Kakkera, A.; Koèová, M.; Vadez, V. Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [Pennisetum glaucum (L.) R. Br.]. J. Exp. Bot. 2010, 61, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Schoppach, R.; Sadok, W. Transpiration sensitivities to evaporative demand and leaf areas vary with night and day warming regimes among wheat genotypes. Funct. Plant Biol. 2013, 40, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Shekoofa, A.; Sinclair, T.R.; Messina, C.D.; Cooper, M. Variation among maize hybrids in response to high vapor pressure deficit at high temperatures. Crop Sci. 2016, 56, 392–396. [Google Scholar] [CrossRef]
- Beseli, A.; Shekoofa, A.; Mujahid, A.; Sinclair, T.R. Temporal water use by two maize lines differing in leaf osmotic potential. Crop Sci. 2020, 60, 945–953. [Google Scholar] [CrossRef]
- Shekoofa, A.; Devi, J.M.; Sinclair, T.R.; Holbrook, C.C.; Isleib, T.G. Divergence in drought-resistance traits among parents of recombinant peanut inbred lines. Crop Sci. 2013, 53, 2569–2576. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 1989, 29, 230–233. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Chopra, R. Osmotic adjustment in chickpea in relation to seed yield and yield parameters. Crop Sci. 2004, 44, 449–455. [Google Scholar] [CrossRef]
- Taiz, I.; Zeiger, E.; Meller, E.I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2015; p. 761. [Google Scholar]
- Chimenti, C.A.; Marcantonio, M.; Hall, A.J. Divergent selection for osmotic adjustment results in improved drought tolerance in maize (Zea mays L.) in both early growth and flowering phases. Field Crops Res. 2006, 95, 305–315. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 2002, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhang, J. Controlled alternate partial root-zone irrigation: Its physiological consequences and impact on water use efficiency. J. Exp. Bot. 2004, 55, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shahnazari, A.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Physiological responses of potato (Solanum tuberosum L.) to partial root-zone drying: ABA signalling, leaf gas exchange, and water use efficiency. J. Exp. Bot. 2006, 57, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Sci. Hortic. Amst. 2009, 122, 281–287. [Google Scholar] [CrossRef]
- Jokhan, A.D.; Else, M.A.; Jackson, M.B. Delivery rates of abscisic acid in xylem sap of Ricinus communis L. plants subjected to part-drying of the soil. J. Exp. Bot. 1996, 47, 1595–1599. [Google Scholar] [CrossRef]
- Wojcik-Jagla, M.; Rapacz, M.; Barcik, W.; Janowiak, F. Differential regulation of barley (Hordeum distichon) HVA1 and SRG6 transcript accumulation during the induction of soil and leaf water deficit. Acta Physiol. Plant. 2012, 34, 2069–2078. [Google Scholar] [CrossRef][Green Version]
- Ernst, L.; Goodger, J.Q.D.; Alvarez, S.; Marsh, E.L.; Berla, B.; Lockhart, E.; Jung, J.Y.; Li, P.; Bohnert, H.J.; Schachtman, D.P. Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J. Exp. Bot. 2010, 61, 3395–3405. [Google Scholar] [CrossRef]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA signalling and its relation to other signalling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J. Plant Growth Regul. 2005, 24, 285–295. [Google Scholar] [CrossRef]
- Batool, S.; Uslu, V.V.; Rajab, H.; Ahmad, N.; Waadt, R.; Geiger, D.; Malagoli, M.; Xiang, C.B.; Hedrich, R.; Rennenberg, H.; et al. Sulfate is Incorporated into Cysteine to Trigger ABA Production and Stomatal Closure. Plant Cell 2018, 30, 2973–2987. [Google Scholar] [CrossRef] [PubMed]
- Goodger, J.Q.D.; Sharp, R.E.; Marsh, E.L.; Schachtman, D.P. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J. Exp. Bot. 2005, 56, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Saravitz, C.H.; Downs, R.J.; Thomas, J.F. Phytotron Procedural Manual: For Controlled-Environment Research at the Southeastern Plant Environment Laboratory. 2009. Available online: https://phytotron.ncsu.edu/wp-content/uploads/2015/10/manual.pdf (accessed on 23 October 2015).
- Sadok, W.; Sinclair, T.R. Genetic variability of transpiration response of soybean [Glycine max (L.) Merr.] shoots to leaf hydraulic conductance inhibitor AgNO3. Crop Sci. 2010, 50, 1423–1430. [Google Scholar] [CrossRef]
- Devi, M.J.; Sadok, W.; Sinclair, T.R. Transpiration response of de-rooted peanut plants to aquaporin inhibitors. Environ. Exp. Bot. 2012, 78, 167–172. [Google Scholar] [CrossRef]

| Experiment | |||||||
|---|---|---|---|---|---|---|---|
| Maize Isolines | TR vs. VPD Soil Substrate | TR vs. VPD Hydroponic Substrate | |||||
| HOP | ABA (1.0 µM) | MgSO4 (15 mM) | ABA + MgSO4 (1.0 µM + 15 mM) | ABA (1.0 µM) | MgSO4 (15 mM) | ABA + MgSO4 (1.0 µM + 15 mM) | Water |
| Slope 1 ± SE a | 15.23 ± 2.34 | 15.12 ± 2.86 | 17.33 ± 1.40 | 21.56 ± 2.49 | 18.45 ± 2.36 | 16.06 ± 1.64 | 12.16 ± 1.04 |
| Slope 1- 95% Confidence Interval | 10.0 to 20.4 | 8.7 to 21.5 | 14.2 to 20.4 | 16.3 to 26.7 | 13.5 to 23.3 | 12.6 to 19.4 | 10.0 to 14.3 |
| Slope 2 ± SE | - | - | - | - | - | - | - |
| BP b | - | - | - | - | - | - | - |
| R2 | 0.80 | 0.73 | 0.93 | 0.77 | 0.73 | 0.81 | 0.86 |
| LOP | |||||||
| Slope 1 ± SE | 30.77 ± 12.2 | 14.95 ± 2.18 | 12.79 ± 2.01 | 14.24 ± 1.90 | 29.78 ± 6.42 | 23.19 ± 4.14 | 16.68 ± 3.71 |
| Slope 1- 95% Confidence Interval | 2.59 to 59.0 | 8.29 to 17.2 | 10.0 to 19.8 | 10.2 to 18.1 | 16.3 to 43.1 | 14.5 to 31.8 | 8.87 to 24.4 |
| Slope 2 ± SE | 9.33 ± 2.23 | - | - | - | −15.34 ± 13.38 | −38.09 ± 50.86 | 7.85 ± 3.72 |
| BP | 1.24 ± 0.35 | - | - | - | 2.41 ± 0.264 | 2.60 ± 0.44 | 2.20 ± 0.62 |
| R2 | 0.97 | 0.82 | 0.80 | 0.71 | 0.62 | 0.66 | 0.83 |
| Experiment Description | TR vs. VPD | DTR (%) | ||
|---|---|---|---|---|
| Long Exposure/Intact Plant | Short Exposure | |||
| Soil | Hydroponic | Intact Plant | Detached Leaf | |
| Pot or flask volume | 1.4 L Pot | 1.4 L Pot | 1.0 L Erlenmeyer flask | 2.3 L Pot |
| Substrate during growth | Garden plus topsoil | Hydroponic solution [25] | Hydroponic solution [25] | Garden plus topsoil |
| Substrate during experiment | 1.0 µM ABA 15 mM MgSO4 (1.0 µM + 15 mM) | 1.0 µM ABA 15 mM MgSO4 (1.0 µM + 15 mM) | 1.0 µM ABA 15 mM MgSO4 (1.0 µM + 15 mM) | Deionized water 1.0 µM ABA 15 mM MgSO4 (1.0 µM + 15 mM) |
| Environment during growth | Walk-in growth chamber | Walk-in growth chamber | Greenhouse | Greenhouse |
| Environment during experiment | 21 L chamber inside a walk-in growth chamber | 21 L chamber inside a walk-in growth chamber | Walk-in growth chamber | Walk-in growth chamber |
| VPD (kPa) | 0.5–3.5 | 0.5–3.5 | 3.0–3.2 | 3.0–3.2 |
| Duration of growth | 30 d | 30 d | 29 d | 29 d |
| Duration of chemical treatments | 2 d * | 2 d | 180 min ** | 180 min |
| Duration of experiment | 2 d | 2 d | 1 d | 1 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekoofa, A.; Sinclair, T.R. Abscisic Acid and Sulfate Offer a Possible Explanation for Differences in Physiological Drought Response of Two Maize Near-Isolines. Plants 2020, 9, 1713. https://doi.org/10.3390/plants9121713
Shekoofa A, Sinclair TR. Abscisic Acid and Sulfate Offer a Possible Explanation for Differences in Physiological Drought Response of Two Maize Near-Isolines. Plants. 2020; 9(12):1713. https://doi.org/10.3390/plants9121713
Chicago/Turabian StyleShekoofa, Avat, and Thomas R. Sinclair. 2020. "Abscisic Acid and Sulfate Offer a Possible Explanation for Differences in Physiological Drought Response of Two Maize Near-Isolines" Plants 9, no. 12: 1713. https://doi.org/10.3390/plants9121713
APA StyleShekoofa, A., & Sinclair, T. R. (2020). Abscisic Acid and Sulfate Offer a Possible Explanation for Differences in Physiological Drought Response of Two Maize Near-Isolines. Plants, 9(12), 1713. https://doi.org/10.3390/plants9121713





