Post-Anthesis Water-stressed Barley Maintains Grain Specific Weight Through Altered Grain Composition and Plant Architecture
Abstract
1. Introduction
2. Results
2.1. Quantification of Water Stress and Plant Development
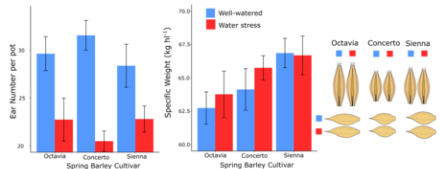
2.2. The Effect of Water Stress on Grain Parameters
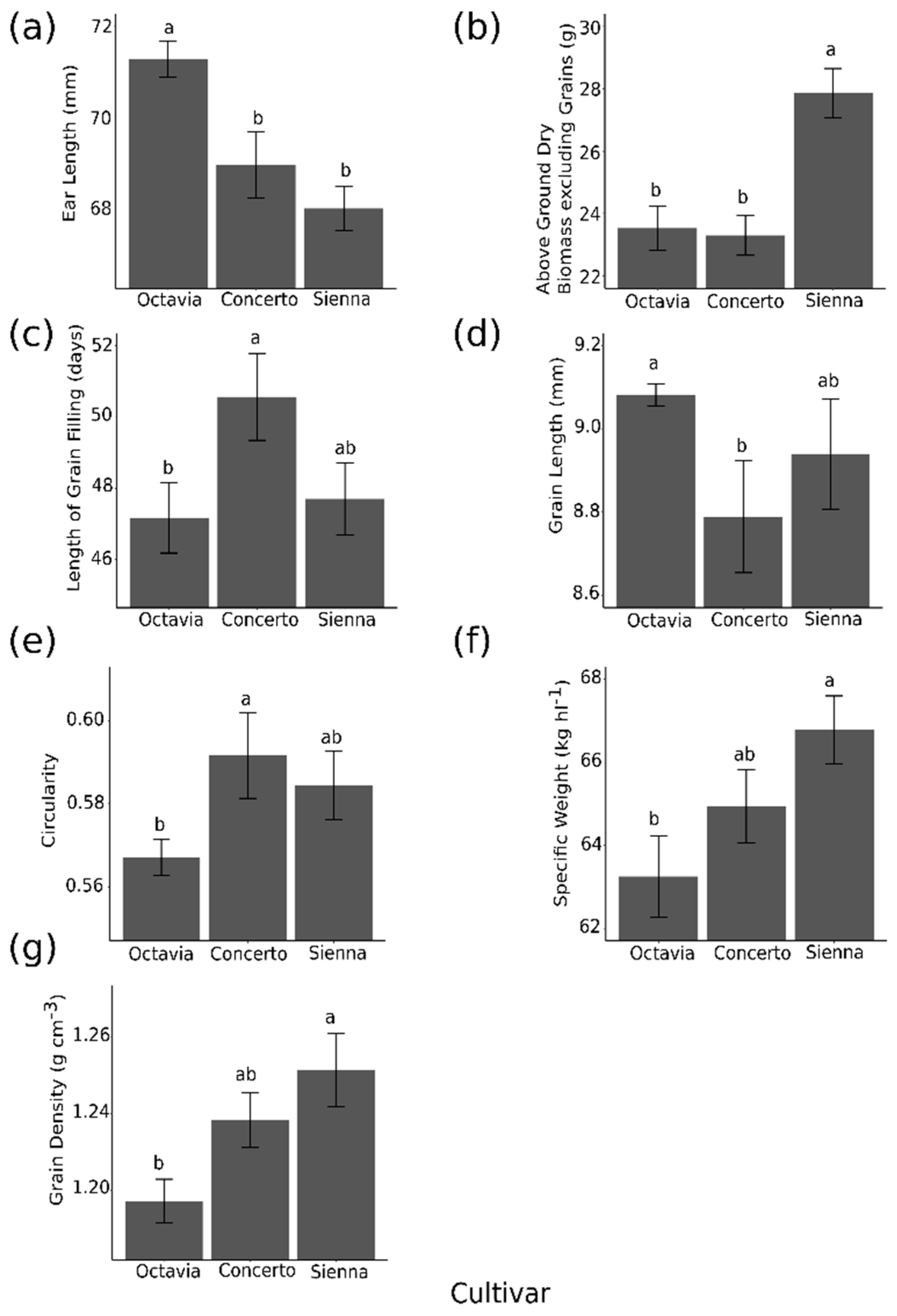
2.3. The Effect of Cultivar on Grain Parameters
2.4. Correlations with Components of Specific Weight
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth
4.2. Water Stress Treatment
4.3. Plant Growth Measurements
4.4. Grain Sampling
4.5. Grain Morphometrics and Specific Weight Components
4.6. Compositional Analysis
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 8 September 2020).
- Hoyle, A.; Brennan, M.; Pitts, N.; Jackson, G.E.; Hoad, S. Relationship between specific weight of spring barley and malt quality. J. Cereal Sci. 2020, 95, 103006. [Google Scholar] [CrossRef]
- Hoyle, A.; Brennan, M.; Jackson, G.; Hoad, S. Specific weight of barley grains is determined by traits affecting packing efficiency and by grain density. J. Sci. Food Agric. 2019, 99, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.K.; Jaiswal, S.; Maley, J.; Båga, M.; Sammynaiken, R.; Rossnagel, B.G.; Chibbar, R.N. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J. Agric. Food Chem. 2011, 59, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Wenwen, Y.; Tao, K.; Gidley, M.J.; Fox, G.P.; Gilbert, R.G. Molecular brewing: Molecular structural effects involved in barley malting and mashing. Carbohydr. Polym. 2019, 206, 583–592. [Google Scholar] [CrossRef]
- Hoyle, A.; Brennan, M.; Jackson, G.E.; Hoad, S. Increased grain density of spring barley (Hordeum vulgare L.) is associated with an increase in grain nitrogen. J. Cereal Sci. 2019, 89, 102797. [Google Scholar] [CrossRef]
- AHDB Barley Growth Guide 2015. Available online: https://ahdb.org.uk/barleygg (accessed on 8 September 2020).
- García, G.A.; Serrago, R.A.; Dreccer, M.F.; Miralles, D.J. Post-anthesis warm nights reduce grain weight in field-grown wheat and barley. Field Crops Res. 2016, 195, 50–59. [Google Scholar] [CrossRef]
- Kennedy, S.P.; Lynch, J.P.; Spink, J.; Bingham, I.J. Grain number and grain filling of two-row malting barley in response to variation in post-anthesis radiation: Analysis by grain position on the ear and its implications for yield improvement and quality. Field Crops Res. 2018, 225, 74–82. [Google Scholar] [CrossRef]
- Macnicol, P.K.; Jacobsen, J.V.; Keys, M.M.; Stuart, I.M. Effects of heat and water stress on malt quality and grain parameters of schooner barley grown in cabinets. J. Cereal Sci. 1993, 18, 61–68. [Google Scholar] [CrossRef]
- Afshari-Behbahanizadeh, S.; Akbari, G.A.; Shahbazi, M.; Alahdadi, I.; Farahani, L.; Tabatabaee, S.A.; Ganji, M. Qualitative and Physical Properties of Barley Grains under Terminal Drought Stress Conditions. Available online: http://ir.jkuat.ac.ke/handle/123456789/1294 (accessed on 8 September 2020).
- Samarah, N.H. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149. [Google Scholar] [CrossRef]
- Thitisaksakul, M.; Jiménez, R.C.; Arias, M.C.; Beckles, D.M. Effects of environmental factors on cereal starch biosynthesis and composition. J. Cereal Sci. 2012, 56, 67–80. [Google Scholar] [CrossRef]
- Beckles, D.M.; Thitisaksakul, M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch 2014, 66, 58–71. [Google Scholar] [CrossRef]
- He, J.-F.; Goyal, R.; Laroche, A.; Zhao, M.-L.; Lu, Z.-X. Water stress during grain development affects starch synthesis, composition and physicochemical properties in triticale. J. Cereal Sci. 2012, 56, 552–560. [Google Scholar] [CrossRef]
- González, A.; Martín, I.; Ayerbe, L. Barley yield in water-stress conditions. F. Crop. Res. 1999, 62, 23–34. [Google Scholar] [CrossRef]
- Gooding, M.J.; Ellis, R.H.; Shewry, P.R.; Schofield, J.D. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Liu, K.; Yang, R.; Lu, J.; Wang, X.; Lu, B.; Tian, X.; Zhang, Y. Radiation use efficiency and source-sink changes of super hybrid rice under shade stress during grain-filling stage. Agron. J. 2019, 111, 1788–1798. [Google Scholar] [CrossRef]
- Mahalingam, R. Phenotypic, physiological and malt quality analyses of US barley varieties subjected to short periods of heat and drought stress. J. Cereal Sci. 2017, 76, 199–205. [Google Scholar] [CrossRef]
- Mahalingam, R.; Bregitzer, P. Impact on physiology and malting quality of barley exposed to heat, drought and their combination during different growth stages under controlled environment. Physiol. Plant. 2019, 165, 277–289. [Google Scholar] [CrossRef]
- Huang, R.; Jiang, L.; Zheng, J.; Wang, T.; Wang, H.; Huang, Y.; Hong, Z. Genetic bases of rice grain shape: So many genes, so little known. Trends Plant Sci. 2013, 18, 218–226. [Google Scholar] [CrossRef]
- Walker, C.K.; Ford, R.; Muñoz-Amatriaín, M.; Panozzo, J.F. The detection of QTLs in barley associated with endosperm hardness, grain density, grain size and malting quality using rapid phenotyping tools. Theor. Appl. Genet. 2013, 126, 2533–2551. [Google Scholar] [CrossRef]
- Mather, D.E.; Tinker, N.A.; LaBerge, D.E.; Edney, M.; Jones, B.L.; Rossnagel, B.G.; Legge, W.G.; Briggs, K.G.; Irvine, R.G.; Falk, D.E.; et al. Regions of the Genome That Affect Grain and Malt Quality in a North American Two-Row Barley Cross. Crop Sci. 1997, 37, 544–554. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, X.; Li, B.; Su, B.; Li, J.; Zhou, D. An improved water-use efficiency for winter wheat grown under reduced irrigation. Field Crops Res. 1998, 59, 91–98. [Google Scholar] [CrossRef]
- Al-Ajlouni, Z.; Al-Abdallat, A.; Al-Ghzawi, A.; Ayad, J.; Abu Elenein, J.; Al-Quraan, N.; Baenziger, P. Impact of pre-anthesis water deficit on yield and yield components in barley (Hordeum vulgare L.) plants grown under controlled conditions. Agronomy 2016, 6, 33. [Google Scholar] [CrossRef]
- Millet, E. Relationships between grain weight and the size of floret cavity in the wheat spike. Ann. Bot. 1986, 58, 417–423. [Google Scholar] [CrossRef]
- Gous, P.W.; Hasjim, J.; Franckowiak, J.; Fox, G.P.; Gilbert, R.G. Barley genotype expressing “stay-green”-like characteristics maintains starch quality of the grain during water stress condition. J. Cereal Sci. 2013, 58, 414–419. [Google Scholar] [CrossRef]
- Atkinson, M.D.; Kettlewell, P.S.; Poulton, P.R.; Hollins, P.D. Grain quality in the Broadbalk wheat experiment and the winter North Atlantic oscillation. J. Agric. Sci. 2008, 146, 541–549. [Google Scholar] [CrossRef]
- Tottman, D.R.; Broad, H. The decimal code for the growth stages of cereals, with illustrations. Ann. Appl. Bot. 1987, 110, 441–454. [Google Scholar] [CrossRef]
- Gibson, T.S.; Solah, V.A.; McCleary, B.V. A procedure to measure amylose in cereal starches and flours with concanavalin A. J. Cereal Sci. 1997, 25, 111–119. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Mccleary, B.V.; Gibson, T.S.; Mugford, D.C.; Lukow, O.; Jackson, D.S.; Rabe, E.; Patel, N.; Williams, P.C.; Gelroth, J.; Camire, M.E.; et al. Measurement of total starch in cereal products by amyloglucosidase-α-amylase method: Collaborative study. J. AOAC Int. 1997, 80, 571–579. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing 2008. Available online: https://www.r-project.org/ (accessed on 8 September 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated marginal means, aka least-squares means. R Packag. Version 2018, 1.1, 3. [Google Scholar]



| Grain Quality Parameters | Octavia | Concerto | Sienna | |||
|---|---|---|---|---|---|---|
| Well-Watered | Water Stress | Well-Watered | Water Stress | Well-Watered | Water Stress | |
| Size Classes | ||||||
| >3.25 mm | 8.29 ± 3.72a | 5.30 ± 3.78a | 5.96 ± 3.80a | 5.95 ± 1.25a | 8.94 ± 5.36a | 8.33 ± 3.29a |
| 3.00–3.25 mm | 20.45 ± 4.93a | 18.90 ± 10.89a | 30.08 ± 21.33a | 33.84 ± 12.90a | 25.43 ± 10.11a | 22.30 ± 8.90a |
| 2.75–3.00 mm | 27.56 ± 6.78a | 35.62 ± 9.02a | 31.09 ± 9.56a | 34.30 ± 3.49a | 30.15 ± 2.53a | 26.36 ± 7.78a |
| 2.50–2.75 mm | 24.70 ± 3.99a | 25.92 ± 7.40a | 17.36 ± 9.91a | 17.38 ± 9.51a | 21.57 ± 6.84a | 24.52 ± 8.01a |
| 2.25–2.50 mm | 18.99 ± 8.30a | 14.26 ± 8.37a | 15.52 ± 18.15a | 8.53 ± 5.23a | 13.91 ± 11.13a | 18.48 ± 8.78a |
| Screenings | 8.06 ± 5.46a | 3.45 ± 2.18a | 6.65 ± 9.33a | 2.34 ± 1.13a | 5.49 ± 6.53a | 5.33 ± 2.89a |
| Dimensions | ||||||
| Length (mm) | 9.09 ± 0.06a | 9.08 ± 0.08a | 8.77 ± 0.34b | 8.80 ± 0.40b | 8.96 ± 0.36ab | 8.92 ± 0.36ab |
| Width (mm) | 3.60 ± 0.08a | 3.61 ± 0.10a | 3.60 ± 0.14a | 3.74 ± 0.04a | 3.68 ± 0.12a | 3.66 ± 0.09a |
| Depth (mm) | 2.86 ± 0.06a | 2.88 ± 0.14a | 2.87 ± 0.15a | 2.98 ± 0.07a | 2.93 ± 0.16a | 2.90 ± 0.10a |
| 2D area (mm2) | 24.20 ± 0.27a | 24.25 ± 0.82a | 23.04 ± 0.99a | 24.08 ± 1.11a | 24.27 ± 0.29a | 23.72 ± 0.17a |
| Circularity | 0.56 ± 0.01b | 0.57 ± 0.01b | 0.59 ± 0.03a | 0.59 ± 0.03a | 0.59 ± 0.03ab | 0.58 ± 0.02ab |
| Specific weight and components | ||||||
| Specific Weight (kg hL−1) | 62.73 ± 2.10b | 63.76 ± 3.01b | 64.12 ± 2.71ab | 65.75 ± 1.57ab | 66.86 ± 1.91a | 66.69 ± 2.52a |
| Packing Efficiency (%) | 52.84 ± 1.95a | 51.95 ± 1.93a | 51.26 ± 0.09a | 52.96 ± 1.21a | 52.72 ± 2.85a | 53.08 ± 0.80a |
| Grain Density (g cm−3) | 1.17 ± 0.02b | 1.21 ± 0.02b | 1.23 ± 0.05ab | 1.25 ± 0.02ab | 1.28 ± 0.05a | 1.25 ± 0.05a |
| Grain Volume (cm−3) | 39.08 ± 0.61a | 37.96 ± 2.04a | 37.16 ± 0.93a | 39.43 ± 0.37a | 39.37 ± 2.17a | 38.55 ± 0.96a |
| Composition | ||||||
| Total starch | 53.80 ± 0.14a | 52.89 ± 1.00a | 52.96 ± 2.04a | 52.88 ± 0.90a | 54.95 ± 2.17a | 54.46 ± 2.35a |
| Amylose (%) | 20.17 ± 1.66a | 19.16 ± 0.86a | 19.24 ± 0.95a | 19.40 ± 2.03a | 18.68 ± 1.32a | 21.05 ± 0.77a |
| Carbon (%) | 39.23 ± 0.89a | 39.84 ± 0.02a | 40.17 ± 0.85a | 39.90 ± 0.12a | 39.90 ± 0.04a | 39.93 ± 0.15a |
| Nitrogen (%) | 1.67 ± 0.23a | 1.84 ± 0.04a | 1.81 ± 0.32a | 1.90 ± 0.12a | 1.61 ± 0.17a | 1.76 ± 0.26a |
| C:N | 23.80 ± 2.87a | 21.70 ± 0.50b | 22.62 ± 3.56a | 20.99 ± 1.21b | 24.92 ± 2.61a | 23.00 ± 3.11b |
| Physical-chemical parameters | ||||||
| Protein Content (mg/grain) | 4.78 ± 0.62b | 5.28 ± 0.57a | 5.10 ± 0.55b | 5.83 ± 0.38a | 5.03 ± 0.38b | 5.24 ± 0.47a |
| Starch Content (mg/grain) | 24.69 ± 0.26a | 24.34 ± 2.66a | 24.23 ± 2.78a | 25.90 ± 0.18a | 27.53 ± 2.73a | 26.15 ± 2.49a |
| Grain weight (mg) | Length (mm) | Width (mm) | Depth (mm) | Volume (mm3) | Area (mm2) | Perimeter (mm) | Circularity | Grain Density (g cm−3) | Packing Efficiency (%) | Specific Weight (kg hL−1) | Nitrogen Content (%) | Carbon Content (%) | Starch Content (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grain weight (mg) | 1 | −0.33 | 0.89 ** | 0.84 ** | 0.83 * | 0.32 | −0.24 | 0.64 ** | 0.48 * | 0.62 ** | 0.84 ** | −0.47 | −0.18 | 0.68 ** |
| Length (mm) | 1 | −0.51 * | −0.53 * | −0.03 | 0.7 ** | 0.91 ** | −0.8 * | −0.56 * | −0.08 | −0.65 ** | 0.35 | 0.04 | −0.30 | |
| Width (mm) | 1 | 0.95 *** | 0.79 | 0.08 | −0.46 | 0.78 *** | 0.36 | 0.57 * | 0.76 *** | −0.55 * | −0.38 | 0.62 ** | ||
| Depth (mm) | 1 | 0.79 | −0.02 | −0.53 * | 0.8 * | 0.27 | 0.62 ** | 0.76 *** | −0.49 * | −0.34 | 0.59 ** | |||
| Volume (mm3) | 1 | 0.48 * | −0.02 | 0.44 | −0.09 | 0.81 * | 0.53 * | −0.43 | −0.31 | 0.63 ** | ||||
| Area (mm2) | 1 | 0.80 | −0.38 | −0.21 | 0.37 | −0.09 | 0.13 | 0.02 | 0.05 | |||||
| Perimeter (mm) | 1 | −0.86 | −0.43 | −0.03 | −0.55 * | 0.42 | 0.10 | −0.30 | ||||||
| Circularity | 1 | 0.47 | 0.34 | 0.76 *** | −0.53 * | −0.14 | 0.5 * | |||||||
| Grain Density (g cm−3) | 1 | −0.17 | 0.67 ** | −0.18 | 0.13 | 0.23 | ||||||||
| Packing Efficiency (%) | 1 | 0.53 * | −0.24 | −0.20 | 0.48 * | |||||||||
| Specific Weight (kg hL−1) | 1 | −0.41 | −0.04 | 0.64 ** | ||||||||||
| Nitrogen Content (%) | 1 | 0.68 ** | −0.76 *** | |||||||||||
| Carbon Content (%) | 1 | −0.28 | ||||||||||||
| Starch Content (%) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoyle, A.; Brennan, M.; Rees, L.; Jackson, G.E.; Hoad, S.P. Post-Anthesis Water-stressed Barley Maintains Grain Specific Weight Through Altered Grain Composition and Plant Architecture. Plants 2020, 9, 1564. https://doi.org/10.3390/plants9111564
Hoyle A, Brennan M, Rees L, Jackson GE, Hoad SP. Post-Anthesis Water-stressed Barley Maintains Grain Specific Weight Through Altered Grain Composition and Plant Architecture. Plants. 2020; 9(11):1564. https://doi.org/10.3390/plants9111564
Chicago/Turabian StyleHoyle, Aaron, Maree Brennan, Logan Rees, Gail E. Jackson, and Stephen P. Hoad. 2020. "Post-Anthesis Water-stressed Barley Maintains Grain Specific Weight Through Altered Grain Composition and Plant Architecture" Plants 9, no. 11: 1564. https://doi.org/10.3390/plants9111564
APA StyleHoyle, A., Brennan, M., Rees, L., Jackson, G. E., & Hoad, S. P. (2020). Post-Anthesis Water-stressed Barley Maintains Grain Specific Weight Through Altered Grain Composition and Plant Architecture. Plants, 9(11), 1564. https://doi.org/10.3390/plants9111564