Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen
Abstract
1. Introduction
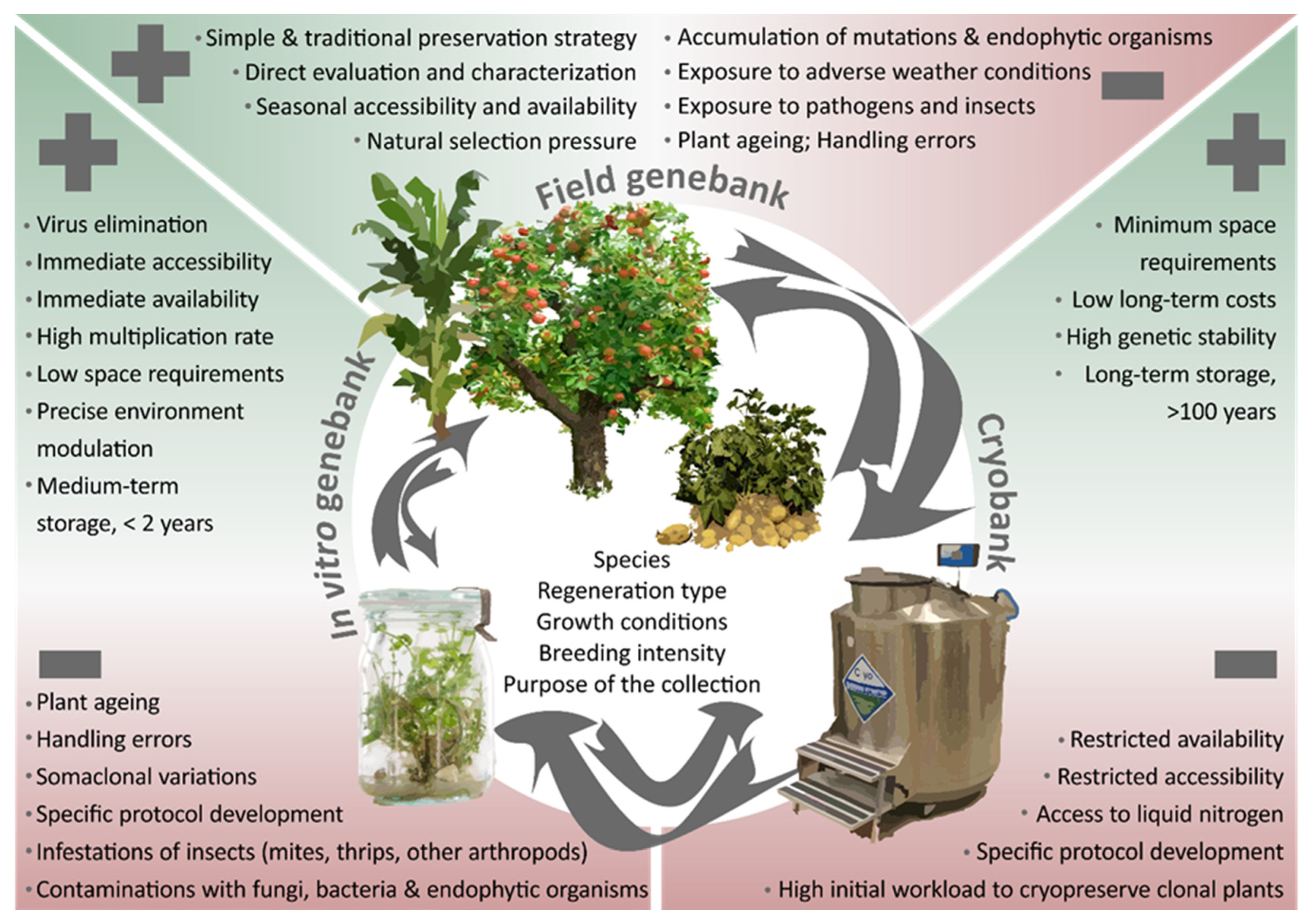
2. Field Genebanks
2.1. Management of Field Genebanks
2.2. Advantage of Field Collections: Characterization and Evaluation
2.3. Problems Associated with Field Collections
3. In Vitro Collections
3.1. Setting Up In Vitro Collections
3.2. Advantages of In Vitro Collection
3.3. Problems Associated with In Vitro Collections

4. Cryopreserved Collections
4.1. Setting-Up Cryopreserved Collections
| Institute | Country | Crop | Cryopreservation Method | Number of Accessions | Ref |
|---|---|---|---|---|---|
| AFOCEL | France | Elm | • Dormant bud freezing | 440 | [80] |
| Bioversity International, Leuven | Belgium | Banana | • Droplet vitrification | 1100 | Panis, personal communication, 2020 |
| Crop Research Institute, Prague | Czech Republic | garlic | • Droplet vitrification | 157 | [81] |
| International Center for Tropical Agriculture (CIAT), Cali | Colombia | cassava | • Droplet vitrification • Encapsulation/dehydration | 480 | [80] |
| International Institute of Tropical Agriculture (IITA), Ibadan | Nigeria | Yam | • Droplet vitrification | 27 | [82] |
| International Potato Center (CIP), Lima | Peru | Potato | • Straw vitrification • Droplet vitrification | 3264 (Situation 14 October 2020) | [83] |
| Julius Kühn-Institut (JKI), Institut für Züchtungsforschung an Obst, Dresden | Germany | Strawberry | • Vitrification | 194 | [84] |
| Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben | Germany | Potato, | • Droplet freezing • Droplet vitrification | 1818 | Nagel, personal communication, 2020 |
| Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben | Germany | Mint | • Droplet vitrification | 157 | Nagel, personal communication, 2020 |
| Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben | Germany | Garlic and shallot | • Droplet vitrification | 213 | Nagel, personal communication, 2020 |
| National Agrobiodiversity Center (NAAS), RDA, Suwon | South Korea | Garlic | • Droplet vitrification | 1158 | [85] |
| National Institute of Agrobiological Sciences (NIAS), Tsukuba | Japan | Mulberry | • Dormant bud freezing | 1236 | [80] |
| Tissue Culture and Cryopreservation Unit, NBPGR, Delhi | India | Mulberry | • Dormant bud freezing | 329 | [80] |
| USDA-ARS, Ford Collins and Corvallis | USA | Citrus | • Droplet vitrification | 451 | [86] |
| USDA-ARS, Ford Collins and Corvallis | USA | Apple | • Dormant bud freezing | 2155 | [87] |
4.1.1. Dormant Bud Cryopreservation
4.1.2. Droplet Vitrification
4.2. Advantages of a Cryopreserved Collection: Safety Backup for Clonally Propagated Crops
4.3. Problems Associated with Cryopreserved Collections
5. Identification of Unique Accessions and Elimination of Duplicates
6. Costs Associated with the Different Conservation Methods
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Genebank Standards for Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014.
- Gepts, P.; Hancock, J. The future of plant breeding. Crop Sci. 2006, 46, 1630–1634. [Google Scholar] [CrossRef]
- Building on Gender, Agrobiodiversity and Local Knowledge; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004.
- Thrupp, L.A. Linking agricultural biodiversity and food security: The valuable role of agrobiodiversity for sustainable agriculture. Int. Aff. 2000, 76, 265–281. [Google Scholar] [CrossRef] [PubMed]
- The State of the World’s Biodiversity for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019.
- WIEWS—World Information and Early Warning System on Plant Genetic Resources for Food and Agriculture; Food and Agriculture Orgnization of the United Nations: Rome, Italy, 2020.
- Rajasekharan, P.E.; Sahijram, L. In vitro conservation of plant germplasm. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K., Eds.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- McKey, D.; Elias, M.; Pujol, B.; Duputie, A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010, 186, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Bramel, P.J.; Volk, G.M. A Global Strategy for the Conservation and Use of Apple Genetic Resources; Global Crop Diversity Trust: Bonn, Germany, 2019. [Google Scholar]
- Damania, A.B. History, achievements, and current status of genetic resources conservation. Agron. J. 2008, 100, S27–S39. [Google Scholar] [CrossRef]
- Müntz, K.; Wobus, U. Das Institut Gatersleben und Seine Geschichte Genetik und Kulturpflanzenforschung in Drei Politischen Systemen; Springer spectrum: Berlin/Heidelberg, Germany, 2013; p. 459. [Google Scholar] [CrossRef]
- Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The Ex Situ Conservation of Plant Genetic Resources; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Acker, J.P.; Adkins, S.; Alves, A.; Horna, D.; Toll, J. Feasibility Study for a Safety Backup Cryopreservation Facility; Bioversity International: Maccarese-Stazione, Italy, 2017. [Google Scholar]
- Reed, B.M.; Engelmann, F.; Dulloo, M.E.; Engels, J.M.M. Technical Guidelines for the Management of Field and In Vitro Germplasm Collections; International Plant Genetics Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Volk, G.; Henk, A. Historic american apple cultivars: Identification and availability. J. Am. Soc. Hortic. Sci. 2016, 141, 292–301. [Google Scholar] [CrossRef]
- Höfer, M.; Flachowsky, H.; Hanke, M.V. German Fruit Genebank–looking back 10 years after launching a national network for sustainable preservation of fruit genetic resources. J. Cultiv. Plants 2019, 71. [Google Scholar] [CrossRef]
- Huaman, S.; Salas, A.; Gomez, R.; Panta, A.; Toledo, J. Conservation of potato genetic resources at CIP. In Potato, Global Research & Development; Khurana, S.M.P., Shekhawat, G.S., Singh, B.P., Pandey, S.K., Eds.; Indian Potato Association: Shimla, India, 2000; Volume 1. [Google Scholar]
- Forbes, G.A.; Charkowski, A.; Andrade-Piedra, J.; Parker, M.L.; Schulte-Geldermann, E. Potato Seed Systems. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 431–447. [Google Scholar] [CrossRef]
- Bachmann-Pfabe, S.; Hammann, T.; Kruse, J.; Dehmer, K.J. Screening of wild potato genetic resources for combined resistance to late blight on tubers and pale potato cyst nematodes. Euphytica 2019, 215, 48. [Google Scholar] [CrossRef]
- Keller, E.R.J.; Kik, C. Allium genetic resources. Compend. Plant Genome 2018. [Google Scholar] [CrossRef]
- Keller, E.R.J.; Zanke, C.D.; Senula, A.; Breuing, A.; Hardeweg, B.; Winkelmann, T. Comparing costs for different conservation strategies of garlic (Allium sativum L.) germplasm in genebanks. Genet. Resour. Crop Evol. 2013, 60, 913–926. [Google Scholar] [CrossRef][Green Version]
- Takagi, H. Garlic Allium sativum L. In Onions and Allied Crops; Brewster, J.L., Rabinowitch, H.D., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume 3, pp. 109–157. [Google Scholar]
- Sharma, K.; Lee, Y.R.; Park, S.W.; Nile, S.H. Importance of growth hormones and temperature for physiological regulation of dormancy and sprouting in onions. Food Rev. Int. 2016, 32, 233–255. [Google Scholar] [CrossRef]
- Senula, A.; Nagel, M. Cryopreservation of plant shoot tips of potato, mint, garlic, and shallot using Plant Vitrification Solution 3. In Cryopreservation and Freeze-Drying Protocols; Humana: New York, NY, USA, 2020; Volume 2180, pp. 647–661. [Google Scholar]
- Keller, E.R.J.; Zanke, C.D.; Blattner, F.R.; Kik, C.; Stavelikova, H.; Zamecnik, J.; Esnault, F.; Kotlinska, T.; Solberg, S.; Miccolis, V. EURALLIVEG: Establishment of a european core collection by cryopreservation and virus elimination in garlic. Acta Hortic. 2012, 969, 319–327. [Google Scholar] [CrossRef]
- Engels, J.M.M.; Visser, L. A Guide to Effective Management of Germplasm Collections; International Plant Genetic Resources Institute: Rome, Italy, 2003. [Google Scholar]
- Volk, G.M.; Chao, C.T.; Norelli, J.; Brown, S.K.; Fazio, G.; Peace, C.; McFerson, J.; Zhong, G.Y.; Bretting, P. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop Evol. 2015, 62, 765–794. [Google Scholar] [CrossRef]
- Herbarium, WU. Available online: https://herbarium.univie.ac.at (accessed on 20 November 2020).
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- The Alliance of Bioversity International and CIAT. Available online: www.bioversityinternational.org (accessed on 20 November 2020).
- Meiyalaghan, S.; Paget, M.; Thompson, S.; Thomson, S.; Baldwin, S.; Anderson, J.; Genet, R.; Lewthwaite, S. High resolution DNA melting markers for identification of H1-linked resistance to potato cyst nematode. Mol. Breed. 2018, 38. [Google Scholar] [CrossRef]
- Zuo, C.; Deng, G.; Li, B.; Huo, H.; Li, C.; Hu, C.; Kuang, R.; Yang, Q.; Dong, T.; Sheng, O.; et al. Germplasm screening of Musa spp. for resistance to Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). Eur. J. Plant Pathol. 2018, 151, 723–734. [Google Scholar] [CrossRef]
- Peil, A.; Emeriewen, O.F.; Khan, A.; Kostick, S.; Malnoy, M. Status of fire blight resistance breeding in Malus. J. Plant Pathol. 2020. [Google Scholar] [CrossRef]
- Höfer, M. Cryopreservation of winter-dormant apple buds: Establishment of a duplicate collection of Malus germplasm. Plant Cell Tissue Organ Cult. 2015, 121, 647–656. [Google Scholar] [CrossRef]
- Bramel, P.; Krishnan, S.; Horna, D.; Lainoff, B.; Montagnon, C. Global Conservation Strategy for Coffee Genetic Resources; Crop Trust: Bonn, Germany, 2017. [Google Scholar]
- Ellis, D.; Chavez, O.; Coombs, J.; Soto, J.; Gomez, R.; Douches, D.; Panta, A.; Silvestre, R.; Anglin, N.L. Genetic identity in genebanks: Application of the SolCAP 12K SNP array in fingerprinting and diversity analysis in the global in trust potato collection. Genome 2018, 61, 523–537. [Google Scholar] [CrossRef]
- Anastasio, A.E.; Platt, A.; Horton, M.; Grotewold, E.; Scholl, R.; Borevitz, J.O.; Nordborg, M.; Bergelson, J. Source verification of mis-identified Arabidopsis thaliana accessions. Plant J. 2011, 67, 554–566. [Google Scholar] [CrossRef]
- Jiang, C.; Mithani, A.; Belfield, E.J.; Mott, R.; Hurst, L.D.; Harberd, N.P. Environmentally responsive genome-wide accumulation of de novo Arabidopsis thaliana mutations and epimutations. Genome Res. 2014, 24, 1821–1829. [Google Scholar] [CrossRef]
- Withers, L.A. Tissue Culture Storage for Genetic Conservation; IBPGR: Rome, Italy, 1980. [Google Scholar]
- Benson, E.E.; Harding, K.; Debouck, D.; Dumet, D.; Escobar, R.; Mafla, G.; Panis, B.; Panta, A.; Tay, D.; Vandenhouwe, I.; et al. Refinement and Standardization of Storage Procedures for Clonal Crops = Global Public Goods Phase 2: Part 1. Project Landscape and General Status of Clonal Crop In Vitro Conservation Technologies; System-wide Genetic Resources Programme: Rome, Italy, 2011. [Google Scholar]
- Genesys. Available online: https://www.genesys-pgr.org/ (accessed on 20 November 2020).
- Bamberg, J.B.; Martin, M.W.; Abad, J.; Jenderek, M.M.; Tanner, J.; Donnelly, D.J.; Nassar, A.M.K.; Veilleux, R.E.; Novy, R.G. In vitro technology at the US Potato Genebank. In Vitro Cell Dev. Plant 2016, 52, 213–225. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M. In vitro plant propagation: A review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar]
- Chen, C.; Chen, J.-J. Measurement of gas exchange rates in plant tissue culture vessels. Plant Cell Tissue Organ Cult. 2002, 71, 103–109. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G.; Luna-Sánchez, I.J. Light quality affects growth and development of in vitro plantlet of Vanilla planifolia Jacks. S. Afr. J. Bot. 2017, 109, 288–293. [Google Scholar] [CrossRef]
- Keller, E.R.J.; Senula, A.; Leunufna, S.; Grube, M. Slow growth storage and cryopreservation—Tools to facilitate germplasm maintenance of vegetatively propagated crops in living plant collections. Int. J. Refrig. 2006, 29, 411–417. [Google Scholar] [CrossRef]
- Vandenhouwe, I.; Desmet, K.; Dumontcel, H.T.; Swennen, R. Variability in storage potential of banana shoot cultures under medium-term storage-conditions. Plant Cell Tissue Organ Cult. 1995, 42, 269–274. [Google Scholar] [CrossRef]
- Jackson, D.M.; Harrison, H.F.; Jarret, R.L.; Wadl, P.A. Phenotypic variation in leaf morphology of the USDA, ARS Sweetpotato (Ipomoea batatas) germplasm collection. Hortscience 2020, 55, 465–475. [Google Scholar] [CrossRef]
- da Silva, R.L.; Ferreira, C.F.; da Silva Ledo, C.A.; de Souza, E.H.; da Silva, P.H.; de Carvalho Costa, M.A.P.; Souza, F.V.D. Viability and genetic stability of pineapple germplasm after 10 years of in vitro conservation. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 123–133. [Google Scholar] [CrossRef]
- Sarkar, D.; Chakrabarti, S.K.; Naik, P.S. Slow-growth conservation of potato microplants: Efficacy of ancymidol for long-term storage in vitro. Euphytica 2001, 117, 133–142. [Google Scholar] [CrossRef]
- Sarkar, D.; Kaushik, S.; Naik, P.S. Minimal growth conservation of potato microplants: Silver thiosulfate reduces ethylene-induced growth abnormalities during prolonged storage in vitro. Plant Cell Rep. 1999, 18, 897–903. [Google Scholar] [CrossRef]
- Sarkar, D.; Naik, P.S. Factors affecting minimal growth conservation of potato microplants in vitro. Euphytica 1998, 102, 275–280. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, V.; Quraishi, A. In vitro conservation through slow-growth storage. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A.A., Eds.; Springer International Publishing: Cham, Swizerland, 2019; pp. 397–416. [Google Scholar] [CrossRef]
- Guta, I.C.; Buciumenau, E.C.; Tataru, L.D.; Oprescu, B.; Topala, C.M. New approach of electrotherapy for grapevine virus elimination. Acta Hortic. 2019, 1242, 697–702. [Google Scholar] [CrossRef]
- Wang, Q.C.; Valkonen, J.P.T. Cryotherapy of shoot tips: Novel pathogen eradication method. Trends Plant Sci. 2009, 14, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Helliot, B.; Panis, B.; Poumay, Y.; Swennen, R.; Lepoivre, P.; Frison, E. Cryopreservation for the elimination of cucumber mosaic and banana streak viruses from banana (Musa spp.). Plant Cell Rep. 2002, 20, 1117–1122. [Google Scholar] [CrossRef]
- Singh, D.H.; Uma, S.; Selvarajan, R.; Karihaloo, J. Micropropagation for Production of Quality Banana Planting Material in Asia-Pacific; Asia-Pacific Consortium on Agricultural Biotechnology (APCoAB): New Delhi, India, 2011; p. 92. [Google Scholar]
- Bhende, S.; Kurien, S. Sucker production in banana. J. Trop. Agric. 2015, 53, 97–106. [Google Scholar]
- Bairu, M.W.; Aremu, A.O.; van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Miguel, C.; Marum, L. An epigenetic view of plant cells cultured in vitro: Somaclonal variation and beyond. J. Exp. Bot. 2011, 62, 3713–3725. [Google Scholar] [CrossRef]
- Roels, S.; Escalona, M.; Cejas, I.; Noceda, C.; Rodriguez, R.; Canal, M.J.; Sandoval, J.; Debergh, P. Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell Tissue Organ Cult. 2005, 82, 57–66. [Google Scholar] [CrossRef]
- Mohanty, S.; Panda, M.K.; Subudhi, E.; Nayak, S. Plant regeneration from callus culture of Curcuma aromatica and in vitro detection of somaclonal variation through cytophotometric analysis. Biol. Plant. 2008, 52, 783–786. [Google Scholar] [CrossRef]
- Bairu, M.W.; Fennell, C.W.; van Staden, J. The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv. ‘Zelig’). Sci. Hortic. Amst. 2006, 108, 347–351. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Houben, A.; Keller, E.R.J.; Mette, M.F. Influence of cryopreservation on the cytosine methylation state of potato genomic DNA. Cryoletters 2010, 31, 380–391. [Google Scholar] [PubMed]
- Smykal, P.; Valledor, L.; Rodriguez, R.; Griga, M. Assessment of genetic and epigenetic stability in long-term in vitro shoot culture of pea (Pisum sativum L.). Plant Cell Rep. 2007, 26, 1985–1998. [Google Scholar] [CrossRef]
- Skirvin, R.M.; Mcpheeters, K.D.; Norton, M. Sources and frequency of somaclonal variation. Hortscience 1994, 29, 1232–1237. [Google Scholar] [CrossRef]
- Senula, A.; Büchner, D.; Keller, E.R.; Nagel, M. An improved cryopreservation protocol for mentha spp. Based on Pvs3 as the cryoprotectant. Cryo Lett. 2018, 39, 345–353. [Google Scholar]
- Graner, E.M.; Brondani, G.E.; de Almeida, C.V.; Batagin-Piotto, K.D.; de Almeida, M. Study of senescence in old cultures of the Bactris gasipaes Kunth in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 120, 1169–1189. [Google Scholar] [CrossRef]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Keller, E.R.J.; Panis, B.; Engelmann, F. In vitro storage and cryopreservation as substantial complements in concerted actions to better maintain and use crop germplasm. Acta Hortic. 2012, 35–50. [Google Scholar] [CrossRef]
- Van den houwe, I.; Swennen, R. Characterization and control of bacterial contaminants in in vitro cultures of banana (Mus spp.). In Proceedings of the International Symposium on Methods and Markers for Quality Assurance in Micropopagation, Cork, Ireland, 24–27 August 1999; pp. 69–79. [Google Scholar]
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. mBio 2015, 6, e00621-15. [Google Scholar] [CrossRef]
- Jarret, R.L.; Florkowski, W.J. In vitro active vs field genebank maintenance of sweet-potato germplasm—major costs and considerations. Hortscience 1990, 25, 141–146. [Google Scholar] [CrossRef]
- Christelova, P.; de Langhe, E.; Hribova, E.; Cizkova, J.; Sardos, J.; Husakova, M.; van den Houwe, I.; Sutanto, A.; Kepler, A.K.; Swennen, R.; et al. Molecular and cytological characterization of the global Musa germplasm collection provides insights into the treasure of banana diversity. Biodivers. Conserv. 2017, 26, 801–824. [Google Scholar] [CrossRef]
- Thomas, J.E. MusaNet Technical Guidelines for the Safe Movement of Musa Germplasm, 3rd ed.; Bioversity International: Rome, Italy, 2015. [Google Scholar]
- Panis, B.; Totte, N.; VanNimmen, K.; Withers, L.A.; Swennen, R. Cryopreservation of banana (Musa spp.) meristem cultures after preculture on sucrose. Plant Sci. 1996, 121, 95–106. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Sakai, A. Survival of plant tissue at super-low temperature III. relation between effective prefreezing temperatures and the degree of front hardiness. Plant Physiol. 1965, 40, 882–887. [Google Scholar] [CrossRef]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. In Proceedings of the III International Symposium on Plant Cryopreservation, Bangkok, Thailand, 26–28 March 2018; pp. 1–8. [Google Scholar]
- Niino, T.; Arizaga, M.V. Cryopreservation for preservation of potato genetic resources. Breed Sci. 2015, 65, 41–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Org. 2020. [Google Scholar] [CrossRef]
- Dumet, D.; Diebiru, E.; Adeyemi, A.; Akinyemi, O.; Gueye, B.; Franco, J. Cryopreservation for the ‘in perpetuity’ conservation of yam and cassava genetic resources. Cryoletters 2013, 34, 107–118. [Google Scholar]
- CIP. Cryopreservation. Available online: https://cipotato.org/genebankcip/process/cryopreservation/ (accessed on 14 October 2020).
- Hofer, M.; Hanke, M.V. Cryopreservation of fruit germplasm. In Vitro Cell Dev. Plant 2017, 53, 372–381. [Google Scholar] [CrossRef]
- Kim, H.H.; Popova, E.; Shin, D.J.; Yi, J.Y.; Kim, C.H.; Lee, J.S.; Yoon, M.K.; Engelmann, F. Cryobanking of Korean Allium Germplasm Collections: Results from a 10 Year Experience. Cryoletters 2012, 33, 45–57. [Google Scholar]
- Volk, G.M.; Jenderek, M.M.; Walters, C.; Bonnart, R.; Shepherd, A.; Skogerboe, D.; Hall, B.D.; Moreland, B.; Krueger, R.; Polek, M. Implementation of Citrus shoot tip cryopreservation in the USDA-ARS National Plant Germplasm System. In Proceedings of the III International Symposium on Plant Cryopreservation, Bangkok, Thailand, 26–28 March 2018. [Google Scholar]
- Jenderek, M.M.; Tanner, J.D.; Ambruzs, B.D.; West, M.; Postman, J.D.; Hummer, K.E. Twig pre-harvest temperature significantly influences effective cryopreservation of Vaccinium dormant buds. Cryobiology 2017, 74, 154–159. [Google Scholar] [CrossRef]
- Maurya, J.P.; Bhalerao, R.P. Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: A molecular perspective. Ann. Bot. Lond. 2017, 120, 351–360. [Google Scholar] [CrossRef]
- Stushnoff, C. Cryopreservation of apple genetic-resources. Can. J. Plant Sci. 1987, 67, 1151–1154. [Google Scholar] [CrossRef]
- Lambardi, M.; Benelli, C.; de Carlo, A.; Ozudogru, E.A.; Previati, A.; Ellis, D. Cryopreservation of ancient apple cultivars of veneto: A Comparison between PVS2-vitrification and dormant-bud techniques. Int. Symp. Cryopreserv. Hortic. Species 2011, 908, 191–198. [Google Scholar] [CrossRef]
- Guyader, A.; Guisnel, R.; Simmoneau, F.; Rocand, B.; Le Bras, C.; Grapin, A.; Chatelet, P.; Dussert, S.; Engelmann, F.; Feugey, L.; et al. First results on cryopreservation by dormant bud technique of a set of Malus and Pyrus cultivars from the INRA biological resources centre. In Proceedings of the Cryopreservation of crop species in Europe: COST Action 871: CryoPlanet: Proceedings of the final meeting, Angers, France, 8–11 February 2011. [Google Scholar]
- Towill, L.E.; Forsline, P.L. Cryopreservation of sour cherry (Prunus cerasus L.) using a dormant vegetative bud method. Cryoletters 1999, 20, 215–222. [Google Scholar]
- Fukui, K.; Shirata, K.; Niino, T.; Kashif, I.M. Cryopreservation of Mulberry Winter Buds in Japan. Int. Symp. Cryopreserv. Hortic. Species 2011, 908, 483–488. [Google Scholar] [CrossRef]
- Rantala, S.; Kaseva, J.; Karhu, S.; Vetelainen, M.; Uosukainen, M.; Haggman, H. Cryopreservation of Ribes nigrum (L.) dormant buds: Recovery via in vitro culture to the field. Plant Cell Tissue Organ Cult. 2019, 138, 109–119. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Andre, E.; van den Houwe, I.; Swennen, R. Droplet vitrification: The first generic cryopreservation protocol for organized plant tissues? Int. Symp. Cryopreserv. Hortic. Species 2011, 908, 157–163. [Google Scholar] [CrossRef]
- Folgado, R.; Panis, B.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.F. Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 2015, 71, 432–441. [Google Scholar] [CrossRef]
- Panta, A.; Panis, B.; Ynouye, C.; Swennen, R.; Roca, W.; Tay, D.; Ellis, D. Improved cryopreservation method for the long-term conservation of the world potato germplasm collection. Plant Cell Tissue Organ Cult. 2015, 120, 117–125. [Google Scholar] [CrossRef]
- Kopnick, C.; Grube, M.; Stock, J.; Senula, A.; Mock, H.P.; Nagel, M. Changes of soluble sugars and ATP content during DMSO droplet freezing and PVS3 droplet vitrification of potato shoot tips. Cryobiology 2018, 85, 79–86. [Google Scholar] [CrossRef]
- Sant, R.; Panis, B.; Taylor, M.; Tyagi, A. Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia esculenta var. esculenta) accessions. Plant Cell Tissue Organ Cult. 2008, 92, 107–111. [Google Scholar] [CrossRef]
- Vollmer, R.; Panta, A.; Tay, D.; Roca, W.; Ellis, D. Effect of sucrose preculture and PVS2 exposure on the cryopreservation of sweet potato shoot tips [Ipomoea batatas (L.) Lam.] Using the PVS2 Droplet Vitrification. Ii Int. Symp. Plant Cryopreserv. 2014, 1039, 265–271. [Google Scholar] [CrossRef]
- Gallard, A.; Panis, B.; Dorion, N.; Swennen, R.; Grapin, A. Cryopreservation of pelargonium apices by droplet-vitrification. Cryoletters 2008, 29, 243–251. [Google Scholar] [PubMed]
- Ozudogru, A.; da Silva, D.P.C.; Kaya, E.; Dradi, G.; Paiva, R.; Lambardi, M. In vitro Conservation and Cryopreservation of Nandina domestica, an Outdoor Ornamental Shrub. Not. Bot. Horti Agrobo. 2013, 41, 638–645. [Google Scholar] [CrossRef]
- Mathew, L.; Burritt, D.J.; McLachlan, A.; Pathirana, R. Combined pre-treatments enhance antioxidant metabolism and improve survival of cryopreserved kiwifruit shoot tips. Plant Cell Tissue Organ Cult. 2019, 138, 193–205. [Google Scholar] [CrossRef]
- Silva, L.C.; Paiva, R.; Swennen, R.; Andre, E.; Panis, B. Shoot-tip cryopreservation by droplet vitrification of Byrsonima intermedia A. Juss.: A woody tropical and medicinal plant species from Brazilian cerrado. Cryo Lett. 2013, 34, 338–348. [Google Scholar]
- Ozudogru, E.A.; Kirdok, E.; Kaya, E.; Capuana, M.; Benelli, C.; Engelmann, F. Cryopreservation of Redwood (Sequoia Sempervirens (D. Don.) Endl.) in vitro buds using vitrification-based techniques. Cryoletters 2011, 32, 99–110. [Google Scholar]
- Yamamoto, S.; Rafique, T.; Priyantha, W.S.; Fukui, K.; Matsumoto, T.; Niino, T. Development of a cryopreservation procedure using aluminium cryo-plates. Cryo Lett. 2011, 32, 256–265. [Google Scholar]
- Niino, T.; Yamamoto, S.I.; Fukui, K.; Castillo Martinez, C.R.; Arizaga, M.V.; Matsumoto, T.; Engelmann, F. Dehydration improves cryopreservation of mat rush (Juncus decipiens Nakai) basal stem buds on cryo-plates. Cryo Lett. 2013, 34, 549–560. [Google Scholar]
- Tanaka, D.; Sakuma, Y.; Yamamoto; Valle Arizaga, M.; Niino, T.; Matsumoto, T. Development of −80 °C storage for Allium shoot tips using D cryo-plate method. Plant Cell Tissue Organ Cult. (PCTOC) 2020. [Google Scholar] [CrossRef]
- Ruta, C.; Lambardi, M.; Ozudogru, E.A. Biobanking of vegetable genetic resources by in vitro conservation and cryopreservation. Biodiv. Conserv. 2020, 29, 3495–3532. [Google Scholar] [CrossRef]
- Asdal, A.; Guarino, L. The svalbard global seed vault: 10 Years-1 million samples. Biopreserv. Biobank. 2018, 16, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.; Namuth-Covert, D.; Byrne, P. Training in plant genetic resources management: A way forward. Crop Sci. 2019, 59. [Google Scholar] [CrossRef]
- Greene, S.L.; Pederson, G.A. Eliminating duplicates in germplasm collections: A white clover example. Crop Sci. 1996, 36, 1398–1400. [Google Scholar] [CrossRef]
- van Treuren, R.; Kemp, H.; Ernsting, G.; Jongejans, B.; Houtman, H.; Visser, L. Microsatellite genotyping of apple (Malus x domestica Borkh.) genetic resources in the Netherlands: Application in collection management and variety identification. Genet. Resour. Crop Evol. 2010, 57, 853–865. [Google Scholar] [CrossRef]
- Milner, S.G.; Jost, M.; Taketa, S.; Mazon, E.R.; Himmelbach, A.; Oppermann, M.; Weise, S.; Knupffer, H.; Basterrechea, M.; Konig, P.; et al. Genebank genomics highlights the diversity of a global barley collection. Nat. Genet. 2019, 51, 319–326. [Google Scholar] [CrossRef]
- Gross, B.L.; Volk, G.M.; Richards, C.M.; Forsline, P.L.; Fazio, G.; Chao, C.T. Identification of “duplicate” accessions within the USDA-ARS national plant germplasm system malus collection. J. Am. Soc. Hortic. Sci. 2012, 137, 333–342. [Google Scholar] [CrossRef]
- Zhang, D.; Arevalo-Gardini, E.; Mischke, S.; Zuniga-Cernades, L.; Barreto-Chavez, A.; del Aguila, J.A. Genetic diversity and structure of managed and semi-natural populations of cocoa (Theobroma cacao) in the Huallaga and Ucayali Valleys of Peru. Ann. Bot. Lond. 2006, 98, 647–655. [Google Scholar] [CrossRef]
- Volk, G.M.; Bonnart, R.; Shepherd, A.; Yin, Z.F.; Lee, R.; Polek, M.; Krueger, R. Citrus cryopreservation: Viability of diverse taxa and histological observations. Plant Cell Tissue Organ Cult. 2017, 128, 327–334. [Google Scholar] [CrossRef]
- Ashmore, S.E. Status Report on the Development and Application of In Vitro Techniques for the Conservation and Use of Plant Genetic Resources; International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop Sci. 2011, 5, 778–800. [Google Scholar]
- Pence, V.C. Tissue cryopreservation for plant conservation: Potential and challenges. Int. J. Plant Sci. 2014, 175, 40–45. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panis, B.; Nagel, M.; Van den houwe, I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. https://doi.org/10.3390/plants9121634
Panis B, Nagel M, Van den houwe I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants. 2020; 9(12):1634. https://doi.org/10.3390/plants9121634
Chicago/Turabian StylePanis, Bart, Manuela Nagel, and Ines Van den houwe. 2020. "Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen" Plants 9, no. 12: 1634. https://doi.org/10.3390/plants9121634
APA StylePanis, B., Nagel, M., & Van den houwe, I. (2020). Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants, 9(12), 1634. https://doi.org/10.3390/plants9121634







