The Fungicide Tetramethylthiuram Disulfide Negatively Affects Plant Cell Walls, Infection Thread Walls, and Symbiosomes in Pea (Pisum sativum L.) Symbiotic Nodules
Abstract
1. Introduction
2. Results
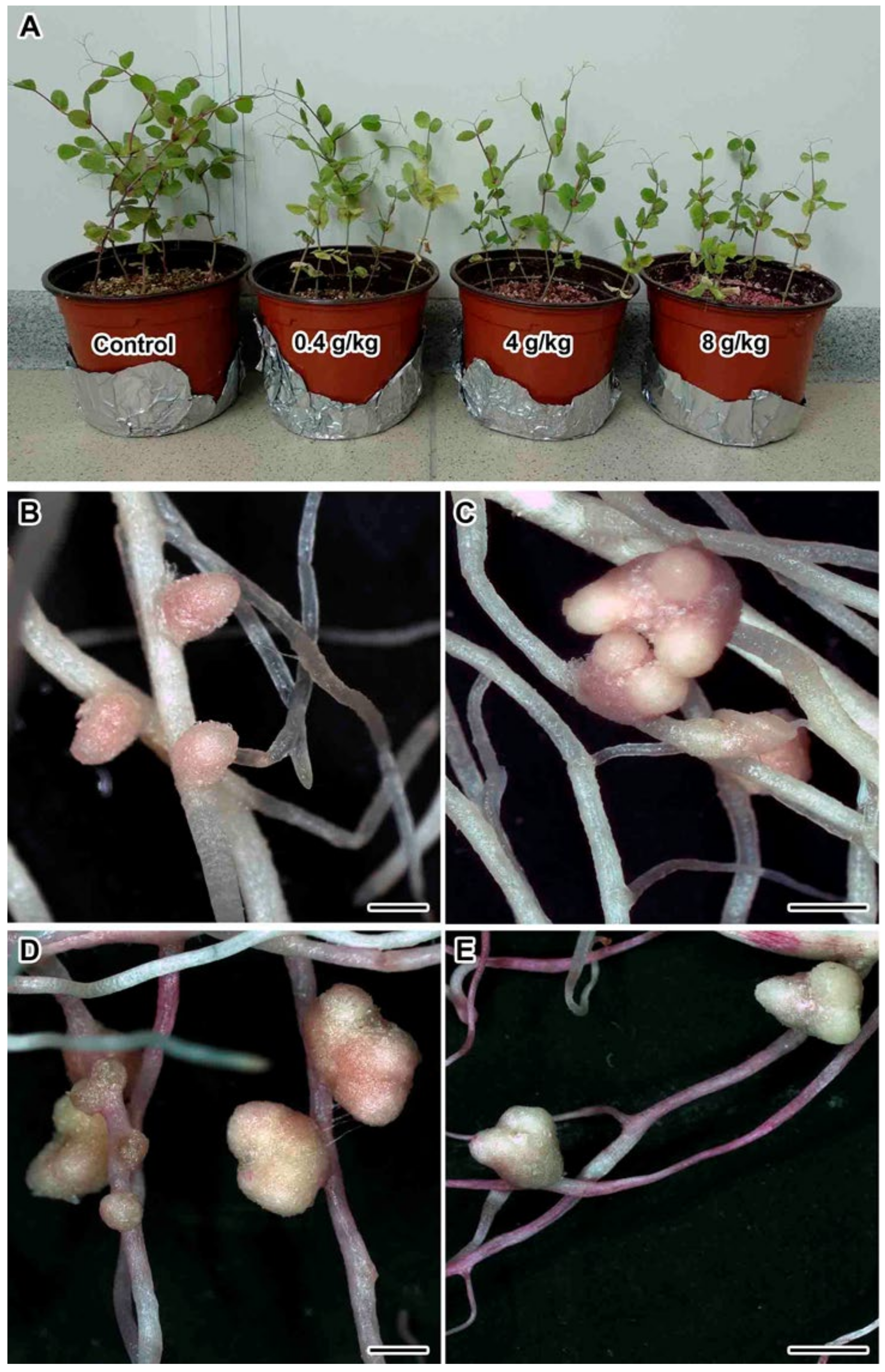
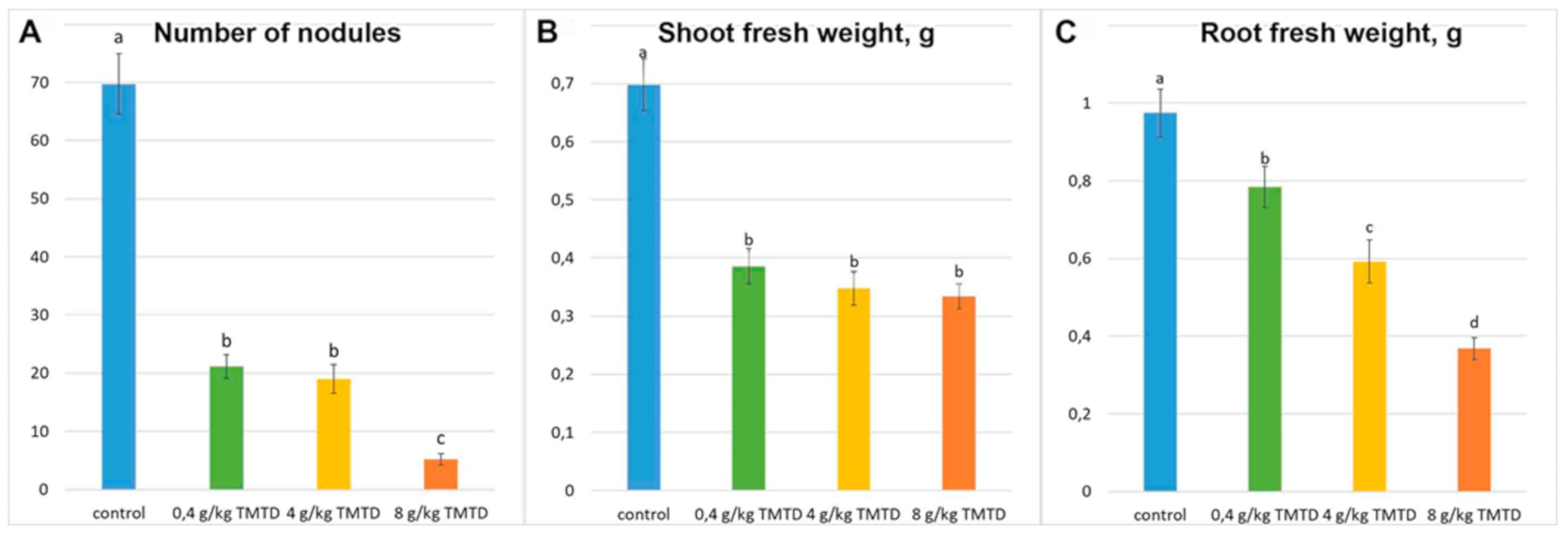
2.1. Nodulation and Plant Growth Parameters
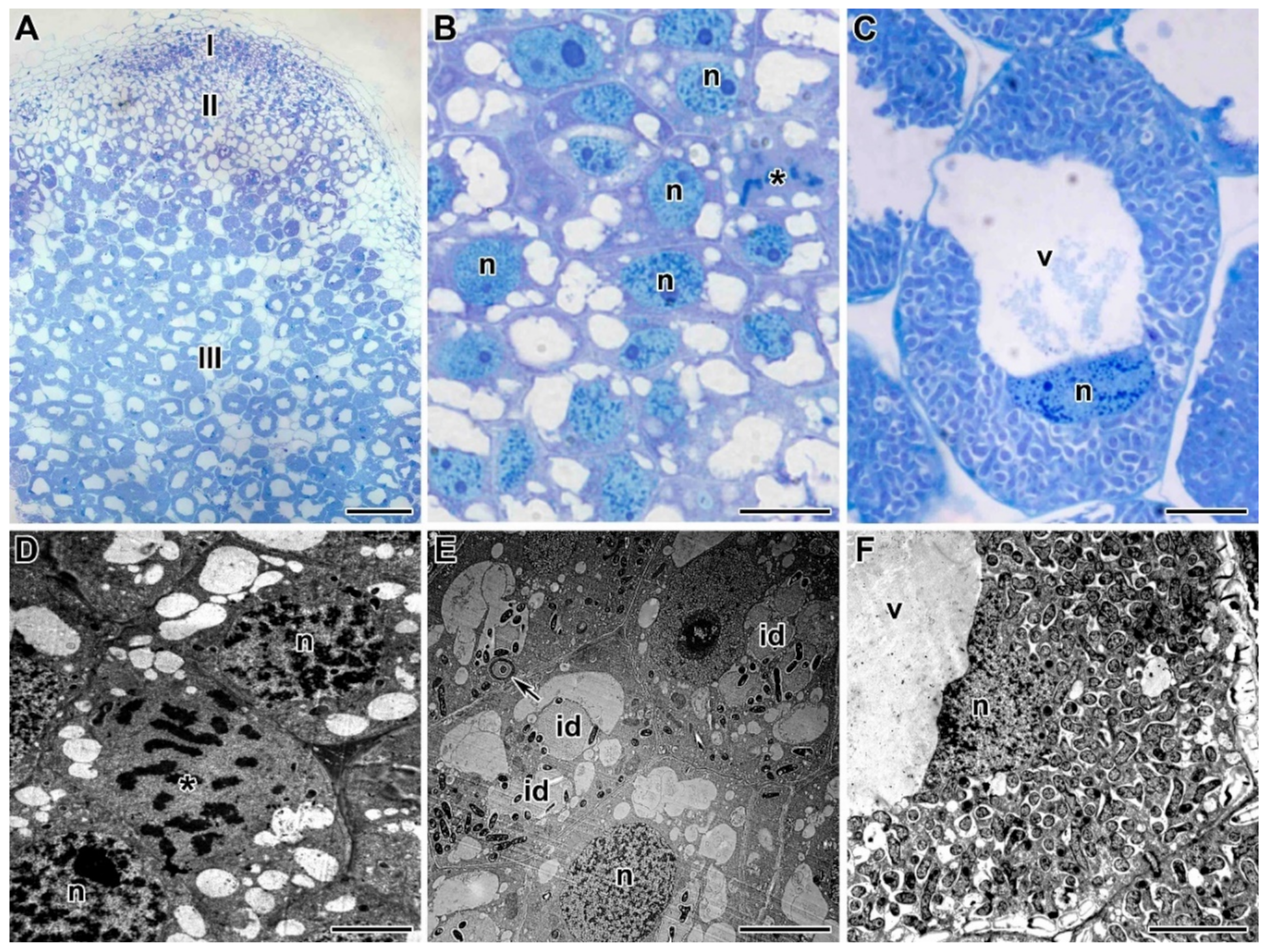
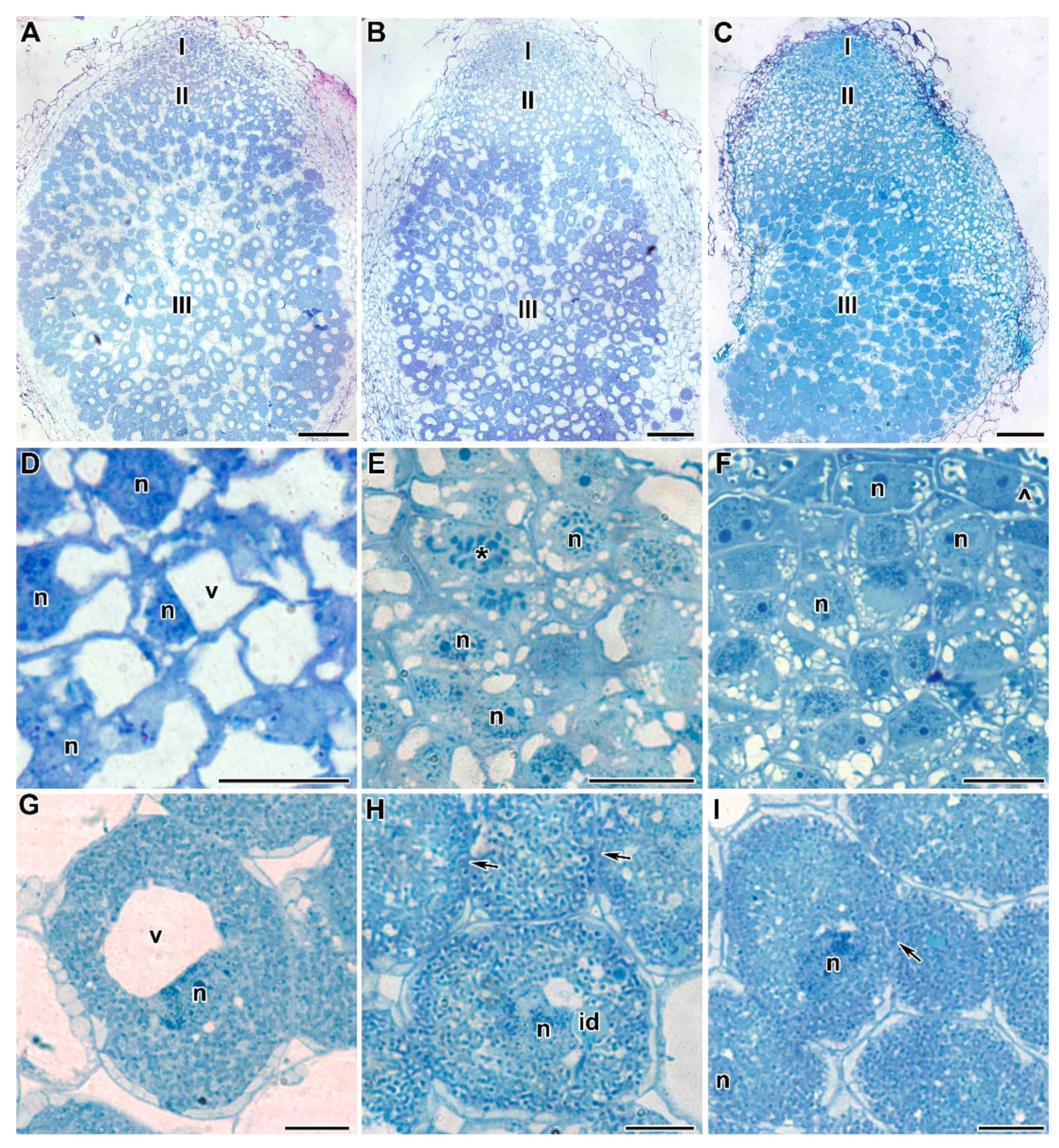
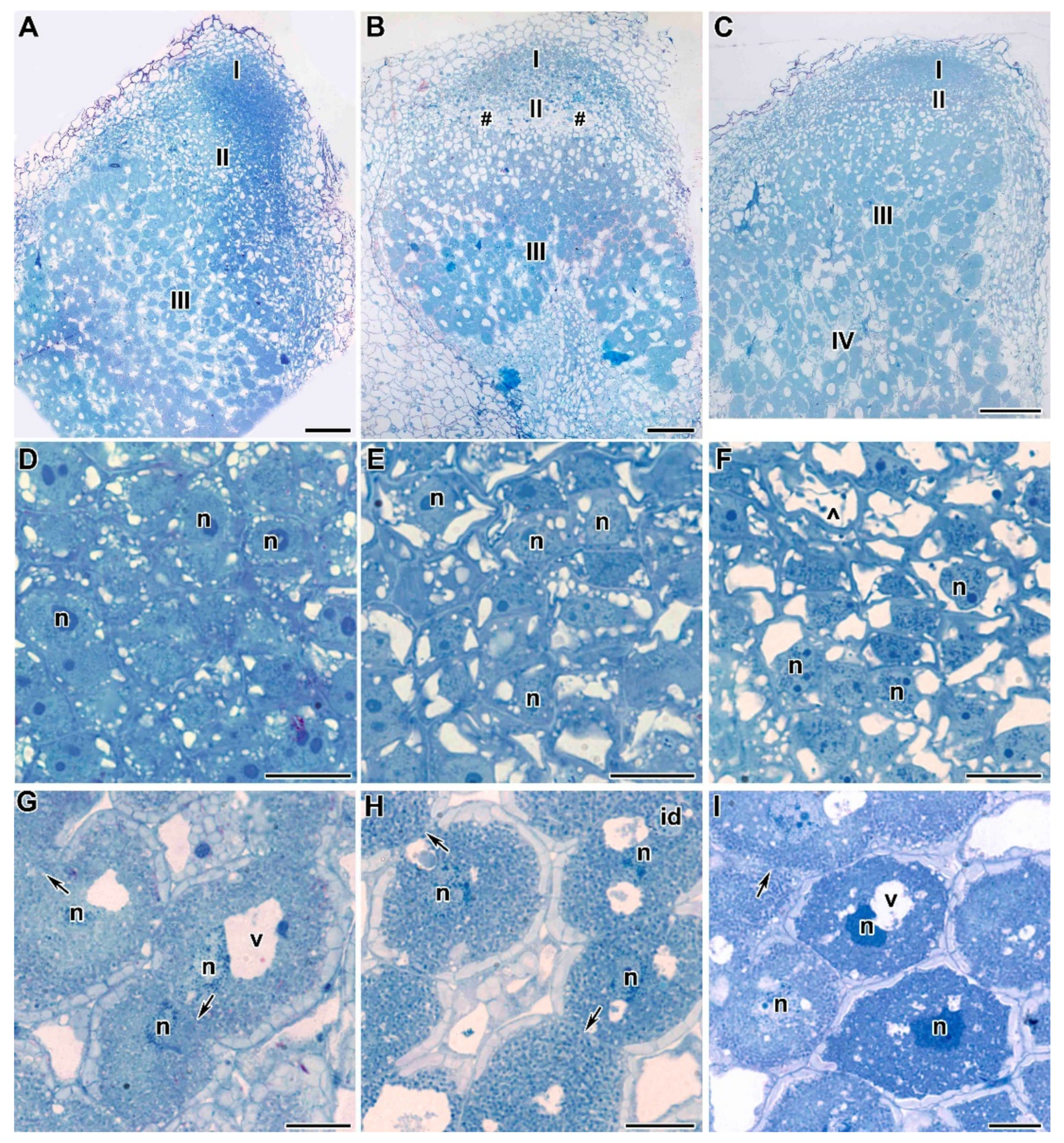
2.2. Light Microscopy Analyses
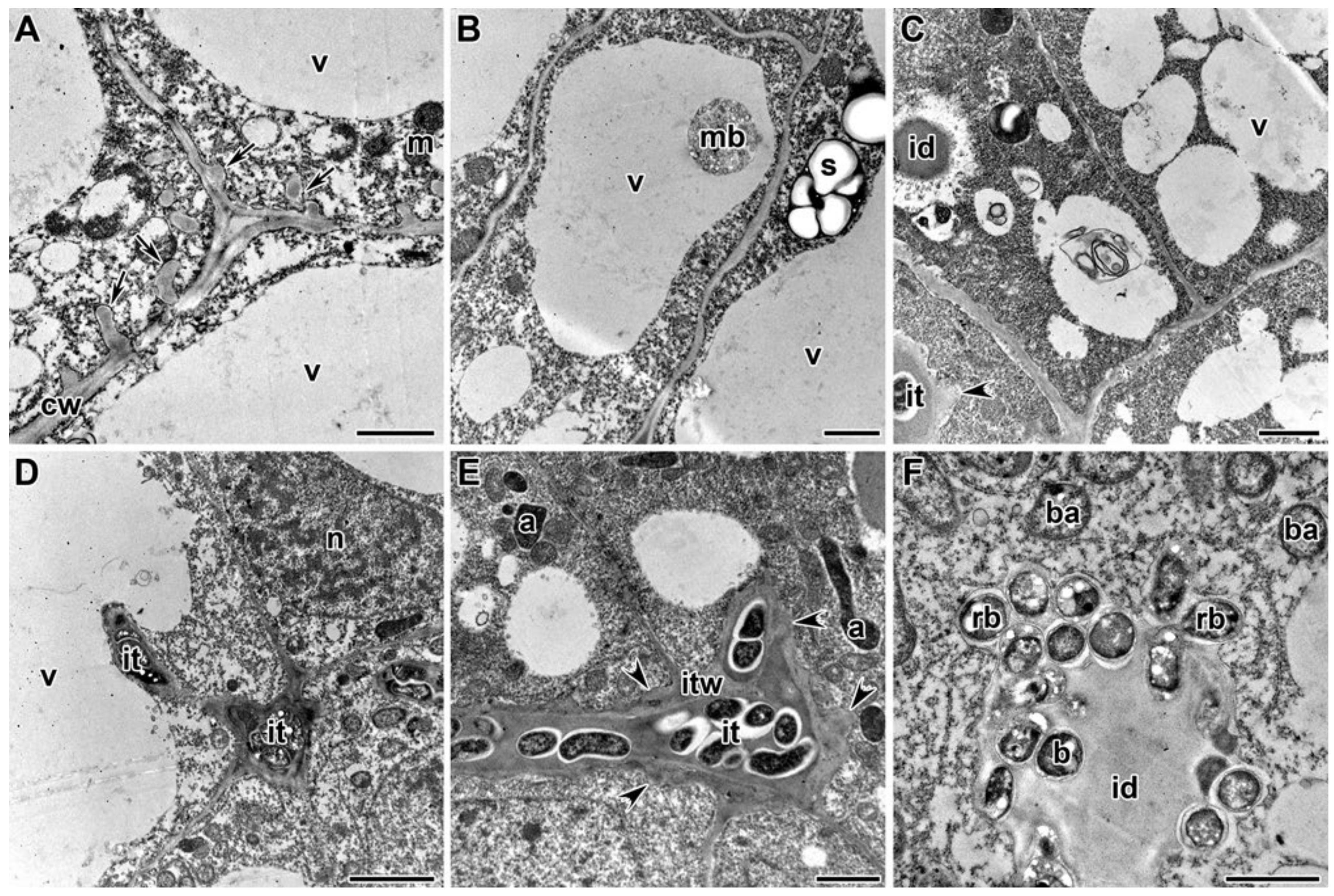
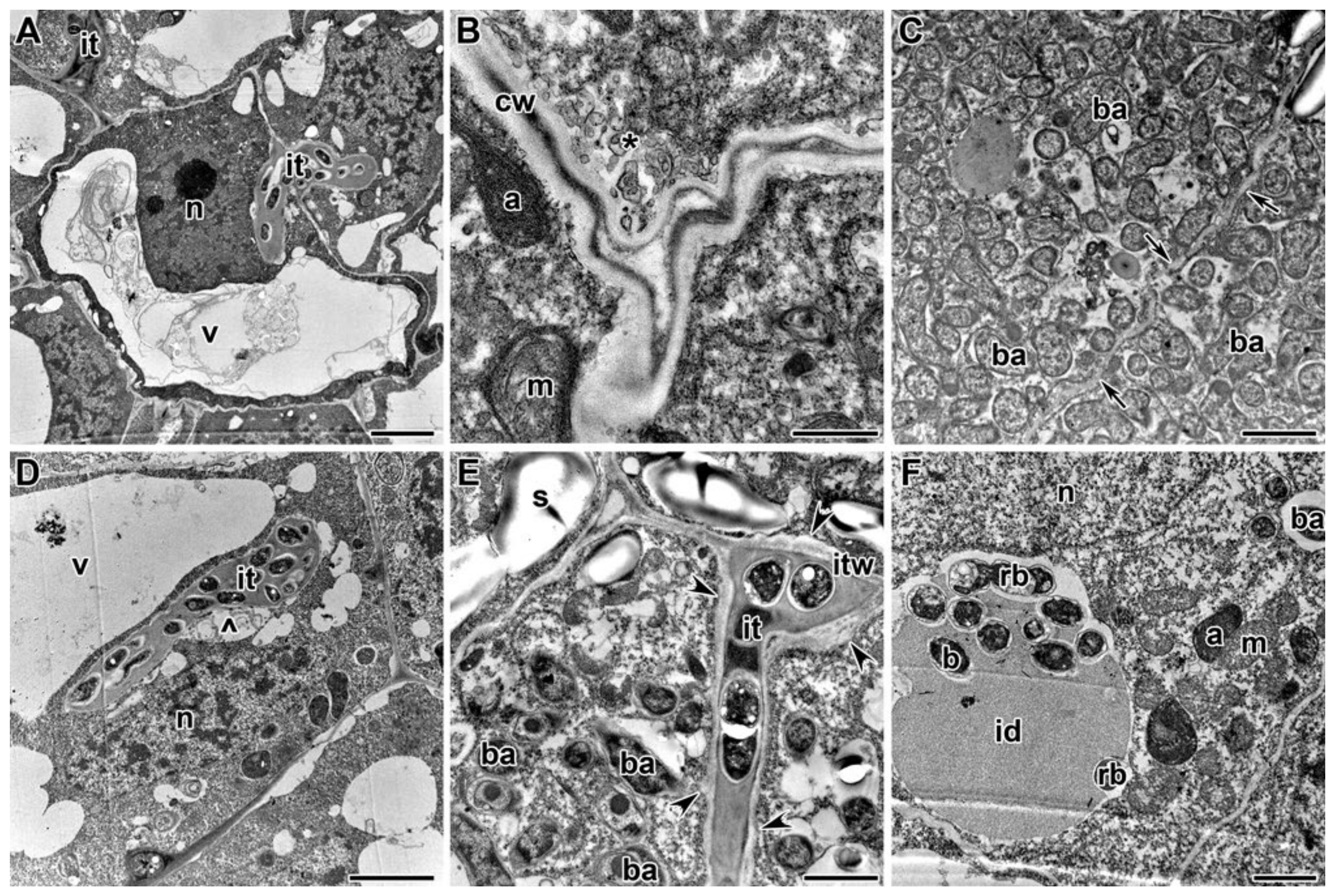
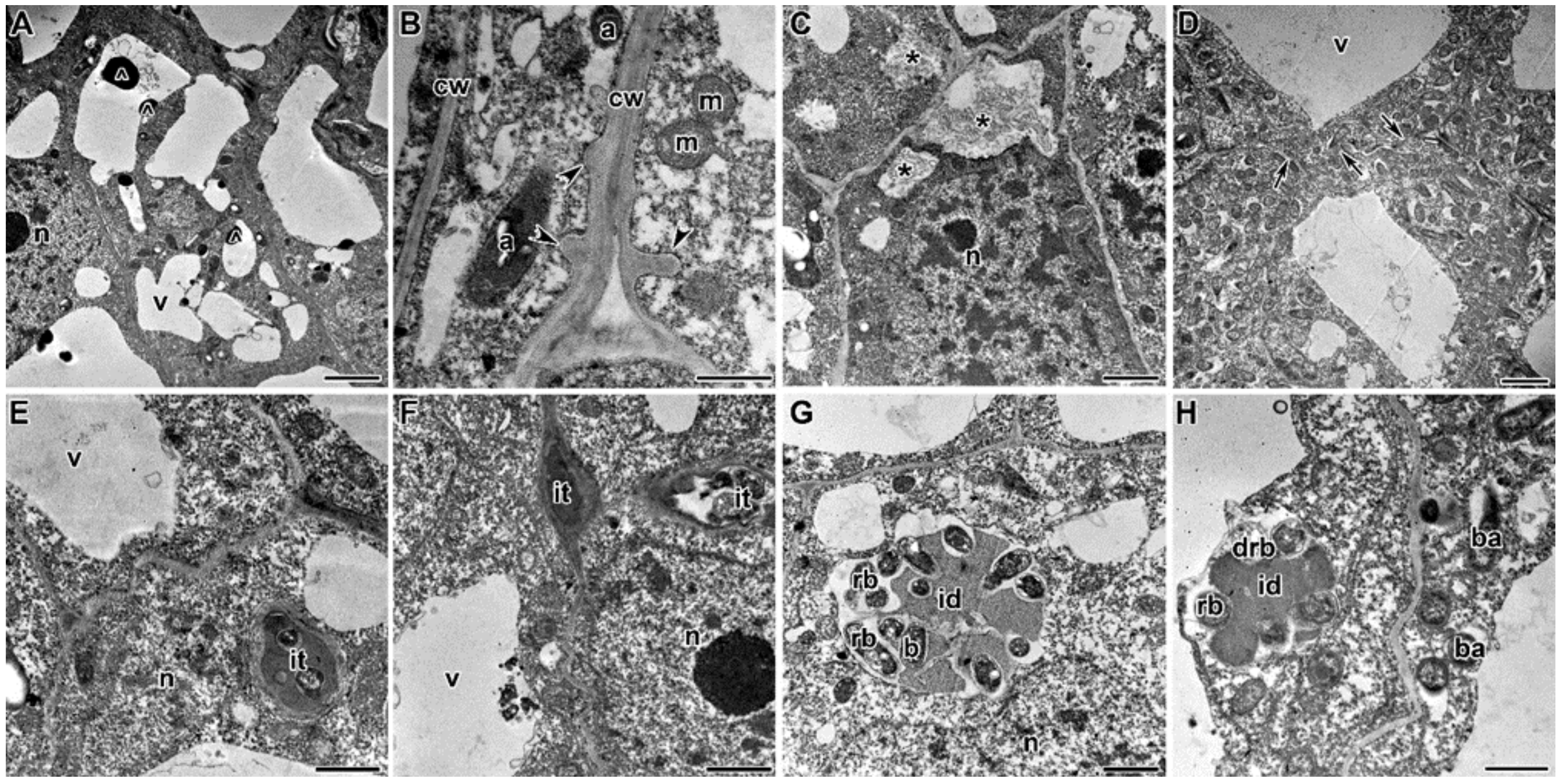
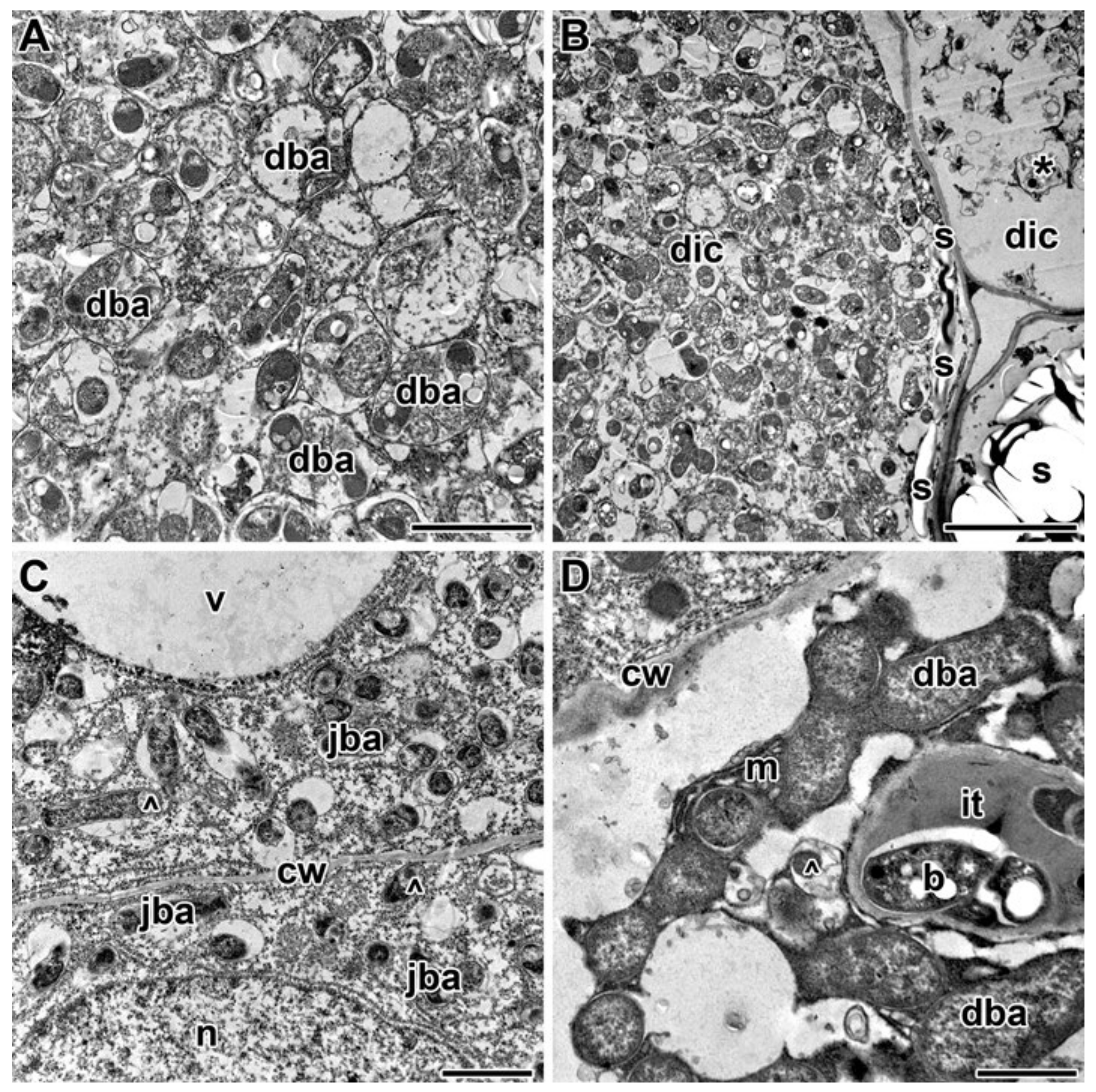
2.3. Ultrastructure of Nodules
3. Discussion
4. Materials and Methods
4.1. Plant Material and Bacterial Strain
4.2. Inoculation and Plant Growth Conditions
4.3. Growth and Nodulation Parameters
4.4. Statistical Analysis
4.5. Light and Transmission Electron Microscopy Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 August 2020).
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/peas-market (accessed on 13 August 2020).
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. 2017, 4, 2. [Google Scholar] [CrossRef]
- Begum, N.; Zeeshan, K.; Haque, M.; Raja, M.; Chohan, S. Evaluation of mycoflora associated with pea seeds and some control measures. Plant Pathol. J. 2004, 3, 48–51. [Google Scholar] [CrossRef][Green Version]
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.; Castillejo, M.; Singh, K.; Rispail, N. Achievements and challenges in legume breeding for pest and disease resistance. Crit. Rev. Plant Sci. 2015, 34, 195–236. [Google Scholar] [CrossRef]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant-microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Zotikov, V.I.; Budarina, G.A. Diseases of peas and the main methods of protecting culture in the conditions of central Russia. Prot. Quarantine Plants 2015, 5, 11–15. (In Russian) [Google Scholar]
- Ellouze, W.; Esmaeili Taheri, A.; Bainard, L.D.; Yang, C.; Bazghaleh, N.; Navarro-Borrell, A.; Hanson, K.; Hamel, C. Soil fungal resources in annual cropping systems and their potential for management. Biomed. Res. Int. 2014, 531824. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting sustainability of fungicide seed treatments for field crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- Mancini, V.; Romanazzi, G. Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Manag. Sci. 2014, 70, 860–868. [Google Scholar] [CrossRef]
- Thind, T.S.; Hollomon, D.W. Thiocarbamate fungicides: Reliable tools in resistance management and future outlook. Pest Manag. Sci. 2018, 74, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Aulakh, J.S.; Malik, A.K. Thiram: Degradation, applications and analytical methods. J. Environ. Monit. 2003, 5, 717–723. [Google Scholar] [CrossRef]
- Kruglov, U.V. Soil microflora and pesticides; Agropromizdat: Moscow, USSR, 1991; pp. 74–89. (In Russian) [Google Scholar]
- Khan, H.; Zeb, A.; Ali, Z.; Shah, S. Impact of five insecticides on chickpea (Cicer arietinum L.) nodulation, yield and nitrogen fixing rhizospheric bacteria. Soil Environ. 2009, 28, 56–59. [Google Scholar]
- Tu, C.M. Effect of fungicides on growth of Rhizobium japonicum in vitro. Bull. Environ. Contam. Toxicol. 1980, 25, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Moorman, T.B. Effects of herbicides on the survival of Rhizobium japonicum strains. Weed Sci. 2017, 34, 628–633. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Shafiani, S.; Malik, A. Tolerance of pesticides and antibiotic resistance in bacteria isolated from wastewater-irrigated soil. World J. Microbiol. Biotechnol. 2003, 19, 897–901. [Google Scholar] [CrossRef]
- Zablotowicz, R.M.; Reddy, K.N. Impact of glyphosate on the Bradyrhizobium japonicum symbiosis with glyphosate-resistant transgenic soybean: A minireview. J. Environ. Qual. 2004, 33, 825–831. [Google Scholar] [CrossRef]
- Santos, J.; Ferreira, E.; Kasuya, M.C.; Silva, A.; Procópio, S. Tolerance of Bradyrhizobium strains to glyphosate formulations. Crop Prot. 2005, 24, 543–547. [Google Scholar] [CrossRef]
- Tesfai, K.; Mallik, M.A.B. Effect of fungicide application on soybean-rhizobia symbiosis and isolation of fungicide-resistant strains of Rhizobia japonicum. Bull. Environ. Contam. Toxicol. 1986, 36, 819–826. [Google Scholar] [CrossRef]
- Tu, C.M. Effects of pesticide seed treatments on Rhizobium japonicum and its symbiotic relationship with soybean. Bull. Environ. Contam. Toxicol. 1977, 18, 190–199. [Google Scholar] [CrossRef]
- Isoi, T.; Yoshida, S. Effect of thiram (tetramethyl-thiuram-disulphide) application on nodulation in soybean and kidney bean plants: Observation using the root-box-culture technique. Soil Sci. Plant Nutr. 1988, 34, 633–637. [Google Scholar] [CrossRef]
- Rathjen, J.R.; Ryder, M.H.; Riley, I.T.; Lai, T.V.; Denton, M.D. Impact of seed-applied pesticides on rhizobial survival and legume nodulation. J. Appl. Microbiol. 2020, 129, 389–399. [Google Scholar] [CrossRef]
- Odeyemi, O.; Alexander, M. Resistance of Rhizobium strains to phygon, spergon, and thiram. Appl. Environ. Microbiol. 1977, 33, 784–790. [Google Scholar] [CrossRef]
- Lennox, L.B.; Alexander, M. Fungicide enhancement of nitrogen fixation and colonization of Phaseolus vulgaris by Rhizobium phaseoli. Appl. Environ. Microbiol. 1981, 41, 404–411. [Google Scholar] [CrossRef]
- Hamdi, Y.A.; Moharram, A.A.; Lofti, M. Effect of certain fungicides on some Rhizobia-legume-symbiotic systems. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 1974, 129, 363–368. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Javed, F.; Ashraf, M.; Hafeez, F.Y. Effect of fungicide seed treatments on N2-fixation and nodulation in pea, Pisum sativum L. Bull. Environ. Contam. Toxicol. 2006, 77, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Erickson, R.S. Effect of seed treatment with Rhizobium leguminosarum on Pythium damping-off, seedling height, root nodulation, root biomass, shoot biomass, and seed yield of pea and lentil. J. Phytopathol. 2007, 155, 31–37. [Google Scholar] [CrossRef]
- Shirkot, P.; Shirkot, C.K.; Gupta, K.G. Effect of tetramethylthiuram disulfide (TMTD) on nodulation, plant yield, and nitrogen fixation by Cicer arietinum in presence of TMTD-utilizing bacteria. Zbl. Mikrobiol. 1991, 146, 413–418. [Google Scholar] [CrossRef]
- Gaind, S.; Rathi, M.S.; Kaushik, B.D.; Nain, L.; Verma, O.P. Survival of bio-inoculants on fungicides-treated seeds of wheat, pea and chickpea and subsequent effect on chickpea yield. J. Environ. Sci. Health B. 2007, 42, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Bikrol, A.; Saxena, N.; Singh, K. Response of Glycine max in relation to nitrogen fixation as influenced by fungicide seed treatment. Afr. J. Biotechnol. 2005, 4, 667–671. [Google Scholar] [CrossRef]
- Kyei-Boahen, S.; Slinkard, A.E.; Walley, F.L. Rhizobial survival and nodulation of chickpea as influenced by fungicide seed treatment. Can. J. Microbiol. 2001, 47, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, A.; Klama, J. Pesticide side effect on the symbiotic efficiency and nitrogenase activity of Rhizobiaceae bacteria family. Pol. J. Microbiol. 2005, 54, 43–48. [Google Scholar]
- Yang, C.; Hamel, C.; Vujanovic, V.; Gan, Y. Nontarget effects of foliar fungicide application on the rhizosphere: Diversity of nifH gene and nodulation in chickpea field. J. Appl. Microbiol. 2012, 112, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, S.K.; Kim, I.D.; Kwak, H.S.; Shin, D.H. Unraveling the effect of structurally different classes of insecticide on germination and early plant growth of soybean [Glycine max (L) Merr]. Pestic. Biochem. Physiol. 2016, 130, 39–43. [Google Scholar] [CrossRef]
- Bragança, I.; Lemos, P.; Barros, P.; Delerue-Matos, C.; Domingues, V. Phytotoxicity of pyrethroid pesticides and its metabolite towards Cucumis sativus. Afr. J. Biotechnol. 2018, 619, 685–691. [Google Scholar] [CrossRef]
- Schneider, M.; Keiblinger, K.M.; Paumann, M.; Soja, G.; Mentler, A.; Golestani-Fard, A.; Retzmann, A.; Prohaska, T.; Zechmeister-Boltenstern, S.; Wenzel, W.; et al. Fungicide application increased copper-bioavailability and impaired nitrogen fixation through reduced root nodule formation on alfalfa. Ecotoxicology 2019, 28, 599–611. [Google Scholar] [CrossRef]
- Baby, U.I.; Balasubramanian, S.; Ajay, D.; Premkumar, R. Effect of ergosterol biosynthesis inhibitors on blister blight disease, the tea plant and quality of made tea. Crop Prot. 2004, 23, 795–800. [Google Scholar] [CrossRef]
- Kurek, M.; Barchańska, H.; Turek, M. Degradation processes of pesticides used in potato cultivations. Rev. Environ. Contam. Toxicol. 2017, 242, 105–151. [Google Scholar] [CrossRef]
- Maznah, Z.; Halimah, M.; Ismail, B.S. Evaluation of the persistence and leaching behaviour of thiram fungicide in soil, water and oil palm leaves. Bull. Environ. Contam. Toxicol. 2018, 100, 677–682. [Google Scholar] [CrossRef]
- Çağlayan, C.; Taslimi, P.; Türk, C.; Gülçin, I.; Kandemir, F.; Demir, Y.; Beydemir, Ş. Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environ. Sci. Pollut. Res. 2020, 27, 10607–10616. [Google Scholar] [CrossRef]
- Salam, S.; Arif, A.; Mahmood, R. Thiram-induced cytotoxicity and oxidative stress in human erythrocytes: An in vitro study. Pestic. Biochem. Physiol. 2020, 164, 14–25. [Google Scholar] [CrossRef]
- Gupta, B.; Rani, M.; Kumar, R. Degradation of thiram in water, soil and plants: A study by high-performance liquid chromatography. Biomed. Chromatogr. 2012, 26, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Duval, R.; Xu, X.; Rodrigues-Lima, F.; Dupret, J.M. Effects of pesticide chemicals on the activity of metabolic enzymes: Focus on thiocarbamates. Expert. Opin. Drug. Metab. Toxicol. 2015, 11, 81–94. [Google Scholar] [CrossRef]
- Sirois, J.C.; Peterson, E.A.; Miller, R.W. Potential effects of Thiram on Medicago—R. meliloti symbiotic association. J. Environ. Sci. Health. B 1981, 16, 293–307. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yang, H. Bioaccumulation and degradation of pentachloronitrobenzene in Medicago sativa. J. Environ. Manage. 2013, 119, 143–150. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Baird, L.M.; Escuredo, P.R.; Dalton, D.A.; Minchin, F.R.; Iturbe-Ormaetxe, I.; Rubio, M.C.; Moran, J.F.; Gordon, A.J.; Becana, M. Stress-induced legume root nodule senescence. Physiological, biochemical, and structural alterations. Plant Physiol. 1999, 121, 97–112. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Tsyganov, V.E. Influence of mutation in pea (Pisum sativum L) cdt (cadmium tolerance) gene on histological and ultrastructural nodule organization. Ekol. Genet. 2019, 17, 71–80. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Tsyganova, A.V.; Gorshkov, A.P.; Seliverstova, E.V.; Kim, V.E.; Chizhevskaya, E.P.; Belimov, A.A.; Serova, T.A.; Ivanova, K.A.; Kulaeva, O.A.; et al. Efficacy of a plant-microbe system: Pisum sativum (L) cadmium-tolerant mutant and Rhizobium leguminosarum strains, expressing pea metallothionein genes PsMT1 and PsMT2, for cadmium phytoremediation. Front. Microbiol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Carpita, N.C. Progress in the biological synthesis of the plant cell wall: New ideas for improving biomass for bioenergy. Curr. Opin. Biotechnol. 2012, 23, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Barnes, W.J.; Anderson, C.T. Release, recycle, rebuild: Cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol. Plant 2018, 11, 31–46. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Diffuse growth of plant cell walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Tsyganova, A.V.; Tsyganov, V.E. Plant cell wall in symbiotic interactions. Pectins. Agric. Biol. 2019, 54, 446–457. [Google Scholar] [CrossRef]
- Keegstra, K. Plant cell walls. Plant Phys. 2010, 154, 483–486. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant. Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Novák, K.; Škrdleta, V.; Němcová, M.; Lisá, L. Behavior of pea nodulation mutants as affected by increasing nitrate level. Symbiosis 1993, 15, 195–206. [Google Scholar]
- Morzhina, E.V.; Tsyganov, V.E.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. Four developmental stages identified by genetic dissection of pea (Pisum sativum L.) root nodule morphogenesis. Plant Sci. 2000, 155, 75–83. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Morzhina, E.V.; Stefanov, S.Y.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol. Gen. Genet. 1998, 259, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, V.E.; Tsyganova, A.V.; Voroshilova, V.A.; Borisov, A.Y.; Tikhonovich, I.A. Analysis of the interaction of pea (Pisum sativum L.) symbiotic genes Sym33 and Sym42 whose mutations result in abnormalities during infection thread development. Russ. J. Genet. Appl. Res. 2014, 4, 83–87. [Google Scholar] [CrossRef]
- Ivanova, K.A.; Tsyganova, A.V.; Brewin, N.J.; Tikhonovich, I.A.; Tsyganov, V.E. Induction of host defences by Rhizobium during ineffective nodulation of pea (Pisum sativum L.) carrying symbiotically defective mutations sym40 (PsEFD), sym33 (PsIPD3/PsCYCLOPS) and sym42. Protoplasma 2015, 252, 1505–1517. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Brewin, N.J.; Tsyganov, V.E. Comparative analysis of remodelling of the plant-microbe interface in Pisum sativum and Medicago truncatula symbiotic nodules. Protoplasma 2019, 256, 983–996. [Google Scholar] [CrossRef]
- Niehaus, K.; Kapp, D.; Pühler, A. Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPSI)-deficient Rhizobium meliloti mutant. Planta 1993, 190, 415–425. [Google Scholar] [CrossRef]
- Perotto, S.; Brewin, N.J.; Kannenberg, E.L. Cytological evidence for a host defense response that reduces cell and tissue invasion in pea nodules by lipopolysaccharide-defective mutants of Rhizobium leguminosarum strain 3841. Mol. Plant Microbe Interact. 1994, 7, 99–112. [Google Scholar] [CrossRef]
- Niehaus, K.; Lagares, A.; Pühler, A. A Sinorhizobium meliloti lipopolysaccharide mutant induces effective nodules on the host plant Medicago sativa (alfalfa) but fails to establish a symbiosis with Medicago truncatula. Mol. Plant Microbe Interact. 1998, 11, 906–914. [Google Scholar] [CrossRef]
- Eleftheriou, E.; Adamakis, I.-D.; Panteris, E.; Fatsiou, M. Chromium-induced ultrastructural changes and oxidative stress in roots of Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 15852–15871. [Google Scholar] [CrossRef]
- Narendrula-Kotha, R.; Theriault, G.; Mehes-Smith, M.; Kalubi, K.; Nkongolo, K. Metal toxicity and resistance in plants and microorganisms in terrestrial ecosystems. Rev. Environ. Contam. Toxicol. 2020, 249, 1–27. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M. Chromium morpho-phytotoxicity. Plants 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Sujkowska-Rybkowska, M.; Kasowska, D.; Gediga, K.; Banasiewicz, J.; Stępkowski, T. Lotus corniculatus-rhizobia symbiosis under Ni, Co and Cr stress on ultramafic soil. Plant Soil 2020, 451, 459–484. [Google Scholar] [CrossRef]
- Chou, M.; Sun, Y.; Yang, J.; Wang, Y.; Li, Y.; Yuan, G.; Zhang, D.; Wang, J.; Wei, G. Comprehensive analysis of phenotype, microstructure and global transcriptional profiling to unravel the effect of excess copper on the symbiosis between nitrogen-fixing bacteria and Medicago lupulina. Sci. Total. Environ. 2019, 656, 1346–1357. [Google Scholar] [CrossRef]
- Colzi, I.; Doumett, S.; Del Bubba, M.; Fornaini, J.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. On the role of the cell wall in the phenomenon of copper tolerance in Silene paradoxa L. Environ. Exp. Bot. 2011, 72, 77–83. [Google Scholar] [CrossRef]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative stress and heavy metals in plants. Rev. Environ. Contam. Toxicol. 2018, 245, 129–156. [Google Scholar] [CrossRef]
- Díaz-Cacho, P.; Moral, R.; Encina, A.; Acebes, J.; Fernandez, J.A. Cell wall modifications in bean (Phaseolus vulgaris) callus cultures tolerant to isoxaben. Physiol. Plant. 1999, 107, 54–59. [Google Scholar] [CrossRef]
- Manfield, I.W.; Orfila, C.; McCartney, L.; Harholt, J.; Bernal, A.J.; Scheller, H.V.; Gilmartin, P.M.; Mikkelsen, J.D.; Paul Knox, J.; Willats, W.G. Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: Global transcript profiling and cellular analysis. Plant J. 2004, 40, 260–275. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.; Dubey, R.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current scenario of Pb toxicity in plants: Unraveling plethora of physiological responses. Rev. Environ. Contam. Toxicol. 2020, 249, 153–197. [Google Scholar] [CrossRef]
- Benhamou, N.; Bélanger, R.R. Induction of systemic resistance to Pythium damping-off in cucumber plants by benzothiadiazole: Ultrastructure and cytochemistry of the host response. Plant J. 1998, 14, 13–21. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Lafuente, A.; Pérez-Palacios, P.; Doukkali, B.; Molina-Sánchez, M.D.; Jiménez-Zurdo, J.I.; Caviedes, M.A.; Rodríguez-Llorente, I.D.; Pajuelo, E. Unraveling the effect of arsenic on the model Medicago-Ensifer interaction: A transcriptomic meta-analysis. New Phytol. 2015, 205, 255–272. [Google Scholar] [CrossRef]
- Raklami, A.; Oufdou, K.; Tahiri, A.-I.; Mateos-Naranjo, E.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Meddich, A.; Redondo-Gómez, S.; Pajuelo, E. Safe cultivation of Medicago sativa in metal-polluted soils from semi-arid regions assisted by heat- and metallo-resistant PGPR. Microorganisms 2019, 7, 212. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Brewin, N.J.; Tsyganov, V.E. Bacterial release is accompanied by ectopic accumulation of cell wall material around the vacuole in nodules of Pisum sativum sym33-3 allele encoding transcription factor PsCYCLOPS/PsIPD3. Protoplasma 2019, 256, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Lodwig, E.M.; Leonard, M.; Marroqui, S.; Wheeler, T.R.; Findlay, K.; Downie, J.A.; Poole, P.S. Role of polyhydroxybutyrate and glycogen as carbon storage compounds in pea and bean bacteroids. Mol. Plant Microbe Interact. 2005, 18, 67–74. [Google Scholar] [CrossRef][Green Version]
- Trainer, M.A.; Charles, T.C. The role of PHB metabolism in the symbiosis of rhizobia with legumes. Appl. Microbiol. Biotechnol. 2006, 71, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Saldanha, M.; Sheng, X.; Shelswell, K.J.; Walsh, K.T.; Sobral, B.W.S.; Charles, T.C. Roles of poly-3-hydroxybutyrate (PHB) and glycogen in symbiosis of Sinorhizobium meliloti with Medicago sp. Microbiology 2007, 153, 388–398. [Google Scholar] [CrossRef][Green Version]
- Ratcliff, W.C.; Kadam, S.V.; Denison, R.F. Poly-3-hydroxybutyrate (PHB) supports survival and reproduction in starving rhizobia. FEMS Microbiol. Ecol. 2008, 65, 391–399. [Google Scholar] [CrossRef]
- Fernandes, P.I.; de Oliveira, P.J.; Rumjanek, N.G.; Xavier, G.R. Poly-β-hydroxybutyrate and exopolysaccharide biosynthesis by bacterial isolates from pigeonpea [Cajanus cajan (L.) Millsp] root nodules. Appl. Biochem. Biotechnol. 2011, 163, 473–484. [Google Scholar] [CrossRef]
- Quelas, J.I.; Mongiardini, E.J.; Pérez-Giménez, J.; Parisi, G.; Lodeiro, A.R. Analysis of two polyhydroxyalkanoate synthases in Bradyrhizobium japonicum USDA 110. J. Bacteriol. 2013, 195, 3145–3155. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tsyganov, V.E. Early nodule senescence is activated in symbiotic mutants of pea (Pisum sativum L) forming ineffective nodules blocked at different nodule developmental stages. Protoplasma 2018, 255, 1443–1459. [Google Scholar] [CrossRef]
- Kong, Z.; Mohamad, O.A.; Deng, Z.; Liu, X.; Glick, B.R.; Wei, G. Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ. Sci. Pollut. Res. Int. 2015, 22, 12479–12489. [Google Scholar] [CrossRef]
- Tsyganova, A.; Ivanova, K.; Tsyganov, V. Histological and ultrastructural nodule organization of the pea (Pisum sativum) mutant SGEFix–-5 in the Sym33 gene encoding the transcription factor PsCYCLOPS/PsIPD3. Ekol. Genet. 2019, 17, 65–70. [Google Scholar] [CrossRef]
- Hou, X.; Lee, L.Y.C.; Xia, K.; Yan, Y.; Yu, H. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 2010, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Borucki, W.; Znojek, E. Structural changes in Medicago truncatula root nodules caused by short-term aluminum stress. Symbiosis 2012, 58, 161–170. [Google Scholar] [CrossRef][Green Version]
- Forrest, S.I.; Verma, D.P.S.; Dhindsa, R.S. Starch content and activities of starch-metabolizing enzymes in effective and ineffective root nodules of soybean. Can. J. Bot. 1991, 69, 697–701. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins inhibit nodule senescence and stimulate nodule meristem bifurcation in pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 285. [Google Scholar] [CrossRef]
- Kosterin, O.E.; Rozov, S.M. Mapping of the new mutation blb and the problem of integrity of linkage group I. Pisum Genet. 1993, 25, 27–31. [Google Scholar]
- Berdnikov, V.A.; Rozov, S.M.; Bogdanova, V.S. Construction of a series of laboratory pea lines. In Special Plant Genetics; Academy of Sciences of the Ukrainian SSR: Kiev, Ukraine, 1989; Volume 1, pp. 26–27. [Google Scholar]
- Engvild, K.C. Nodulation and nitrogen fixation mutants of pea, Pisum sativum. Theor. Appl. Genet. 1987, 74, 711–713. [Google Scholar] [CrossRef]
- Glenn, A.R.; Poole, P.S.; Hudman, J.F. Succinate uptake by free-living and bacteroid forms of Rhizobium leguminosarum. Microbiology 1980, 119, 267–271. [Google Scholar] [CrossRef][Green Version]
- Fåhraeus, G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef]
- Humphrey, C.D.; Pittman, F.E. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974, 49, 9–14. [Google Scholar] [CrossRef]



| Number of Nodules | Shoot Fresh Weight | Root Fresh Weight | TMTD | |
|---|---|---|---|---|
| Number of nodules | 1 | |||
| Shoot fresh weight | 0.807 | 1 | ||
| Root fresh weight | 0.726 | 0.825 | 1 | |
| TMTD | −0.661 | −0.505 | −0.733 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorshkov, A.P.; Tsyganova, A.V.; Vorobiev, M.G.; Tsyganov, V.E. The Fungicide Tetramethylthiuram Disulfide Negatively Affects Plant Cell Walls, Infection Thread Walls, and Symbiosomes in Pea (Pisum sativum L.) Symbiotic Nodules. Plants 2020, 9, 1488. https://doi.org/10.3390/plants9111488
Gorshkov AP, Tsyganova AV, Vorobiev MG, Tsyganov VE. The Fungicide Tetramethylthiuram Disulfide Negatively Affects Plant Cell Walls, Infection Thread Walls, and Symbiosomes in Pea (Pisum sativum L.) Symbiotic Nodules. Plants. 2020; 9(11):1488. https://doi.org/10.3390/plants9111488
Chicago/Turabian StyleGorshkov, Artemii P., Anna V. Tsyganova, Maxim G. Vorobiev, and Viktor E. Tsyganov. 2020. "The Fungicide Tetramethylthiuram Disulfide Negatively Affects Plant Cell Walls, Infection Thread Walls, and Symbiosomes in Pea (Pisum sativum L.) Symbiotic Nodules" Plants 9, no. 11: 1488. https://doi.org/10.3390/plants9111488
APA StyleGorshkov, A. P., Tsyganova, A. V., Vorobiev, M. G., & Tsyganov, V. E. (2020). The Fungicide Tetramethylthiuram Disulfide Negatively Affects Plant Cell Walls, Infection Thread Walls, and Symbiosomes in Pea (Pisum sativum L.) Symbiotic Nodules. Plants, 9(11), 1488. https://doi.org/10.3390/plants9111488








