Nutrients Leaching in Response to Long-Term Fertigation and Broadcast Nitrogen in Blueberry Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Treatments
2.3. Leachate Samples
2.4. Sawdust Mulch and Soil Sampling
2.5. Berry Yield
2.6. Chemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Weather Conditions
3.2. Concentrations of Nitrate, Ammonium and Sulfate in Leachate
3.3. Acidity and Electrical Conductivity of Leachate
3.4. Ammonium, Nitrate and Mineral N in the Soil
3.5. Acidity and Electrical Conductivity in the Soil
3.6. Blueberry Yields
3.7. Relationships between Soil And Leachate Chemical Properties and Berry Yield
4. Discussion
4.1. Nutrient Concentrations in Leachates
4.2. Yield
4.3. Relationship between Yield and the Nutrient Concentration in Leachate and Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Padilla, F.M.; Gallardo, M.; Manzano-Agugliaro, F. Global trends in nitrate leaching research in the 1960–2017 period. Sci. Total Environ. 2018, 643, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, L.; Wei, A.; Gao, J.; Lu, Y.; Zhou, J. Land-use change from arable lands to orchards reduced soil erosion and increased nutrient loss in a small catchment. Sci. Total Environ. 2019, 648, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Zadeh, H.G.; Van Damme, P. Impacts of inorganic nitrogen fertiliser on soil fertility and groundwater quality of touba orchards (western Iran). J. Environ. Prot. Ecol. 2013, 14, 71–79. [Google Scholar]
- Zebarth, B.J.; Kowalenko, C.G.; Harding, B. Soil inorganic nitrogen content and indices of red raspberry yield, vigor, and nitrogen status as affected by rate and source of nitrogen fertilizer. Comm. Soil Sci. Plant Anal. 2007, 38, 637–660. [Google Scholar] [CrossRef]
- Kiani, M.; Raave, H.; Simojoki, A.; Tammeorg, O.; Tammeorg, P. Recycling lake sediment to agriculture: Effects on plant growth, nutrient availability, and leaching. Sci. Total Environ. 2020, 753, 141984. [Google Scholar] [CrossRef]
- Hester, R.E.; Harrison, R.M.; Addiscott, T.M. Fertilizers and nitrate leaching. In Agricultural Chemicals and the Environment; Hester, R.E., Harrison, R.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1996; pp. 1–26. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality Summary Table. 2019. Available online: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/guidelines-canadian-drinking-water-quality-summary-table.html (accessed on 24 October 2020).
- U.S. EPA. Nitrates and Nitrites. TEACH Chemical Summary. U.S. EPA, Toxicity and Exposure Assessment for Children’s Health; Environmental Protection Agency: Washington, DC, USA, 2007. Available online: www.epa.gov/teach/chem_summ/Nitrates_summary.pdf (accessed on 24 October 2020).
- Moreno, F.; Cabrera, F.; Murillo, J.M.; Fernandez, J.E.; Fernandez-Boy, E.; Cayuela, J.A. Nitrate leaching under irrigated agriculture. In Sustainability of Irrigated Agriculture; Pereira, L.S., Feddes, R.A., Gilley, J.R., Lesaffre, B., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 407–415. [Google Scholar]
- Lamers, L.P.M.; Tomassen, H.B.M.; Roelofs, J.G.M. Sulfate–induced eutrophication and phytotoxicity in freshwater wetlands. Environ. Sci. Technol. 1998, 32, 199–205. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.C.; Luedtke, R.J.; Smith, C.E.; Meyn, E.L.; Chakoumakos, C.; Wang, K.C.; Brown, C.J.D. Chronic toxicity of ammonia to rainbow trout. Trans. Am. Fish. Society. 1984, 113, 56–73. [Google Scholar] [CrossRef]
- Ercoli, L.; Arduini, I.; Mariotti, M.; Lulli, L.; Masoni, A. Management of sulphur fertilizer to improve durum wheat production and minimise S leaching. Eur. J. Argon. 2012, 38, 74–82. [Google Scholar]
- Messiga, A.; Haak, D.; Dorais, M. Blueberry yield and soil properties response to long-term fertigation and broadcast nitrogen. Sci. Hortic. 2018, 230, 92–101. [Google Scholar] [CrossRef]
- Eriksen, J.; Askegaard, M. Sulphate leaching in an organic crop rotation on sandy soil in Denmark. Agric. Ecosyst. Environ. 2000, 78, 107–114. [Google Scholar] [CrossRef]
- Eriksen, J.; Murphy, M.D.; Schnug, E. The soil sulphur cycle. In Sulphur in Agroecosystems; Schnug, E., Ed.; Kluwer Academic Press: Dordrecht, The Netherlands, 1998; pp. 39–73. [Google Scholar]
- Guzys, S.; Aksomaitiene, R. Migration of sulphur in limed soils differing in agricultural management. Nutr. Cycl. Agroecosyst. 2005, 71, 191–201. [Google Scholar] [CrossRef]
- Poonnachit, U.; Darnell, R. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Ann. Bot. 2004, 93, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Forge, T.; Ehret, D.; Messiga, A.; Dorais, M. Influences of nitrogen inputs on nematode populations under highbush blueberry. J. Nematol. 2020, 52, e2020-56. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, E.A.; Hepp, R.F.; Mariño, M.A. Effect of irrigation on fruit production in blueberry. Agric. Water Manage. 2004, 67, 173–184. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, H.; Yin, Y.; Zheng, H.; Cui, Z. Estimating soil nitrate leaching of nitrogen fertilizer from global meta-analysis. Sci. Total Environ. 2019, 657, 96–102. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef]
- Ehret, D.L.; Frey, B.; Forge, T.; Helmer, T.; Bryla, D.R.; Zebarth, B.J. Effects of nitrogen rate and application method on early production and fruit quality in highbush blueberry. Can. J. Plant. Sci. 2014, 94, 1165–1179. [Google Scholar] [CrossRef]
- Adu-Gyamfi, R.; Agyin-Birikorang, S.; Tindjina, I.; Manu, Y.; Singh, U. Minimizing nutrient leaching from maize production systems in northern Ghana with one-time application of multi-nutrient fertilizer briquettes. Sci. Tot. Environ. 2019, 694, 133667. [Google Scholar] [CrossRef]
- Zvomuya, F.; Rosen, C.J.; Russelle, M.P.; Gupta, S.C. Nitrate leaching and nitrogen recovery following application of polyolefin-coated urea to potato. J. Environ. Qual. 2003, 32, 480–489. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.; NRCS: Washington, DC, USA, 2010. [Google Scholar]
- BCMAL. British Columbia Ministry of Agriculture and Lands, BC Berry Production Guides. 2020. Available online: https://www2.gov.bc.ca/gov/content/industry/agriservice-bc/production-guides/berries (accessed on 3 September 2020).
- Woodard, K.R.; French, E.C.; Sweat, L.A.; Graetz, D.A.; Sollenberger, L.A.; Macoon, B.; Portier, K.M.; Rymph, S.J.; Wade, B.L.; Prine, G.M.; et al. Nitrogen removal and nitrate leaching for two perennial, sod-based forage systems receiving dairy effluent. J. Environ. Qual. 2003, 32, 996–1007. [Google Scholar] [CrossRef]
- Maynard, D.G.; Kalra, Y.P.; Crambaugh, J.A. Nitrate and exchangeable ammonium nitrogen. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 607–616. [Google Scholar]
- Tabatabai, M.A. A rapid method for determination of sulfate in water samples. Environ. Lett. 1974, 7, 237–243. [Google Scholar] [CrossRef]
- Statistical Analysis System. User’s Guide: Statistics, Version 9, 3rd ed.; SAS Inst.: Cary, NC, USA, 2010. [Google Scholar]
- Malekani, F.; Ryan, M.C.; Zebarth, B.J.; Loo, S.E.; Suchy, M.; Cey, E.E. A field scale approach to estimate nitrate loading to groundwater. J. Environ. Qual. 2018, 47, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, J.-E.; Li, X.-H.; Zhang, S.-L.; Wang, H.-J. Nitrate accumulation and leaching in surface and ground water based on simulated rainfall experiments. PLoS ONE 2015, 10, E136274. [Google Scholar] [CrossRef] [PubMed]
- Kowalenko, C.G. The dynamics of inorganic nitrogen in a Fraser Valley soil with and without spring or fall ammonium nitrate applications. Can. J. Soil Sci. 1987, 67, 367–382. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Directive, C. Concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. Eur. Commun. 2013, 375, 1–8. [Google Scholar]
- Carey, B.M.; Cummings, R. Sumas-Blaine Aquifer Nitrate Contamination Summary; Department of Ecology State of Washington: Washington, DC, USA, 2013. [Google Scholar]
- Lamers, L.P.M.; Vile, M.A.; Grootjans, A.P.; Acreman, M.C.; van Diggelen, R.; Evans, M.G.; Richardson, C.J.; Rochefort, L.; Kooijman, A.M.; Roelofs, J.G.M.; et al. Ecological restoration of rich fens in Europe and North America: From trial and error to an evidence-based approach. Biol. Rev. 2015, 90, 182–203. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Bryla, D.R.; Vargas, O. Effects of salinity induced ammonium sulfate fertilizer on root and shoot growth of highbush blueberry. Acta Hortic. 2014, 1017, 407–414. [Google Scholar] [CrossRef]
- Bryla, D.R.; Machado, R.M.A. Comparative effects of nitrogen fertigation and granular fertilizer application on growth and availability of soil nitrogen during establishment of highbush blueberry. Front. Plant Sci. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Bryla, D.R.; Shireman, A.D.; Machado, R. Effects of method and level of nitrogen fertilizer application on soil pH, electrical conductivity, and availability of ammonium and nitrate in blueberry. Acta. Hort. 2010, 868, 95–101. [Google Scholar] [CrossRef]


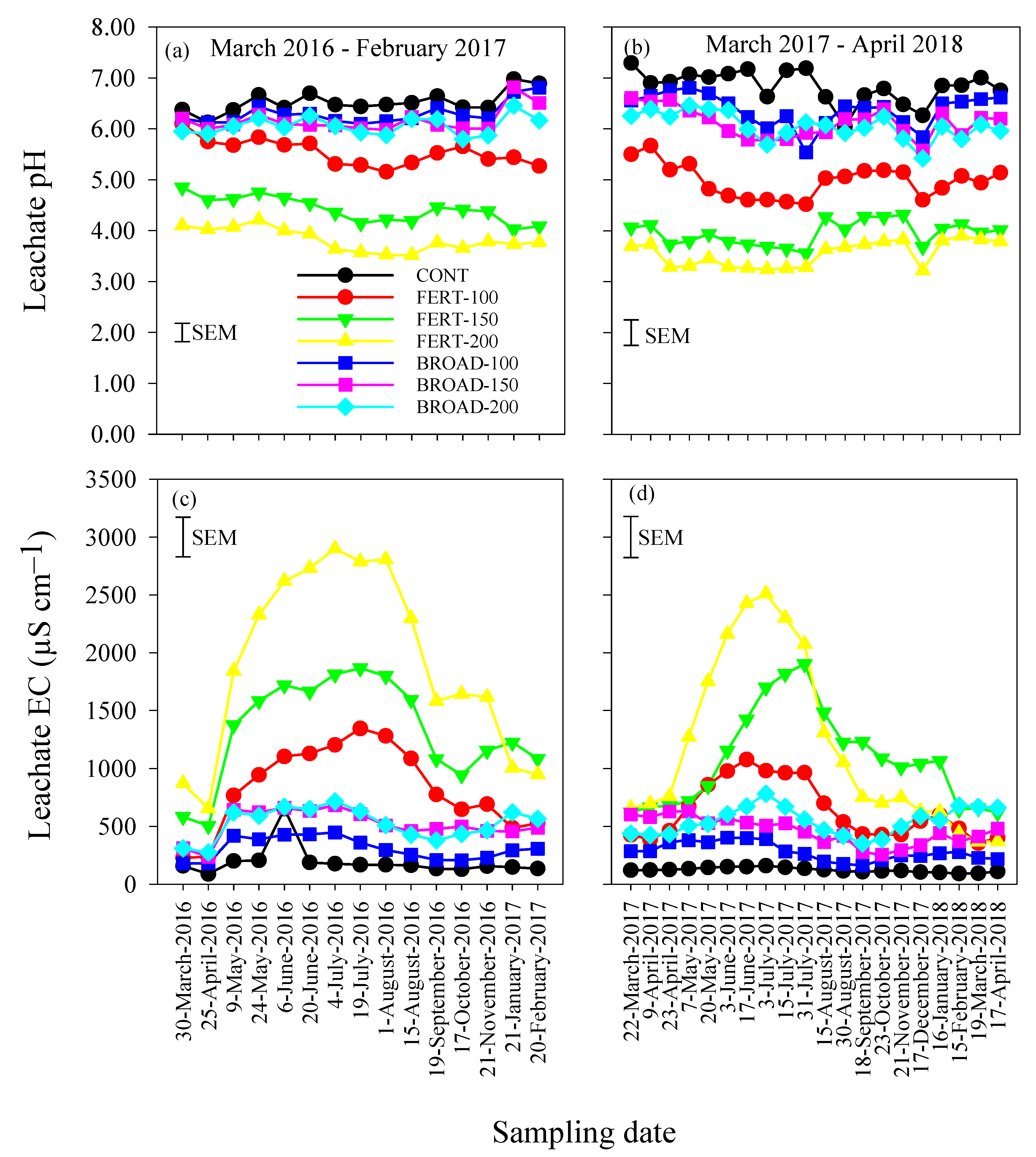
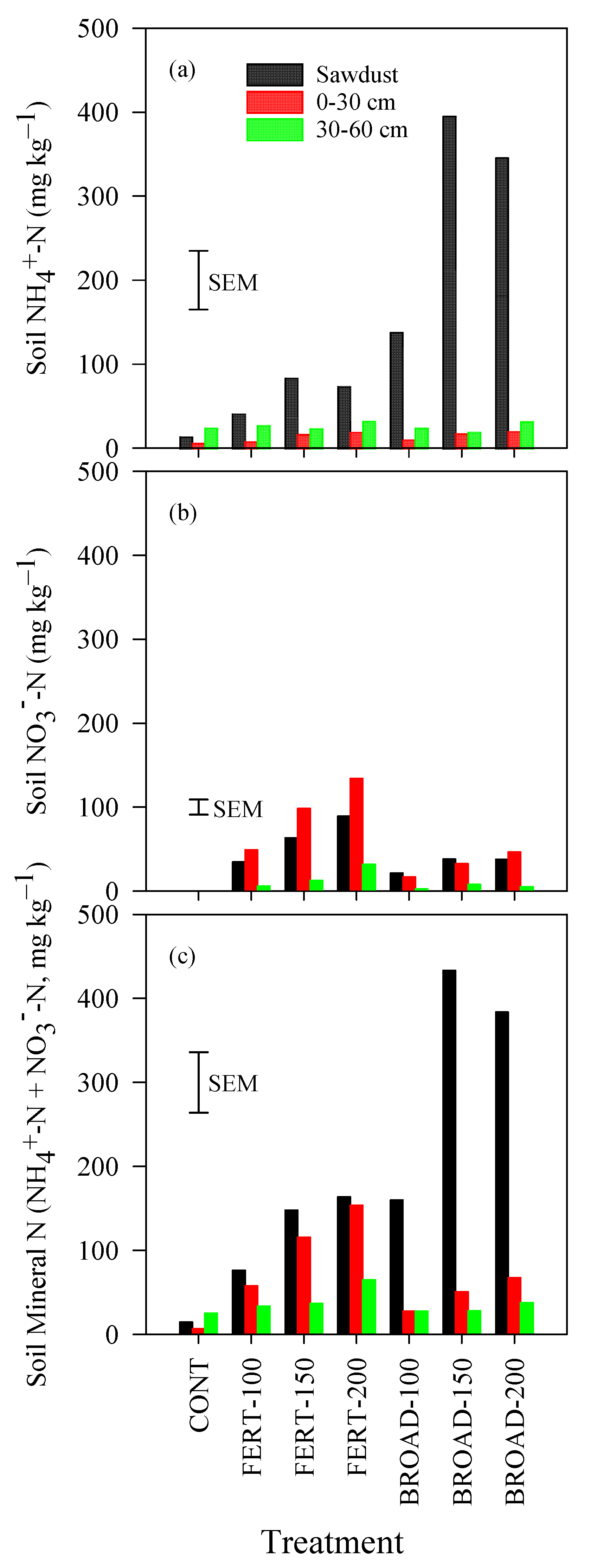
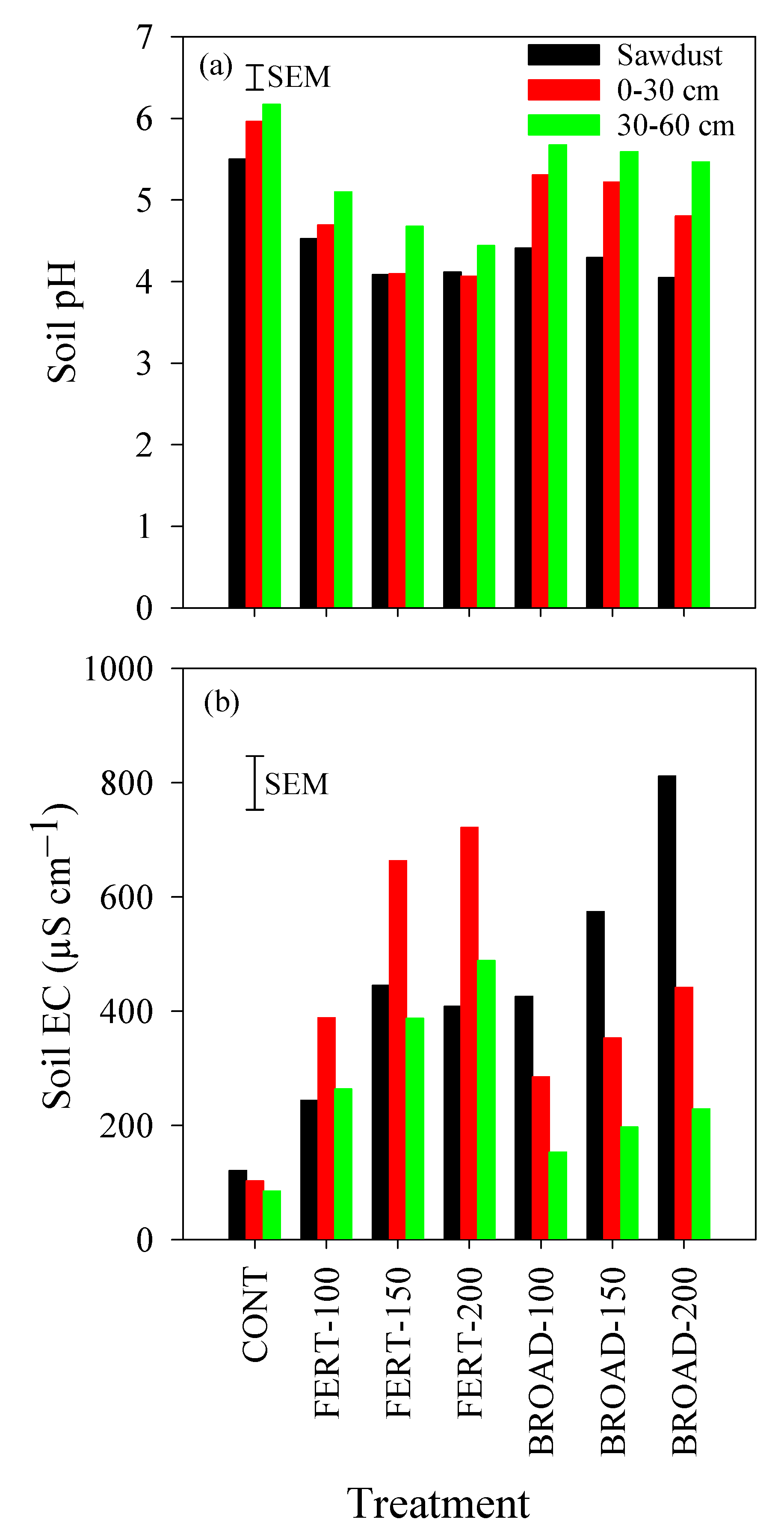

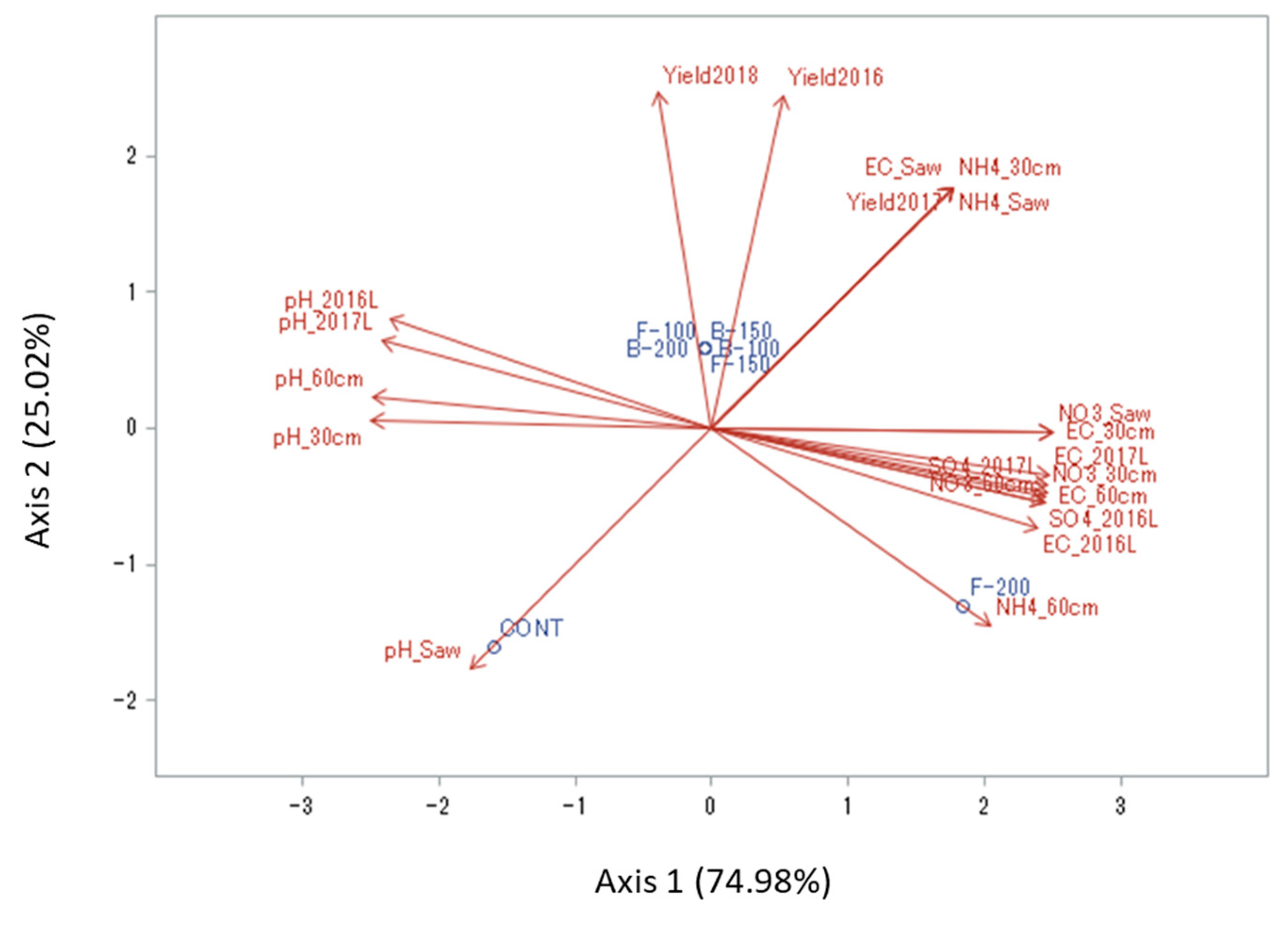
| March 2016–February 2017 | March 2017–April 2018 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NO3−-N | NH4+-N | SO4−-S | pH | EC 1 | NO3−-N | NH4+-N | SO4−-S | pH | EC 1 | |
| (mg L−1) | (mg L−1) | (mg L−1) | (µS cm−1) | (mg L−1) | (mg L−1) | (mg L−1) | (µS cm−1) | |||
| Sources of variation | p values 2 | |||||||||
| Treatment (T) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Sampling periods (SP) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| T × SP | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Contrast | ||||||||||
| CONT vs. All | <0.001 | 0.146 | 0.001 | <0.001 | <0.001 | <0.001 | 0.034 | <0.001 | 0.002 | <0.001 |
| FERT vs. BROAD | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Linear | 0.001 | 0.852 | 0.032 | 0.001 | 0.003 | 0.004 | 0.694 | 0.003 | 0.004 | <0.001 |
| Quadratic | <0.001 | 0.019 | 0.004 | <0.001 | <0.001 | 0.008 | 0.002 | 0.002 | <0.002 | 0.006 |
| NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | Mineral N (NH4+-N + NO3−-N, mg kg−1) | pH | EC | |
|---|---|---|---|---|---|
| Sources of variation | p values 1 | ||||
| N applications (N) | 0.039 | <0.001 | 0.016 | <0.001 | <0.001 |
| Depth | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| N applications × Depth | 0.002 | 0.020 | 0.002 | <0.001 | <0.001 |
| Contrast | |||||
| CONT 2 vs. All | 0.882 | 0.005 | 0.114 | 0.003 | 0.045 |
| FERT vs. BROAD | 0.029 | <0.001 | 0.881 | <0.001 | 0.007 |
| Linear | 0.477 | 0.045 | 0.015 | <0.001 | 0.001 |
| Quadratic | 0.676 | 0.025 | 0.097 | 0.002 | 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by Her Majesty the Queen in Right of Canada, as represented by the Minister of Agriculture and Agri-Food Canada; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messiga, A.J.; Dyck, K.; Ronda, K.; van Baar, K.; Haak, D.; Yu, S.; Dorais, M. Nutrients Leaching in Response to Long-Term Fertigation and Broadcast Nitrogen in Blueberry Production. Plants 2020, 9, 1530. https://doi.org/10.3390/plants9111530
Messiga AJ, Dyck K, Ronda K, van Baar K, Haak D, Yu S, Dorais M. Nutrients Leaching in Response to Long-Term Fertigation and Broadcast Nitrogen in Blueberry Production. Plants. 2020; 9(11):1530. https://doi.org/10.3390/plants9111530
Chicago/Turabian StyleMessiga, Aimé J., Kathryn Dyck, Kiera Ronda, Kolden van Baar, Dennis Haak, Shaobing Yu, and Martine Dorais. 2020. "Nutrients Leaching in Response to Long-Term Fertigation and Broadcast Nitrogen in Blueberry Production" Plants 9, no. 11: 1530. https://doi.org/10.3390/plants9111530
APA StyleMessiga, A. J., Dyck, K., Ronda, K., van Baar, K., Haak, D., Yu, S., & Dorais, M. (2020). Nutrients Leaching in Response to Long-Term Fertigation and Broadcast Nitrogen in Blueberry Production. Plants, 9(11), 1530. https://doi.org/10.3390/plants9111530





