Assessment of Water Mimosa (Neptunia oleracea Lour.) Morphological, Physiological, and Removal Efficiency for Phytoremediation of Arsenic-Polluted Water
Abstract
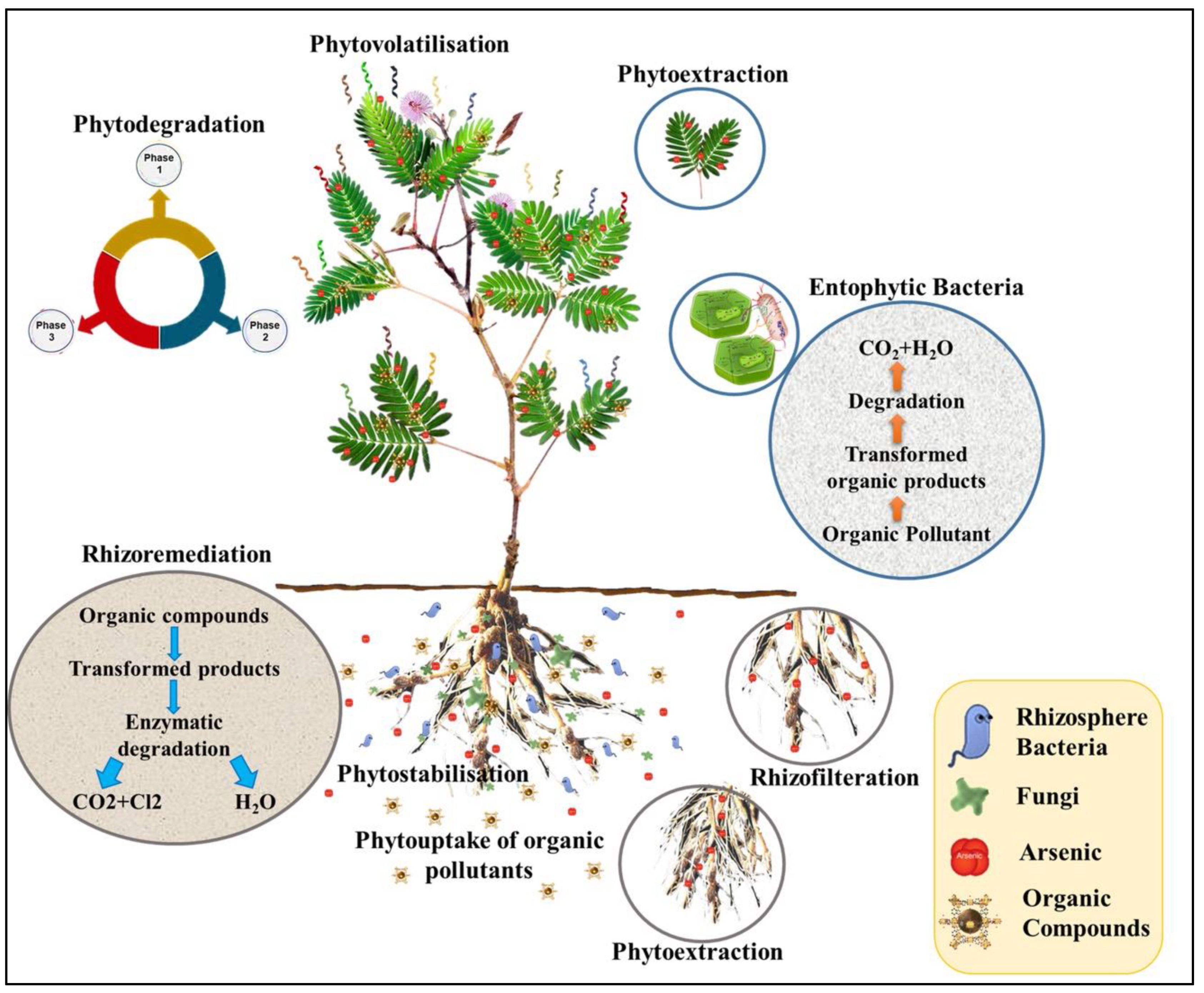
1. Introduction
2. Results and Discussion
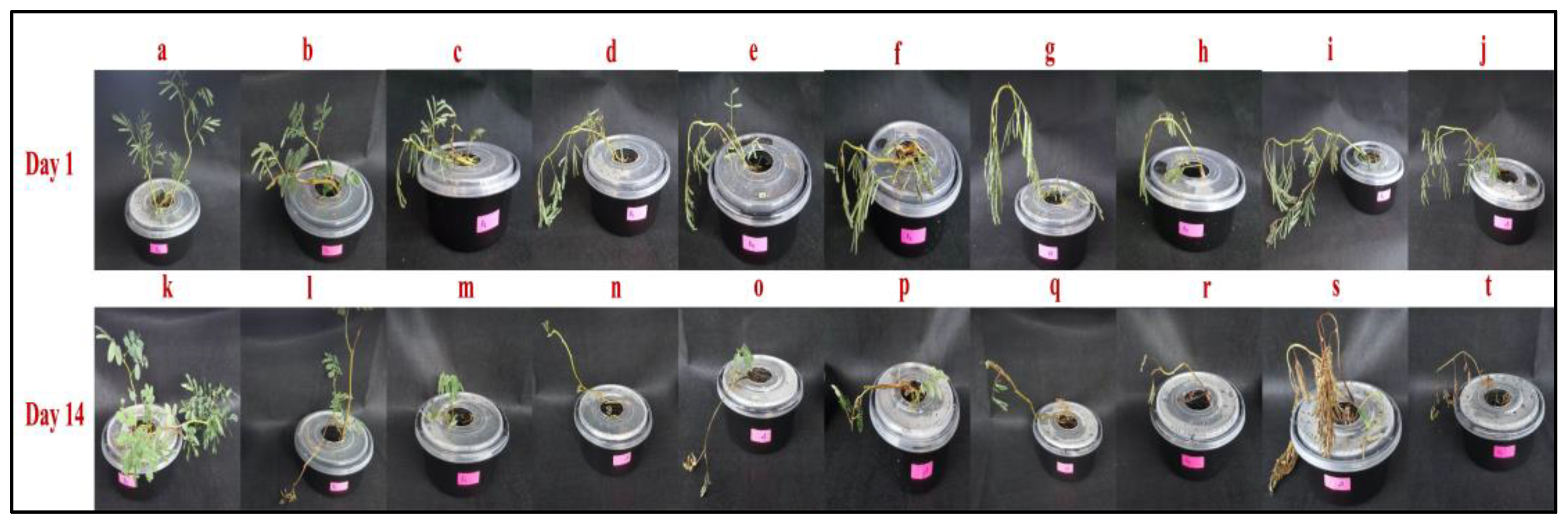
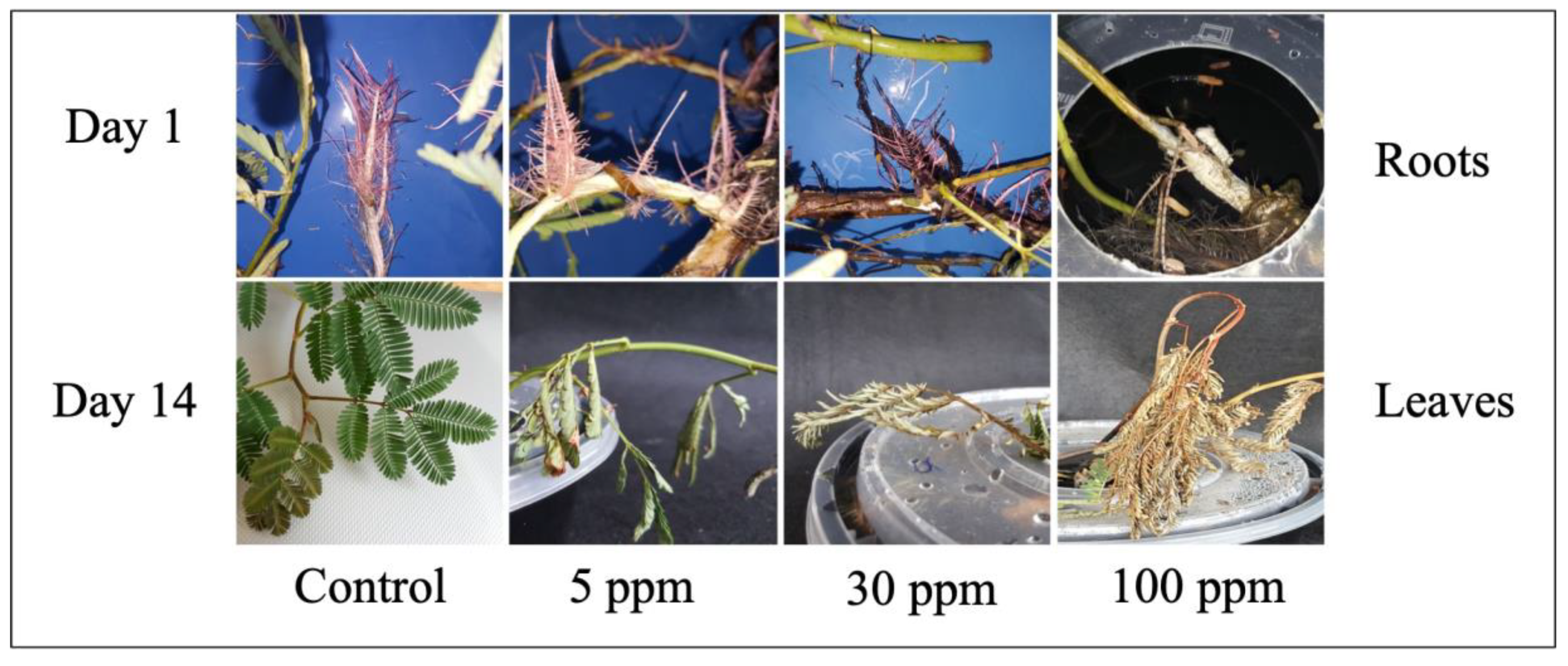
2.1. Macromorphological Observation of Water Mimosa under Different Arsenic Concentrations
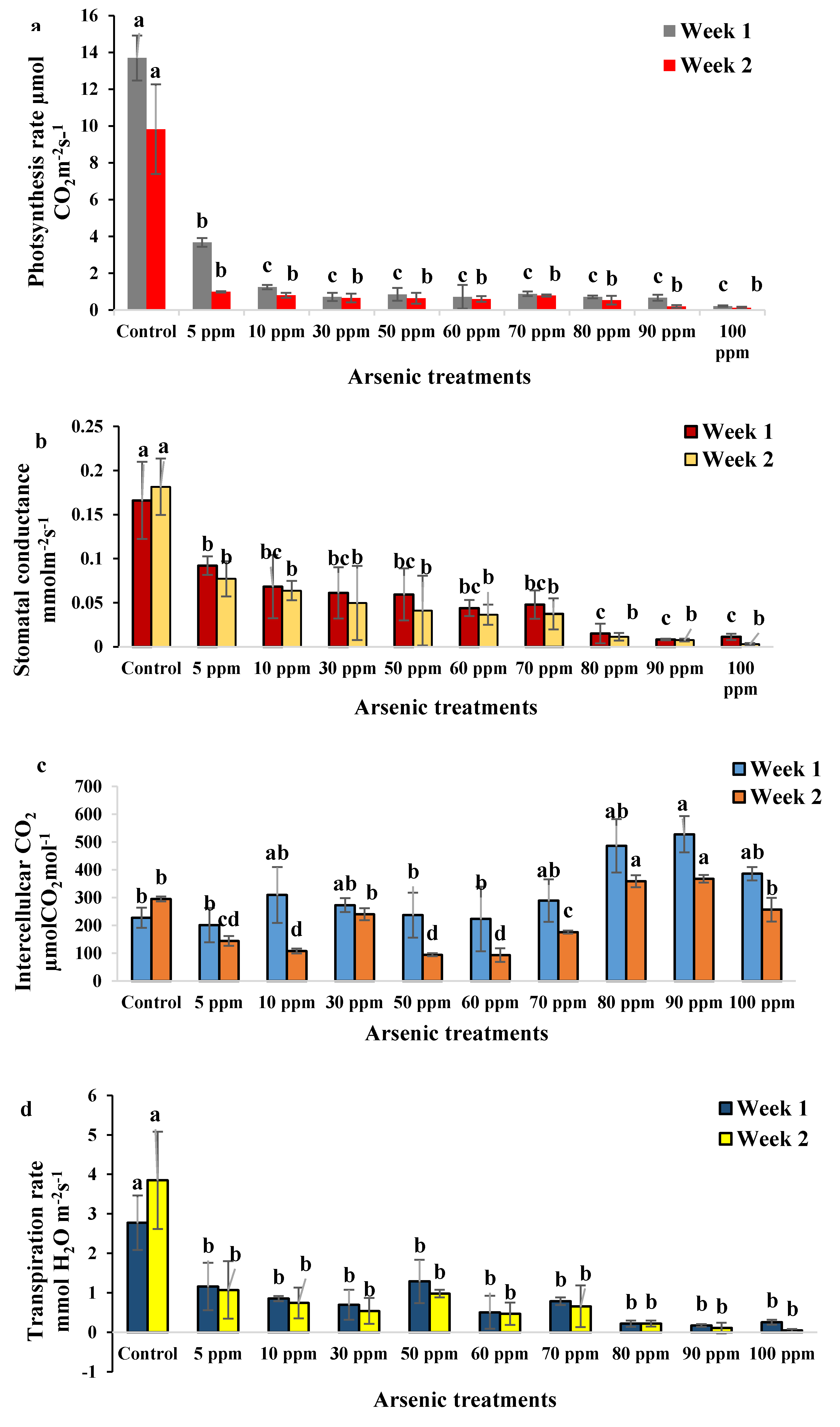
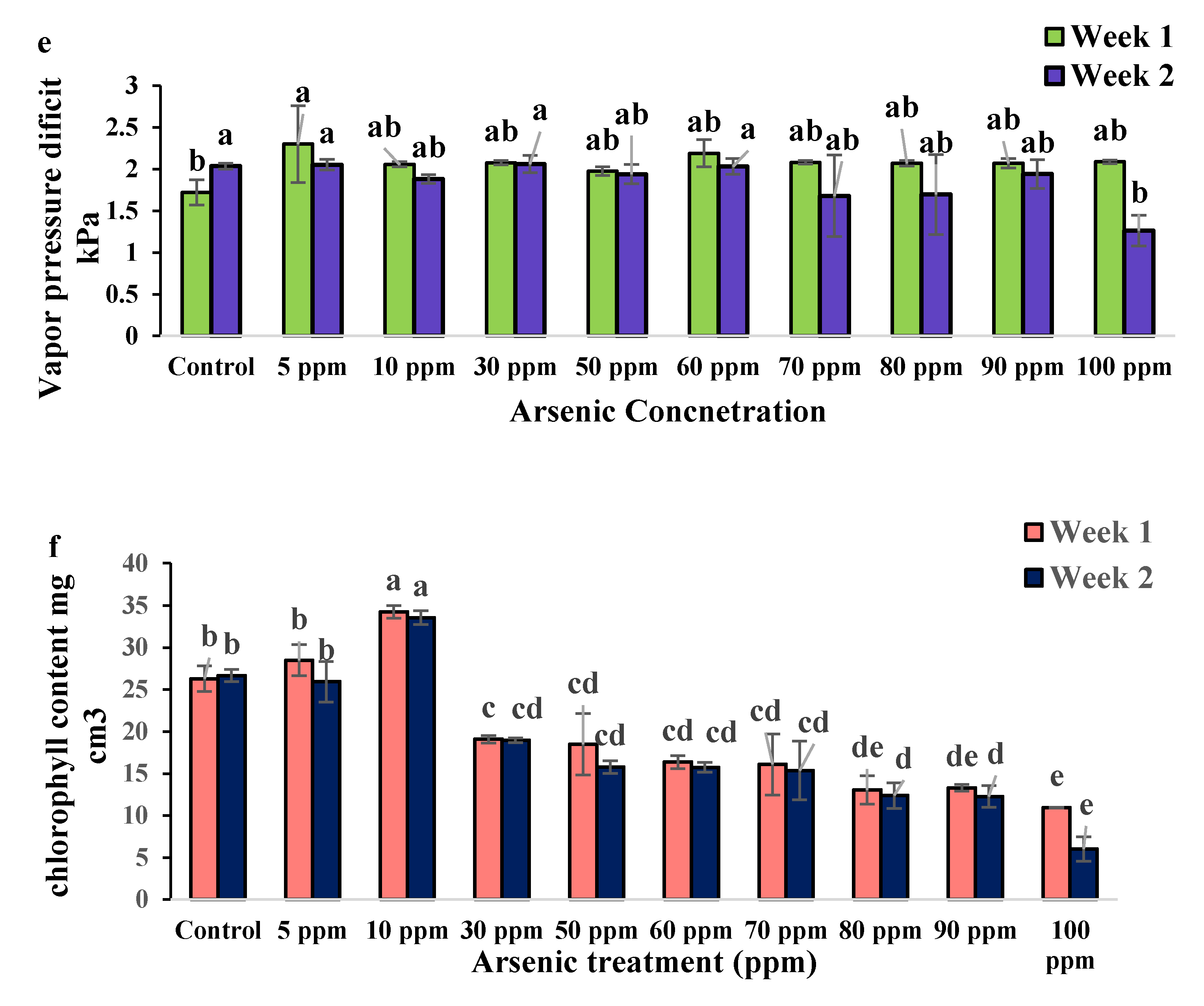
2.2. Physiological Changes of Water Mimosa under Arsenic Treatment
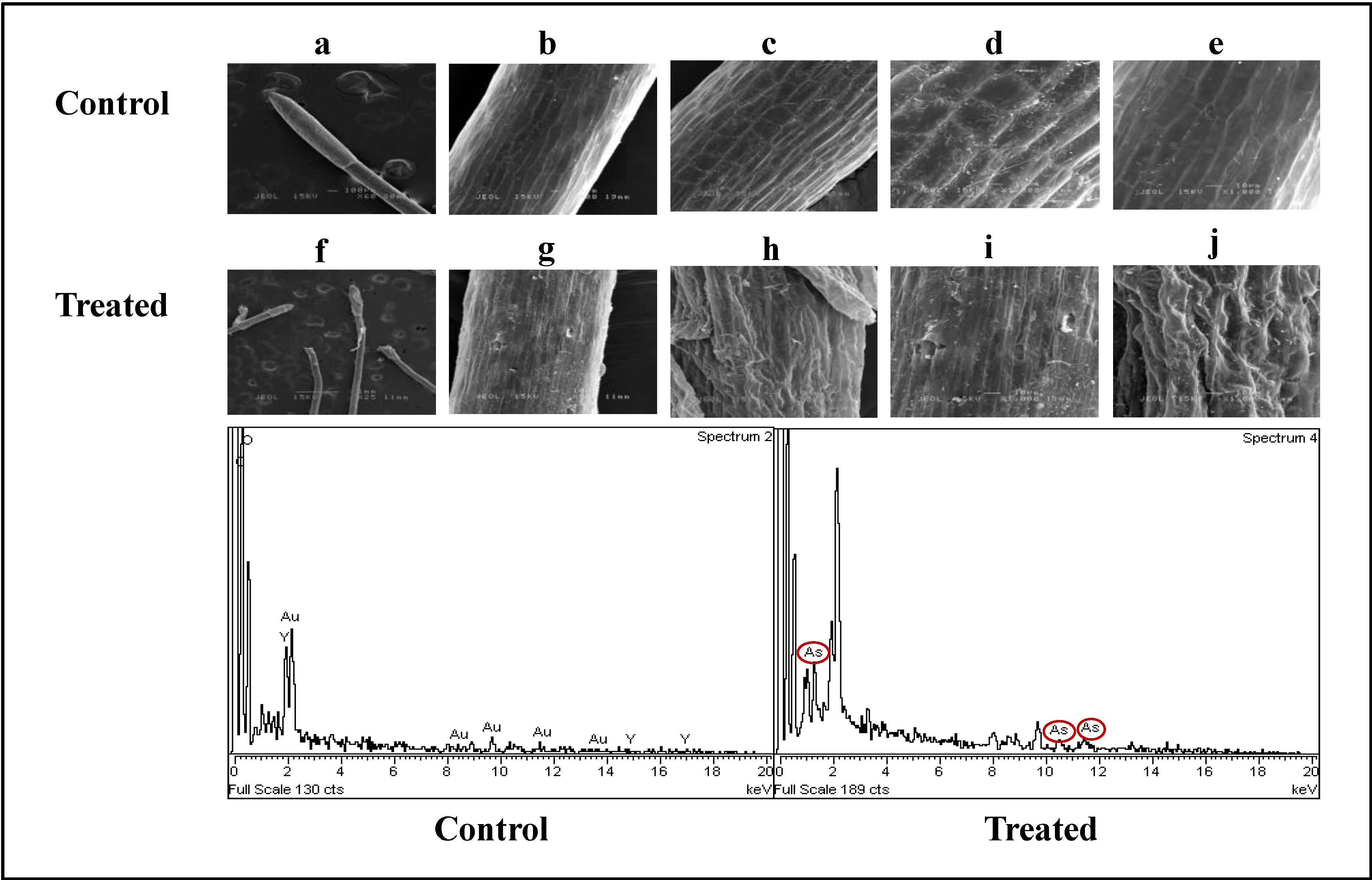
2.2.1. Arsenic’s Impact on Micromorphological Traits of Water Mimosa
2.2.2. Impact of Arsenic on Physiological Traits of Water Mimosa
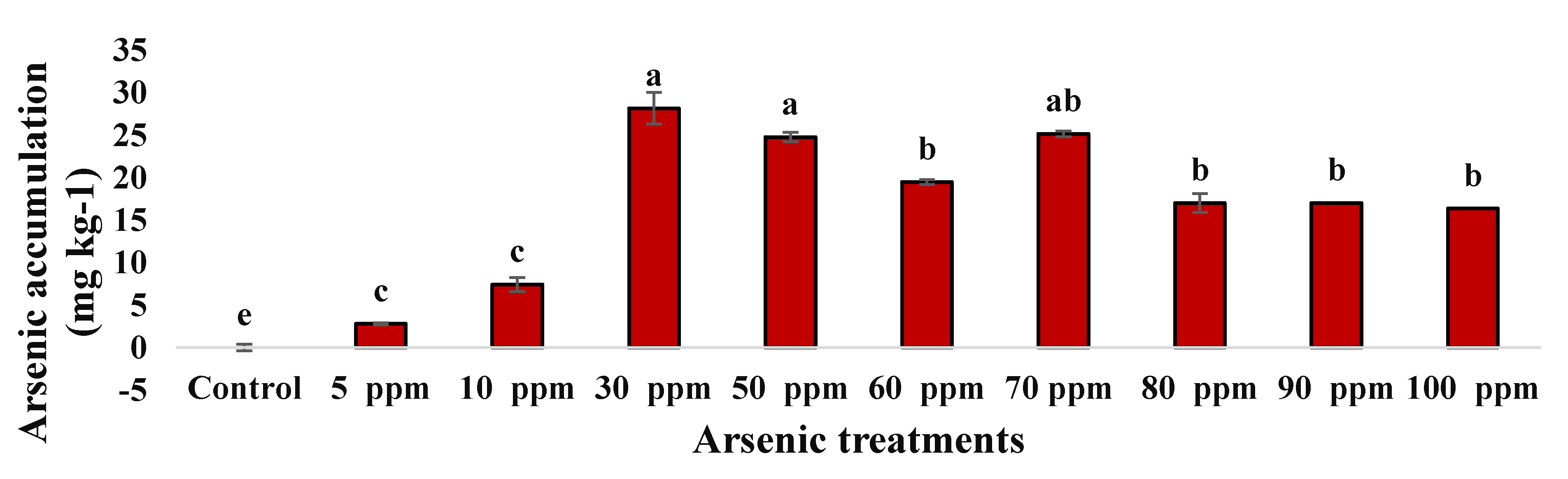
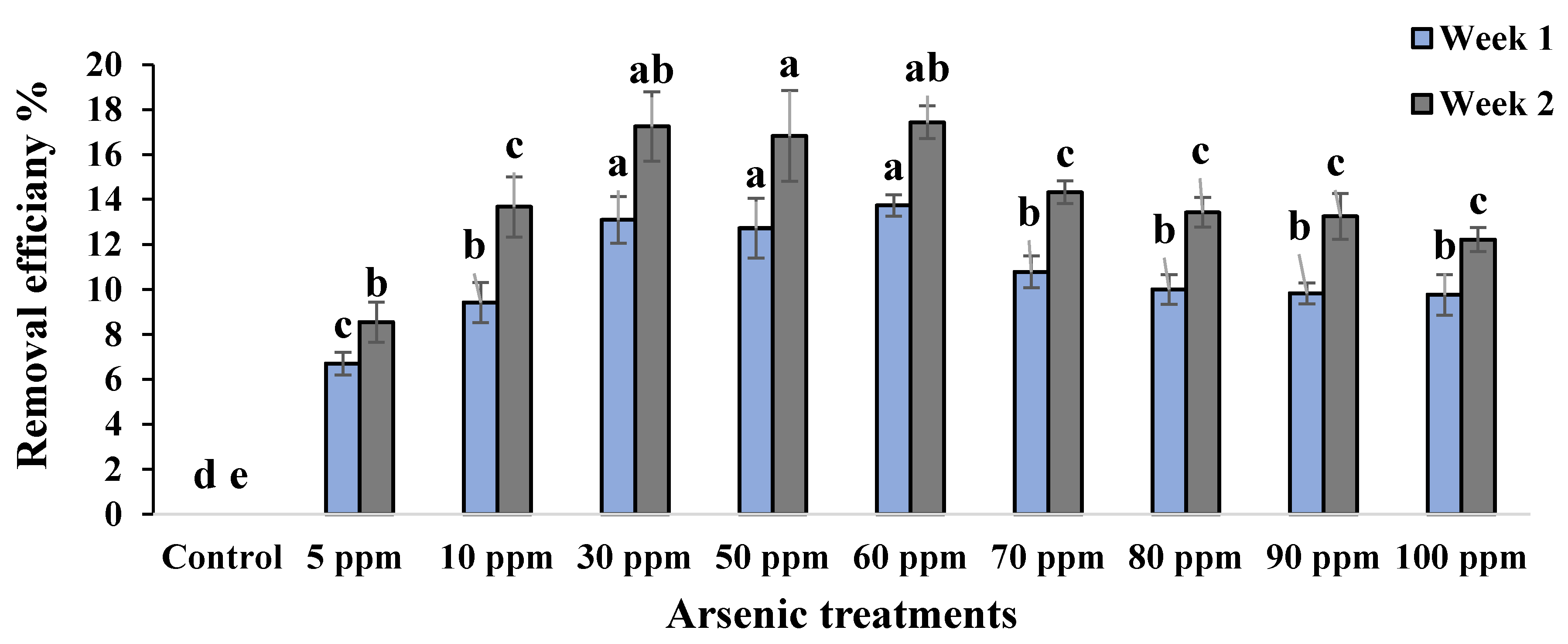
2.3. Phytoremediation Attributes of Water Mimosa under Arsenic Treatment
2.3.1. Arsenic Accumulation of Water Mimosa
2.3.2. Arsenic Effect on Removal Efficiency of Water Mimosa
2.3.3. Impact of Arsenic on Histology of Water Mimosa
3. Materials and Methods
3.1. Plant Materials and Culture Conditions
3.2. Arsenic Stress Treatment
3.3. Arsenic Stock Solution Preparation
3.4. Morphological Attribute Evaluation of N. oleracea under Arsenic Treatment
3.5. Relative Growth Rates
3.6. Decreasing Ratio of Biomass (DRB) and Decreasing Ratio of Dry Weight (DRD)
3.7. Relative Water Content
3.8. Tolerance Index
3.9. Physiological Features Assessment of N. oleracea under Arsenic Treatment
3.9.1. Chlorophyll Contents
3.9.2. Gas Exchange Attribute
3.9.3. Proline Contents
3.9.4. Lipid Peroxidation Contents
3.10. Arsenic Content Analysis Using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
3.10.1. Plant Sample Preparation and Acid Digestion Method
3.10.2. Analysis of Arsenic Uptake from Water
3.11. Detection of Arsenic in N. oleracea Samples Using Electron Microscopy Analysis
3.11.1. Sample Preparation for Electron Microscopy
3.11.2. Localization of Sodium Hepta Hydrate Arsenate in Treated Samples
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weldeslassie, T.; Naz, H.; Singh, B.; Oves, M. Chemical contaminants for soil, air and aquatic ecosystem. In Modern Age Environmental Problems and Their Remediation; Springer: Cham, Switzerland, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Subramaniam, K.; Ahmad, S.A.; Shaharuddin, N.A. Mini review on phenol biodegradation in Antarctica using native microorganisms. Asia Pac. J. Mol. Biol. Biotechnol. 2020, 28, 77–89. [Google Scholar] [CrossRef]
- Utermann, J.; Aydın, C.T.; Bischoff, N.; Böttcher, J.; Eickenscheidt, N.; Gehrmann, J.; König, N.; Scheler, B.; Stange, F. Heavy metal stocks and concentrations in forest soils. In Status and Dynamics of Forests in Germany. Ecological Studies (Analysis and Synthesis); Wellbrock, N., Bolte, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 237, pp. 199–229. [Google Scholar] [CrossRef]
- Zakaria, N.N.; Roslee, A.F.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Abdulrasheed, M.; Sabri, S.; Ramirez-Moreno, N.; Calisto-Ulloa, N.; Ahmad, S.A. Kinetic studies of marine psychrotolerant microorganisms capable of degrading diesel in the presence of heavy metals. Rev. Mex. Ing. Quím. 2020, 19, 1375–1388. [Google Scholar] [CrossRef]
- Izatt, R.M.; Izatt, S.R.; Bruening, R.L.; Izatt, N.E.; Moyer, B.A. Challenges to achievement of metal sustainability in our high-tech society. Chem. Soc. Rev. 2014, 43, 2451–2475. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, L.; Huang, K.; Zhang, J.; Xie, W.-Y.; Lu, Y.; Dong, X.; Zhao, F.J. Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J. 2019, 13, 2523–2535. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Jasrotia, S.; Kansal, A.; Mehra, A. Performance of aquatic plant species for phytoremediation of arsenic-contaminated water. Appl. Water Sci. 2017, 7, 889–896. [Google Scholar] [CrossRef]
- Novo, L.A.; Castro, P.M.; Alvarenga, P.; da Silva, E.F. Plant rhizobacteria-assisted phytoremediation growth–promoting of mine. In Bio-Geotechnologies for Mine Site Rehabilitation; Prasad, M.N.V., Favas, P.J.d.C., Maiti, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 281–295. [Google Scholar] [CrossRef]
- Abolayo, T.T. Comparative Analysis of Heavy Metals Presence in Soil and Water in Relation to Its Environs: A Case Study of Gbagede Dumpsite Area of Kwara State and Ilokun Dumpsite Area of Ekiti State. Ph.D. Thesis, Kwara State University, Malete, Nigeria, 2019. [Google Scholar]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Jayakumar, S.; Yusoff, M.M.; Rahim, M.H.A.; Maniam, G.P.; Govindan, N. The prospect of microalgal biodiesel using agro-industrial and industrial wastes in Malaysia. Renew. Sustain. Energy Rev. 2017, 72, 33–47. [Google Scholar] [CrossRef]
- Sobahan, M.A.; Mir, S.I.; Zakaria, I.; Hossain, M. Surface water contamination due to industrial activities in Gebeng area, Kuantan, Malaysia. In Proceedings of the International Conference on Civil and Architecture Engineering (ICCAE’2013), Kuala Lumpur, Malaysia, 6–7 May 2013; pp. 6–7. [Google Scholar]
- Ab Razak, N.H.; Praveena, S.M.; Aris, A.Z.; Hashim, Z. Drinking water studies: A review on heavy metal, application of biomarker and health risk assessment (a special focus in Malaysia). J. Epidemiol. Glob. Heal. 2015, 5, 297–310. [Google Scholar] [CrossRef]
- Sakai, N.; Alsaad, Z.; Thuong, N.T.; Shiota, K.; Yoneda, M.; Mohd, M.A. Source profiling of arsenic and heavy metals in the Selangor River basin and their maternal and cord blood levels in Selangor State, Malaysia. Chemosphere 2017, 184, 857–865. [Google Scholar] [CrossRef]
- Othman, F.; Uddin Chowdhury, M.; Wan Jaafar, W.Z.; Mohammad Faresh, E.M.; Shirazi, S.M. Assessing risk and sources of heavy metals in a tropical river basin: A case study of the Selangor River, Malaysia. Pol. J. Environ. Stud. 2018, 27, 1659–1671. [Google Scholar] [CrossRef]
- Hwi, T.Y.; Ibrahim, Y.S.; Khalik, W. Microplastic abundance, distribution, and composition in Sungai Dungun, Terengganu, Malaysia. Sains Malays. 2020, 49, 1479–1490. [Google Scholar]
- Fernandez-Luqueno, F.; López-Valdez, F.; Gamero-Melo, P.; Luna-Suárez, S.; Aguilera-González, E.N.; Martínez, A.I.; García-Guillermo, M.d.S.; Hernández-Martínez, G.; Herrera-Mendoza, R.; Álvarez-Garza, M.A.; et al. Heavy metal pollution in drinking water-a global risk for human health: A review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar] [CrossRef]
- Aljaberi, F.Y. Studies of autocatalytic electrocoagulation reactor for lead removal from simulated wastewater. J. Environ. Chem. Eng. 2018, 6, 6069–6078. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Soussi, A.; Ferjani, R.; Marasco, R.; Guesmi, A.; Cherif, H.; Rolli, E.; Mapelli, F.; Ouzari, H.I.; Daffonchio, D.; Cherif, A. Plant-associated microbiomes in arid lands: Diversity, ecology and biotechnological potential. Plant Soil 2016, 405, 357–370. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, Z.; Li, X.; Guan, Z.; Cai, Y.; Liao, X. The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Sci. Total Environ. 2019, 670, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, K.; Lokanathan, M.; Kakade, B. Three dimensional flower like cobalt sulfide (CoS)/functionalized MWCNT composite catalyst for efficient oxygen evolution reactions. Appl. Surf. Sci. 2019, 466, 830–836. [Google Scholar] [CrossRef]
- Arabnezhad, M.; Afarani, M.S.; Jafari, A. Co-precipitation synthesis of ZnO–TiO2 nanostructure composites for arsenic photodegradation from industrial wastewater. Int. J. Environ. Sci. Technol. 2019, 16, 463–468. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Ahmad, S.A.; Mustapha, S.R.; Ong-Abdullah, J. Ability of Ipomoea aquatica Forssk. to remediate phenol in water and effects of phenol on the plant’s growth. Pertanika J. Sci. Technol. 2017, 25, 441–452. [Google Scholar]
- Verasoundarapandian, G.; Darham, S.; Ahmad, S.A. Toxicity of molybdenum and microbial application in molybdenum reduction for bioremediation: A mini review. Malays. J. Biochem. Mol. Biol. 2019, 22, 46–51. [Google Scholar]
- Nasr, M. Phytomanagement in Egypt: A sustainable approach for clean environment coupled with meeting future energy demand. In Waste Management in MENA Regions; Springer: Cham, Switzerland, 2020; pp. 93–109. [Google Scholar] [CrossRef]
- Castaldi, P.; Silvetti, M.; Manzano, R.; Brundu, G.; Roggero, P.P.; Garau, G. Mutual effect of Phragmites australis, Arundo donax and immobilization agents on arsenic and trace metals phytostabilization in polluted soils. Geoderma 2018, 314, 63–72. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. (2020). Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Prabakaran, K.; Li, J.; Anandkumar, A.; Leng, Z.; Zou, C.B.; Du, D. Managing environmental contamination through phytoremediation by invasive plants: A review. Ecol. Eng. 2019, 138, 28–37. [Google Scholar] [CrossRef]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Choppala, G.; Kirkham, M.B.; Bolan, N.S. Rhizoremediation as a green technology for the remediation of petroleum hydrocarbon-contaminated soils. J. Hazard. Mater. 2020, 401, 123282. [Google Scholar] [CrossRef] [PubMed]
- Oniosun, S.; Harbottle, M.; Tripathy, S.; Cleall, P. Plant growth, root distribution and non-aqueous phase liquid phytoremediation at the pore-scale. J. Environ. Manag. 2019, 249, 109378. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Srivastava, S. A review of phytoremediation prospects for arsenic contaminated water and soil. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–254. [Google Scholar] [CrossRef]
- Bhunia, D.; Mondal, A.K. Systematic analysis (morphology, anatomy and palynology) of an aquatic medicinal plant water mimosa (Neptunia oleracea Lour.) in Eastern India. Int. J. Life Sci. Biotechnol. Pharm. Res. 2012, 1, 290–319. [Google Scholar]
- Visoottiviseth, P.; Francesconi, K.; Sridokchan, W. The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ. Pollut. 2002, 118, 453–461. [Google Scholar] [CrossRef]
- Wahab, A.; Ismail, S.S.; Abidin, E.Z.; Praveena, S. Neptunia oleracea (water mimosa) as phytoremediation plant and the risk to human health: A review. Adv. Environ. Biol. 2014, 8, 187–194. [Google Scholar]
- Anokhina, T.O.; Kochetkov, V.V.; Zelenkova, N.F.; Balakshina, V.V.; Boronin, A.M. Biodegradation of phenanthrene by Pseudomonas bacteria bearing rhizospheric plasmids in model plant–microbial associations. Appl. Biochem. Microbiol. 2004, 40, 568–572. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.D. Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Biores. Technol. 2007, 99, 7091–7097. [Google Scholar] [CrossRef] [PubMed]
- Syuhaida, A.W.A.; Norkhadijah, S.I.S.; Praveena, S.M.; Suriyani, A. The comparison of phytoremediation abilities of water mimosa and water hyacinth. ARPN J. Sci. Technol. 2014, 4, 722–731. [Google Scholar]
- Azimi, R.; Hossein Jafari, S.; Kianian, M.K.; Khaksarzade, V.; Amini, A. Studying arbuscular mycorrhiza symbiotic effects on establishment and morphological characteristics of Bromus kopetdaghensis in cadmium contaminated soil. Taiwan Water Conserv. 2016, 64, 82–91. [Google Scholar]
- Muro-González, D.A.; Mussali-Galante, P.; Valencia-Cuevas, L.; Flores-Trujillo, K.; Tovar-Sánchez, E. Morphological, physiological, and genotoxic effects of heavy metal bioaccumulation in Prosopis laevigata reveal its potential for phytoremediation. Environ. Sci. Pollut. Res. 2020, 1–18. [Google Scholar] [CrossRef]
- Applewhite, P.B.; Gardner, F.T. Rapid leaf closure of Mimosa in response to light. Nature 1971, 233, 279–280. [Google Scholar] [CrossRef]
- Hagihara, T.; Toyota, M. Mechanical signaling in the sensitive plant Mimosa pudica L. Plants 2020, 9, 587. [Google Scholar] [CrossRef]
- Yanitch, A.; Brereton, N.J.; Gonzalez, E.; Labrecque, M.; Joly, S.; Pitre, F.E. Transcriptomic response of purple willow (Salix purpurea) to arsenic stress. Front. Plant Sci. 2017, 8, 1115. [Google Scholar] [CrossRef]
- Dushenko, W.T.; Bright, D.A.; Reimer, K.J. Arsenic bioaccumulation and toxicity in aquatic macrophytes to gold-mine effluent: Relationship with environmental partitioning, metal uptake and nutrients. Aquat. Bot. 1995, 50, 141–158. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K. A review of the arsenic concentration in paddy rice from the perspective of geoscience. Geosci. J. 2013, 17, 107–122. [Google Scholar] [CrossRef]
- Lyubenova, L.; Pongrac, P.; Vogel-Mikuš, K.; Mezek, G.K.; Vavpetič, P.; Grlj, N.; Regvar, M.; Pelicon, P.; Schröder, P. The fate of arsenic, cadmium and lead in Typha latifolia: A case study on the applicability of micro-PIXE in plant ionomics. J. Hazard. Mater. 2013, 15, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ingole, N.W.; Bhole, A.G. Removal of heavy metals from aqueous solution by water hyacinth (Eichhornia crassipes). J. Water Supply Res. Technol. AQUA 2003, 52, 119–128. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- Reichman, S.M. The responses of plants to metal toxicity: A review forusing on copper, manganese & zinc. Aust. Miner. Energy Environ. Found. 2002, 14, 22–26. [Google Scholar]
- Bianconi, D.; Pietrini, F.; Massacci, A.; Iannelli, M.A. Uptake of cadmium by Lemna minor, a (hyper?-) accumulator plant involved in phytoremediation applications. E3S Web Conf. 2013, 1, 13002. [Google Scholar] [CrossRef]
- Kartika, K.; Lakitan, B.; Wijaya, A.; Kadir, S.; Widur, L.I.; Siaga, E.; Meihana, M. Effects of particle size and application rate of rice-husk biochar on chemical properties of tropical wetland soil, rice growth and yield. Aust. J. Crop. Sci. 2018, 12, 817–826. [Google Scholar] [CrossRef]
- Wan Rafiekal, W.A.R.; Hazandy, A.H.; Arifin, A.; Mohd Kamil, I. Efficiency of Bambusa vulgaris to clean up heavy metal contaminants. Trans. Malays. Soc. Plant Physiol. 2016, 23, 200–205. [Google Scholar]
- Gautam, A.; Pandey, A.K.; Dubey, R.S. Effect of arsenic toxicity on photosynthesis, oxidative stress and alleviation of toxicity with herbal extracts in growing rice seedlings. Indian J. Agric. Biochem. 2019, 32, 143–148. [Google Scholar] [CrossRef]
- Zu, Y.Q.; Sun, J.J.; He, Y.M.; Wu, J.; Feng, G.Q.; Li, Y. Effects of arsenic on growth, photosynthesis and some antioxidant parameters of Panax notoginseng growing in shaded conditions. Int. J. Adv. Agric. Res. 2016, 4, 78–88. [Google Scholar]
- Vezza, M.E.; Llanes, A.; Traveglia, C.; Agostini, E.; Talano, M.A. Arsenic stress effects on root water absorption in soybean plants: Physiological and morphological aspects. Plant Physiol. Biochem. 2018, 123, 9–17. [Google Scholar] [CrossRef]
- de Freitas-Silva, L.; de Araújo, T.O.; da Silva, L.C.; de Oliveira, J.A.; de Araujo, J.M. Arsenic accumulation in Brassicaceae seedlings and its effects on growth and plant anatomy. Ecotoxicol. Environ. Saf. 2016, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, S.; Spor, A.; Busset, H.; Falchetto, L.; Martin, J.; Bizouard, F.; Bru, D.; Breuil, M.-C.; Philippot, L.; Cordeau, S. Cover crop management practices rather than composition of cover crop mixtures affect bacterial communities in no-till agroecosystems. Front. Microbiol. 2019, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Goomer, S. Arsenic-A hidden poison in water-soil-rice crop continuum. Int. J. Sci. Technol. Res. 2019, 8, 864–877. [Google Scholar]
- Haider, I.; Andreo-Jimenez, B.; Bruno, M.; Bimbo, A.; Floková, K.; Abuauf, H.; Ntui, V.O.; Guo, X.; Charnikhova, T.; al-Babili, S.; et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018, 69, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Pathare, V.S.; Sounderajan, S.; Suprasanna, P. Nitrogen supply influences arsenic accumulation and stress responses of rice (Oryza sativa L.) seedlings. J. Hazard. Mater. 2019, 367, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; John, G.P.; Pan, R.; Bartlett, M.K.; Fletcher, L.R.; Scoffoni, C.; Sack, L. A stomatal safety-efficiency trade-off constrains responses to leaf dehydration. Nat. Commun. 2019, 10, 3398. [Google Scholar] [CrossRef]
- Xu, X.W.; Wang, P.; Zhang, J.; Chen, C.; Wang, Z.; Kopittke, P.M.; Kretzschmar, R.; Zhao, F.-J. Microbial sulfate reduction decreases arsenic mobilization in flooded paddy soils with high potential for microbial Fe reduction. Environ. Pollut. 2019, 251, 952–960. [Google Scholar] [CrossRef]
- Andrade-Linares, D.R.; Grosch, R.; Restrepo, S.; Krumbein, A.; Franken, P. Effects of dark septate endophytes on tomato plant perfor-mance. Mycorrhiza 2011, 21, 413–422. [Google Scholar] [CrossRef]
- Rasheed, A.; Takumi, S.; Hassan, M.A.; Imtiaz, M.; Ali, M.; Morgunov, A.I.; Mahmood, T.; He, Z. Appraisal of wheat genomics for gene discovery and breeding applications: A special emphasis on advances in Asia. Theor. Appl. Genet. 2020, 133, 1503–1520. [Google Scholar] [CrossRef]
- Mishra, V.K.; Upadhyaya, A.R.; Pandey, S.K.; Tripathi, B.D. Heavy metal pollution induced due to coal mining effluent on surrounding aquatic ecosystem and its management through naturally occurring aquatic macrophytes. Biores. Technol. 2008, 99, 930–936. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, S. Adsorptive uptake of arsenic (V) from water by aquatic fern Salvinia natans. J. Water Supply Res. Technol. AQUA 2005, 54, 47–53. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Wang, Y.S.; Lou, Z.P.; Dong, J.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Sun, K.; Su, X.; Pan, Y.X.; Zhang, Y.F.; Wang, X.P. Physiological responses and tolerance mechanisms to Pb in two xerophils: Salsola passerina Bunge and Chenopodium album L. J. Hazard. Mater. 2012, 205, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Mousavi, E.; Manochehri Kalantari, K.; Jafari, S.R. Change of some osmolytes accumulation in water-stressed colza (Brassica napus L.) as affected by 24-epibrassinolide. Iranian J. Sci. Technol. (Sci.) 2009, 33, 1–11. [Google Scholar]
- Monem, R.; Mirsharifi, S.M.; Mirtaheri, S.M. Evaluation allelopathic effects of barley shoot aqueous extract on germination, seedling growth, cell membrance permeability and malondialdehyde content of corn weeds. Adv. Environ. Biol. 2012, 6, 2490–2495. [Google Scholar]
- Zhao, J.; Shi, G.; Yuan, Q. Polyamines content and physiological and biochemical responses to ladder concentration of nickel stress in Hydrocharis dubia (Bl.) Backer leaves. Biometals 2008, 21, 665. [Google Scholar] [CrossRef]
- Ding, C.; Shi, G.; Xu, X.; Yang, H.; Xu, Y. Effect of exogenous spermidine on polyamine metabolism in water hyacinth leaves under mercury stress. Plant Growth Regul. 2010, 60, 61. [Google Scholar] [CrossRef]
- Galston, A.W.; Kaur-Sawhney, R.; Altabella, T.; Tiburcio, A.F. Plant polyamines in reproductive activity and response to abiotic stress. Bot. Acta 1997, 110, 197–207. [Google Scholar] [CrossRef]
- Saygideger, S.; Deniz, F. Effect of 24-epibrassinolide on biomass, growth and free proline concentration in Spirulina platensis (Cyanophyta) under NaCl stress. Plant Growth Regul. 2008, 56, 219–223. [Google Scholar] [CrossRef]
- Sylvain, B.; Mikael, M.H.; Florie, M.; Emmanuel, J.; Marilyne, S.; Sylvain, B.; Domenico, M. Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena 2016, 136, 44–52. [Google Scholar] [CrossRef]
- Bondada, B.R.; Tu, S.; Ma, L.Q. Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.). Sci. Total Environ. 2004, 332, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.T.; Shukor, M.Y.; Wasohm, H. Physical, chemical, and biological methods for the removal of arsenic compounds. Biomed. Res. Int. 2014, 2014, 503784. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.; Phung, L.T. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol. 2005, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Afkar, E. Localization of the dissimilatory arsenate reductase in Sulfurospirillum barnesii strain SeS-3. Am. J. Agric. Biol. Sci. 2012, 7, 97–105. [Google Scholar]
- Prum, C.; Dolphen, R.; Thiravetyan, P. Enhancing arsenic removal from arsenic-contaminated water by Echinodorus cordifolius−Endophytic arthrobacter creatinolyticus interactions. J. Environ. Manag. 2018, 213, 11–19. [Google Scholar] [CrossRef]
- Ogamba, E.N.; Izah, S.C.; Ogbogu, M.J. Cadmium and lead level in Eichhornia crassipes from River Nun, Niger Delta, Nigeria. J. Adv. Biol. Basic Res. 2015, 1, 53–56. [Google Scholar]
- Darajeh, N.; Idris, A.; Truong, P.; Abdul Aziz, A.; Abu Bakar, R.; Che Man, H. Phytoremediation potential of vetiver system technology for improving the quality of palm oil mill effluent. Adv. Mater. Sci. Eng. 2014, 2014, 683579. [Google Scholar] [CrossRef]
- Sumiahadi, A.; Acar, R. A review of phytoremediation technology: Heavy metals uptake by plants. Iop Conf. Ser. Earth Environ. Sci. 2018, 142, 012023. [Google Scholar] [CrossRef]
- Barbosa Felestrino, É.; de Almeida Barbosa Assis, R.; de Carvalho Lemes, C.G.; Ferreira Cordeiro, I.; Peixoto Fonseca, N.; Villa, M.M.; Marcio Moreira, L.; Vieira, I.T.; Kamino, L.H.Y.; do Carmo, F.F.; et al. Alcaligenes faecalis associated with Mimosa calodendron rizhosphere assist plant survival in arsenic rich soils. J. Soil Sci. Plant Nutr. 2017, 17, 1102–1115. [Google Scholar] [CrossRef]
- Sarangi, B.K.; Tiwari, S.; Pandey, R.A. Efficacy of three different plant species for arsenic phytoextraction from hydroponic system. Environ. Eng. Res. 2014, 19, 145–149. [Google Scholar]
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Balia Yusof, Z.N.; Atabaki, N.; Sahebi, M.; Azizi, P. Quantitative assessment of indica rice germination to hydropriming, hormonal priming and polyethylene glycol priming. Chil. J. Agric. Res. 2016, 76, 392–400. [Google Scholar] [CrossRef]
- Chen, J.; Shiyab, S.; Han, F.X.; Monts, D.L.; Waggoner, C.A.; Yang, Z.; Su, Y. Bioaccumulation and physiological effects of mercury in Pteris vittata and Nephrolepis exaltata. Ecotoxicology 2009, 18, 110–121. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Davenport, S.B.; Gallego, S.M.; Benavides, M.P.; Tomaro, M.L. Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annuus L. cells. Plant Growth Regul. 2003, 40, 81–88. [Google Scholar] [CrossRef]
- El Maki, H.B.; Abdel Rahaman, S.M.; Idris, W.H.; Hassan, A.B.; Babiker, E.E.; El Tinay, A.H. Content of antinutritional factors and HCl-extractability of minerals from white bean (Phaseolus vulgaris) cultivars: Influence of soaking and/or cooking. Food Chem. 2007, 100, 362–368. [Google Scholar] [CrossRef]
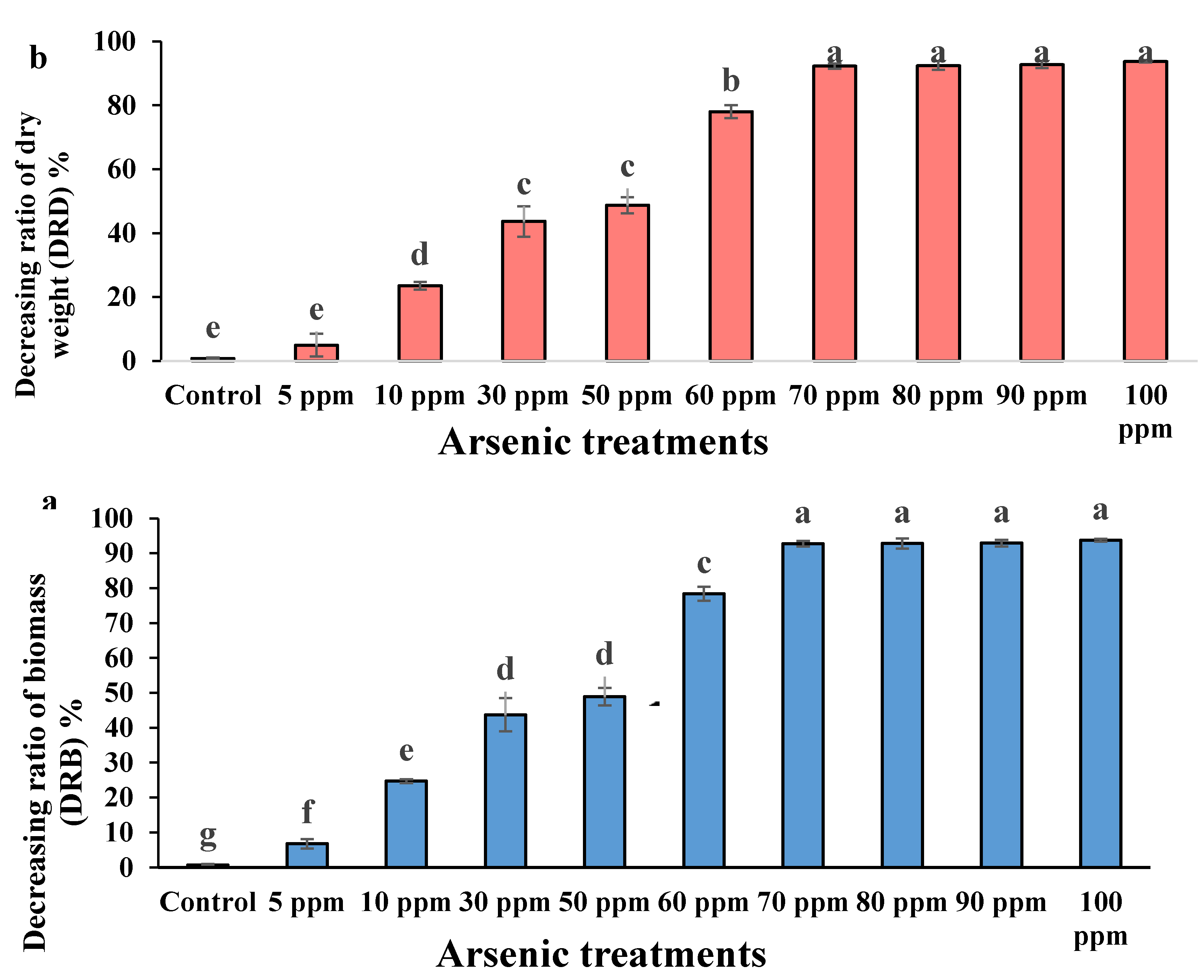
| S.O.V | df | DRB | DRD |
|---|---|---|---|
| Concentration | 9 | 0.6241 ** | 0.66 ** |
| Replicate | 2 | 0.001 ns | 0.004 ns |
| Error | 18 | 0.0006 | 0.0043 |
| Total | 29 | - | - |
| C.V. | - | 3.08 | 7.818 |
| Arsenic Concentrations (ppm) | Height of Frond (%) | Green Leaves (%) | RGR (g/g·day) | RWC (%) | Ti (%) |
|---|---|---|---|---|---|
| Control | 5 ± 0.2 a | 100 ± 0.0 a | 0.004 ± 0.00076 a | 89.34 ± 1.12 d | 100 ± 0.00 a |
| 5 | 5 ± 0.44 a | 96 ±1.43 b | 0.002 ± 0.00023 b | 90.56 ± 1.34 cd | 85 ± 1.2 b |
| 10 | 4.5 ± 0.34 b | 89 ± 1.76 c | 0.001 ± 0.00012 bc | 90.67 ± 2.32 c | 73 ± 1.5 c |
| 30 | 3.7 ± 0.36 c | 71 ± 0.32 d | 0.00 ± 0.0 d | 91.02 ± 1.12 bc | 61± 1.23 d |
| 50 | 2.2 ± 0.24 d | 46 ± 0.24 e | 0.00 ± 0.0 d | 91.03 ± 2.35 bc | 47 ± 1.43 e |
| 60 | 1.5 ± 0.12 e | 32 ±1.43 f | 0.00 ± 0.0 d | 91.2 ± 3.23 ab | 34 ± 1.84 f |
| 70 | 1.5 ± 0.23 e | 29 ± 0.32 f | 0.00 ± 0.0 d | 91.23 ± 2.22 ab | 26 ± 0.98 g |
| 80 | 1.2 ± 0.25 e | 7 ± 1.21 g | 0.00 ± 0.0 d | 91.3 ± 1.33 a | 22 ± 1.72 h |
| 90 | 0.9 ± 0.23 f | 0.00 ± 0.0 e | 0.00 ± 0.0 d | 91.32 ± 1.21 a | 20 ± 1.32 i |
| 100 | 0.7 ± 0.32 g | 0.00 ± 0.0 e | 0.00 ± 0.0 d | 91.32 ± 1.23 a | 16 ± 1.23 j |
| S.O.V | df | Chlorophyll Content | Photosynthesis Rate | Stomata Conductance | Intercellular CO2 Concentrations | Transpiration Rate | Air Pressure Deficit | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 1 | Week 2 | Week 1 | Week 2 | Week 1 | Week 2 | Week 1 | Week 2 | Week 1 | Week 2 | ||
| Concentration | 9 | 173.6 ** | 201.7 ** | 50.53 ** | 25.82 ** | 0.0065 ** | 0.008 ** | 39,106.1 * | 32,985.5 ** | 1.77 ** | 3.64 ** | 0.065 ns | 0.188 * |
| Replicate | 2 | 1.12 ns | 0.36 ns | 0.106 ns | 3.45 ns | 0.0002 ns | 0.001 ns | 6335.9 ns | 383.87 ns | 0.024 ns | 0.02 ns | 0.048 ns | 0.067 ns |
| Error | 18 | 89.53 | 65.14 | 0.358 | 2.99 | 0.0007 | 0.001 | 11,974.96 | 518.67 | 0.202 | 0.372 | 0.032 | 0.07184 |
| Total | 29 | - | - | - | - | - | - | - | - | - | - | - | - |
| C.V. | - | 11.35 | 10.41 | 20.34 | 19.6 | 18.22 | 16.55 | 34.33 | 10.47 | 19.97 | 20.32 | 8.698 | 14.42 |
| MDA Contents (µmol/g FW) | Proline (µmol/g FW) | |||
|---|---|---|---|---|
| Arsenic Concentrations (ppm) | Root | Leave | Root | Leave |
| Control | 12.43 ± 1.3 g | 15.45 ± 0.81 h | 20 ± 0.98 fg | 22 ± 0.54 h |
| 5 | 25.54 ± 1.02 f | 26.84 ± 1.36 g | 23 ± 0.87 e | 35 ± 1.39 e |
| 10 | 26.76 ± 0.9 e | 28.43 ± 1.21 f | 28 ± 0.23 d | 37 ± 1.3 d |
| 30 | 32.87 ± 1.2 b | 31.34 ± 0.98 d | 35 ± 0.4 a | 43 ± 1.3 a |
| 50 | 35.47 ± 1.8 a | 37.23 ± 0.80 a | 33 ± 1.93 b | 41 ± 1.74 b |
| 60 | 31.21 ± 1.01 c | 34.09 ±0.90 b | 30 ± 0.94 c | 39 ± 0.87 c |
| 70 | 28.65 ± 0.87 d | 33.35 ± 1.89 c | 27 ± 0.76 d | 28 ± 0.36 f |
| 80 | 26.67 ± 0.67 e | 30.12 ± 1.24 e | 21 ± 0.89 f | 25 ± 0.87 g |
| 90 | 20.76 ± 0.56 h | 24.34 ± 1.78 i | 19 ± 0.4 g | 22 ± 0.87 h |
| 100 | 18.23 ± 0.76 i | 24.12 ± 1.23 i | 19 ± 0.5 g | 18 ± 0.45 i |
| S.O.V | df | ICP Water Mimosa | Removal Efficiency after 7 Days | Removal Efficiency after 14 Days |
|---|---|---|---|---|
| Concentrations | 9 | 277.330 ** | 47.31 ** | 81.06 ** |
| Replicate | 2 | 1.303 ns | 0.709 ns | 0.750 ns |
| Error | 18 | 0.7125 | 0.661 | 1.46 |
| Total | 29 | - | - | - |
| C.V. | - | 5.290 | 8.419 | 9.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atabaki, N.; Shaharuddin, N.A.; Ahmad, S.A.; Nulit, R.; Abiri, R. Assessment of Water Mimosa (Neptunia oleracea Lour.) Morphological, Physiological, and Removal Efficiency for Phytoremediation of Arsenic-Polluted Water. Plants 2020, 9, 1500. https://doi.org/10.3390/plants9111500
Atabaki N, Shaharuddin NA, Ahmad SA, Nulit R, Abiri R. Assessment of Water Mimosa (Neptunia oleracea Lour.) Morphological, Physiological, and Removal Efficiency for Phytoremediation of Arsenic-Polluted Water. Plants. 2020; 9(11):1500. https://doi.org/10.3390/plants9111500
Chicago/Turabian StyleAtabaki, Narges, Noor Azmi Shaharuddin, Siti Aqlima Ahmad, Rosimah Nulit, and Rambod Abiri. 2020. "Assessment of Water Mimosa (Neptunia oleracea Lour.) Morphological, Physiological, and Removal Efficiency for Phytoremediation of Arsenic-Polluted Water" Plants 9, no. 11: 1500. https://doi.org/10.3390/plants9111500
APA StyleAtabaki, N., Shaharuddin, N. A., Ahmad, S. A., Nulit, R., & Abiri, R. (2020). Assessment of Water Mimosa (Neptunia oleracea Lour.) Morphological, Physiological, and Removal Efficiency for Phytoremediation of Arsenic-Polluted Water. Plants, 9(11), 1500. https://doi.org/10.3390/plants9111500







