Source of Oxygen Fed to Adventitious Roots of Syzygium kunstleri (King) Bahadur and R.C. Gaur Grown in Hypoxic Conditions
Abstract
1. Introduction
2. Results
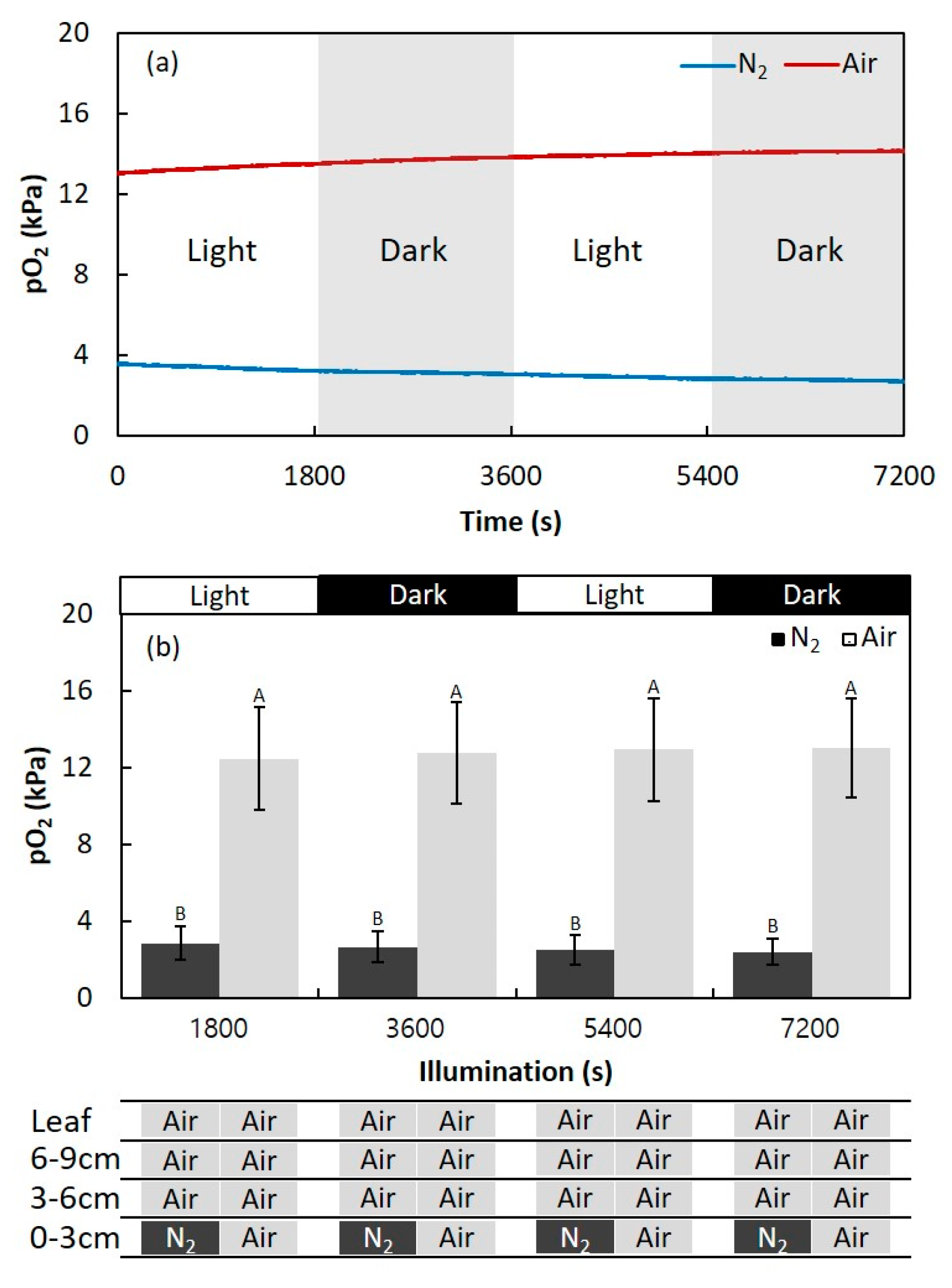
2.1. Effect of Light Changes on the Internal pO2 in Adventitious Roots
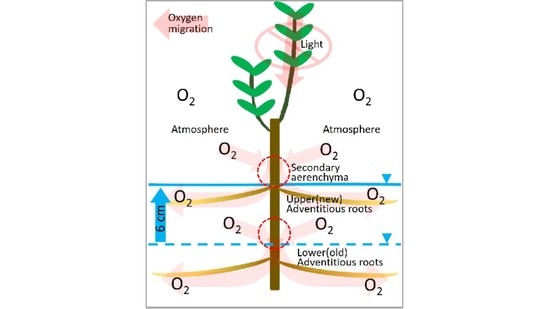
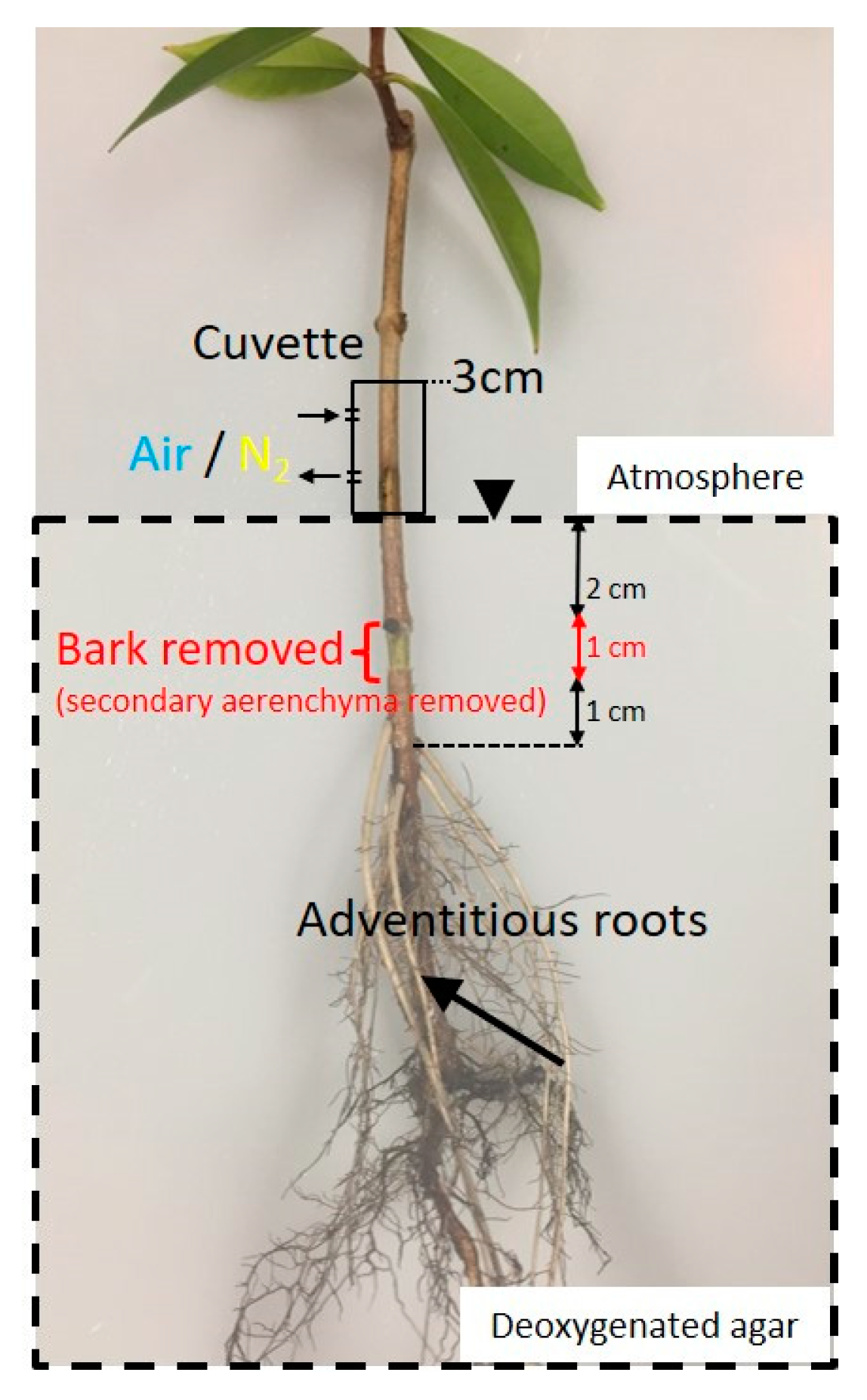
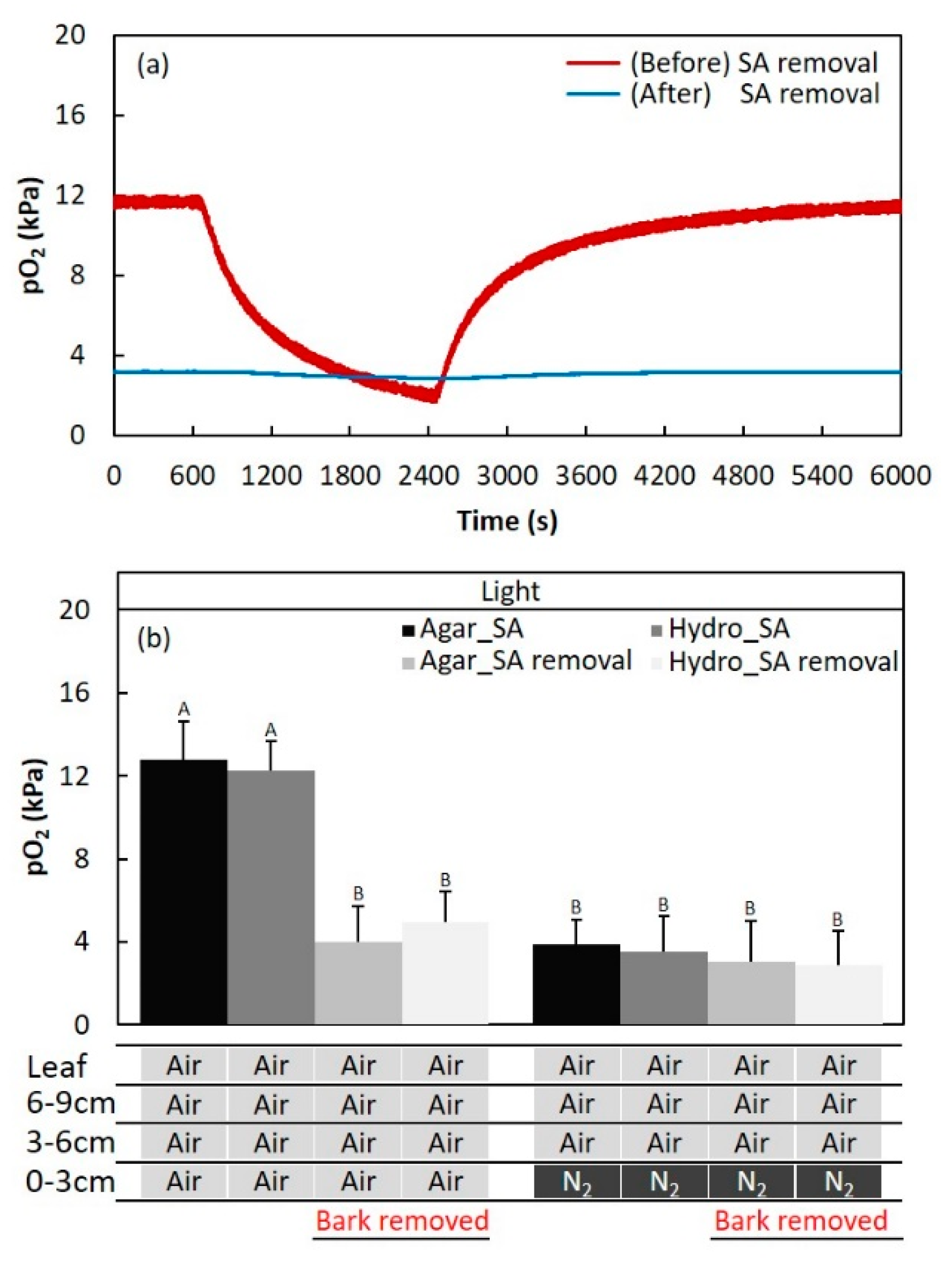
2.2. Secondary Aerenchyma Functioning as Oxygen Pathway in the Stem
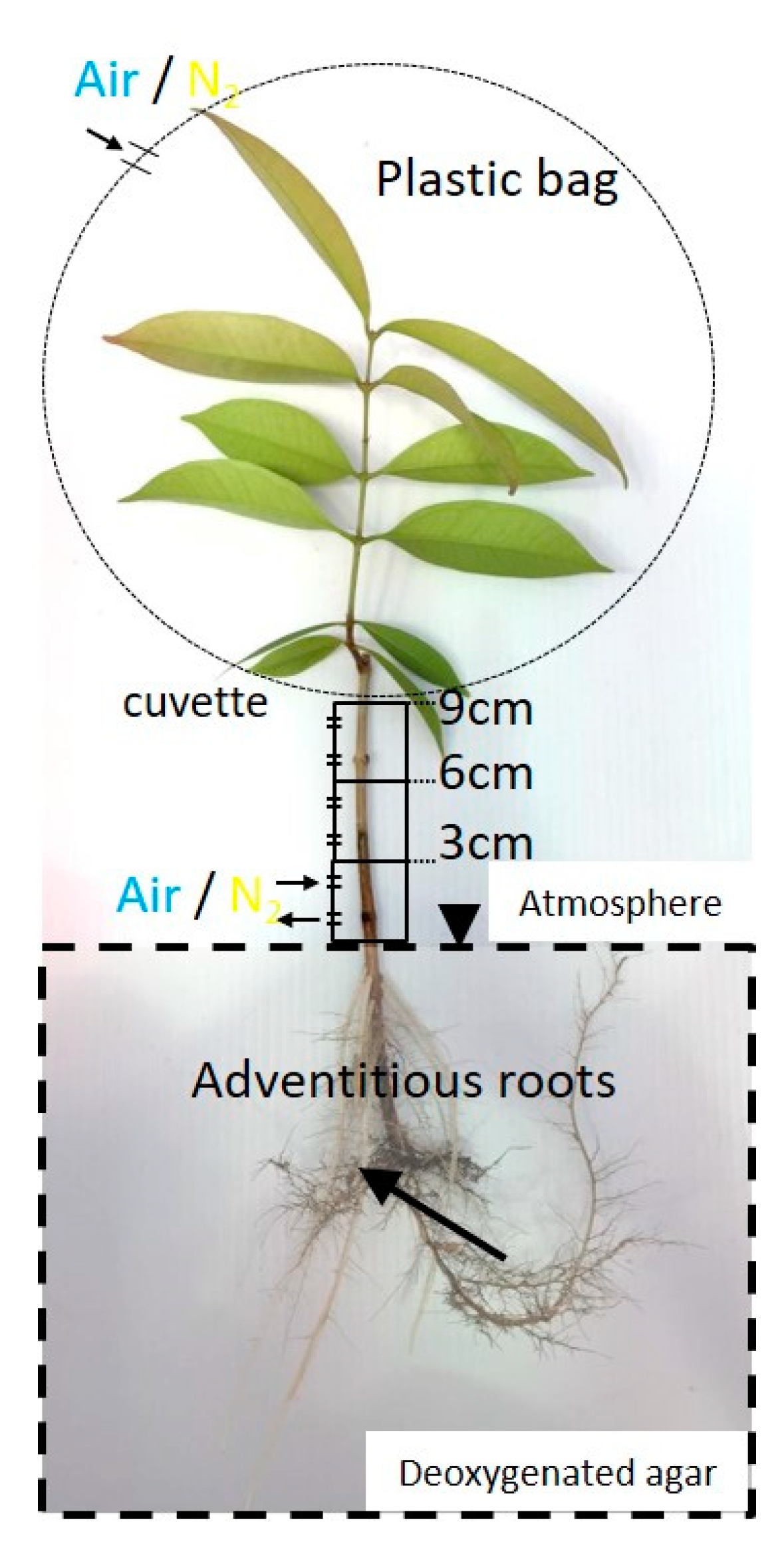
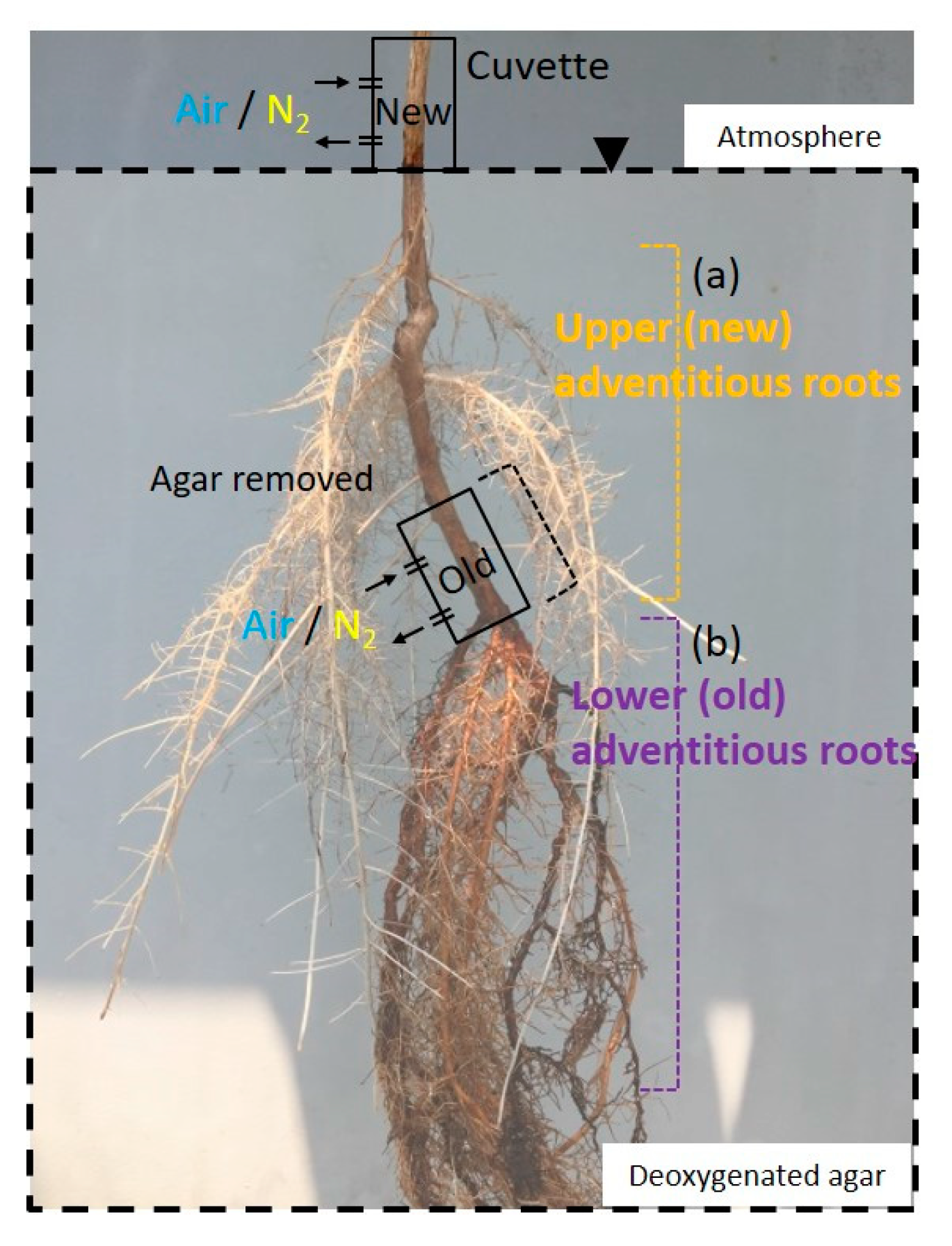
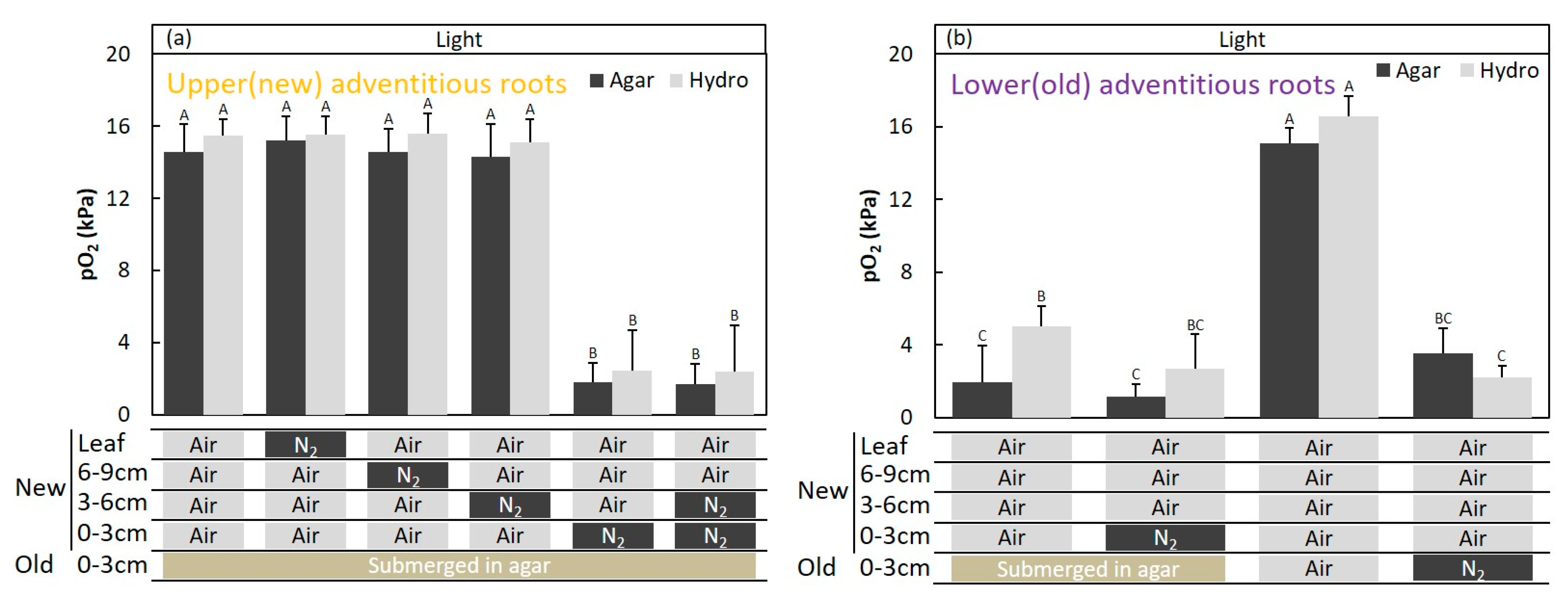
2.3. Entry Portion of Atmospheric Oxygen in the Stem
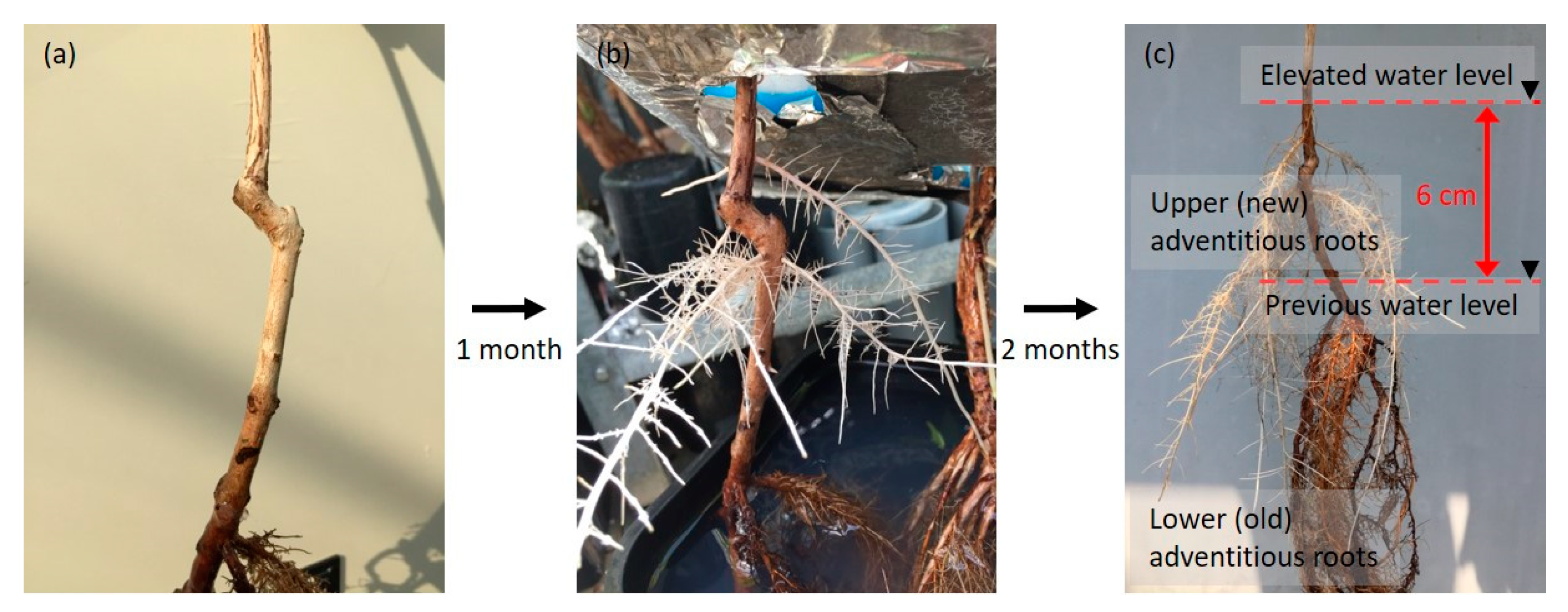
2.4. Entry Portion of Atmospheric Oxygen in the Stem after Water Level Elevation
3. Discussion
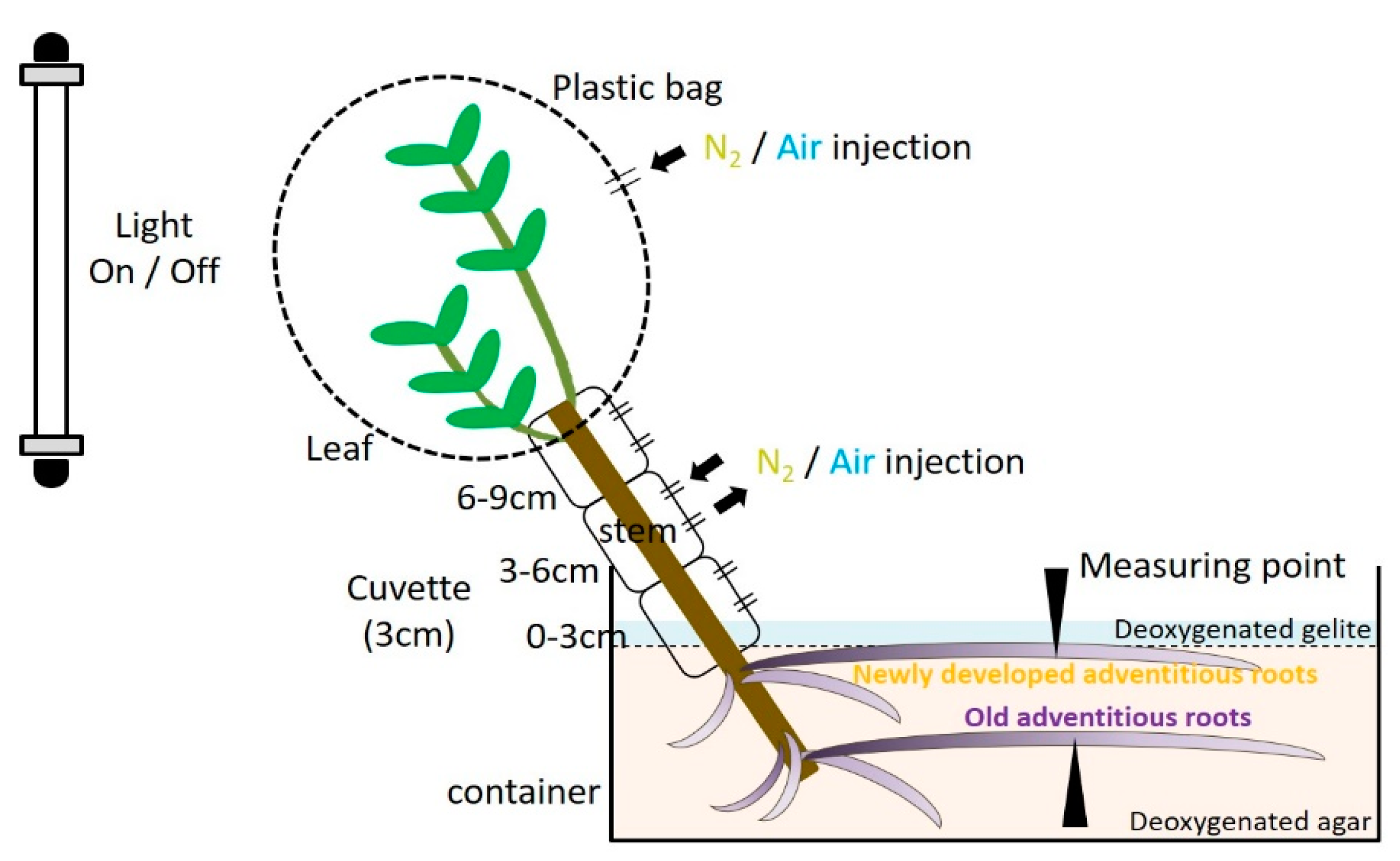
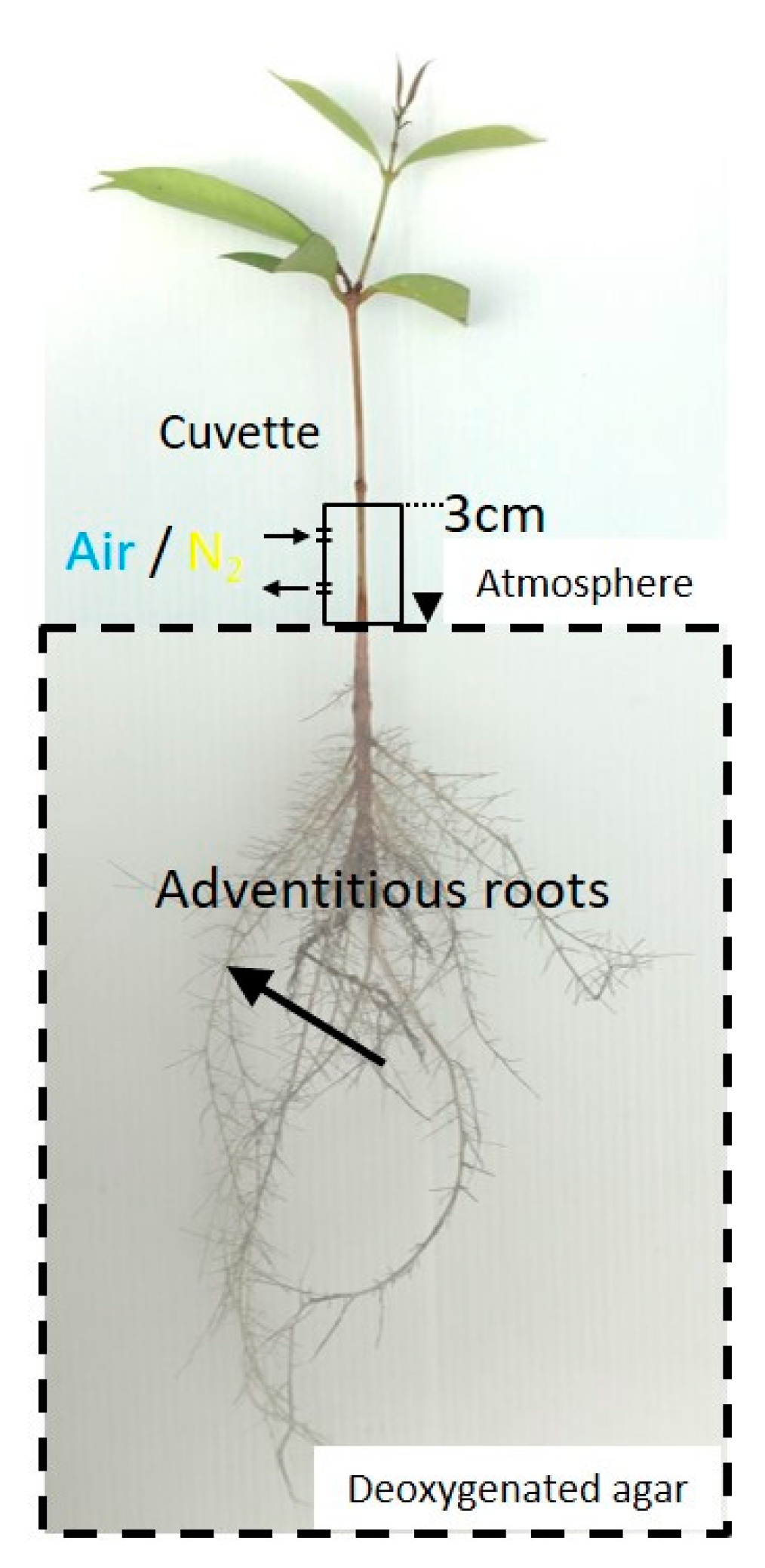
4. Materials and Methods
4.1. Plant Materials
4.2. O2 Microelectrode Measurements
4.3. Effect of Light Conditions on the Root Oxygen Concentration
4.4. Function of Secondary Aerenchyma Developed in the Stem as an Oxygen Pathway
4.5. Entry Portion of Atmospheric Oxygen in the Stem
4.6. Entry Portion of Atmospheric oxygen into the Stem after Water Level Elevation
4.7. Upper (New) Adventitious Roots
4.8. Lower (Old) Adventitious Roots
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vartapetian, B.B.; Jackson, M.B. Plant adaptations to anaerobic stress. Ann. Bot. 1997, 79, 3–20. [Google Scholar] [CrossRef]
- Jackson, M.B.; Armstrong, W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1999, 1, 274–287. [Google Scholar] [CrossRef]
- Colmer, T.D. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann. Bot. 2003, 91, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Mommer, L.; Visser, E.J. Underwater photosynthesis in flooded terrestrial plants: A matter of leaf plasticity. Ann. Bot. 2005, 96, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Pedersen, O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: Gas films improve CO2 and O2 exchange. New Phytol. 2008, 177, 918–926. [Google Scholar] [CrossRef]
- Polko, J.K.; van Zanten, M.; van Rooij, J.A.; Marée, A.F.M.; Voesenek, L.A.C.J.; Peeters, A.J.M.; Pierik, R. Ethylene-induced differential petiole growth in Arabidopsis thaliana involves local microtubule reorientation and cell expansion. New Phytol. 2012, 193, 339–348. [Google Scholar] [CrossRef]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ishizawa, K.; Ito, O. Evolution and mechanisms of plant tolerance to flooding stress. Ann. Bot. 2009, 103, 137–142. [Google Scholar] [CrossRef]
- Pedersen, O.; Vos, H.; Colmer, T.D. Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant Cell Environ. 2006, 29, 1388–1399. [Google Scholar] [CrossRef]
- Colmer, T.D.; Pedersen, O. Oxygen dynamics in submerged rice (Oryza sativa). New Phytol. 2008, 178, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Pedersen, O. Partial versus complete submergence: Snorkelling aids root aeration in Rumex palustris but not in R. acetosa. Plant Cell Environ. 2014, 37, 2381–2390. [Google Scholar] [PubMed]
- Haase, K.; De Simone, O.; Junk, W.J.; Schmidt, W. Internal oxygen transport in cuttings from flood-adapted várzea tree species. Tree Physiol. 2003, 23, 1069–1076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Armstrong, J. Stem photosynthesis not pressurized ventilation is responsible for light-enhanced oxygen supply to submerged roots of Alder (Alnus glutinosa). Ann. Bot. 2005, 96, 591–612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimamura, S.; Mochizuki, T.; Nada, Y.; Fukuyama, M. Secondary aerenchyma formation and its relation to nitrogen fixation in root nodules of soybean plants (Glycine max) grown under flooded conditions. Plant Prod. Sci. 2002, 5, 294–300. [Google Scholar] [CrossRef]
- Shimamura, S.; Yamamoto, R.; Nakamura, T.; Shimada, S.; Komatsu, S. Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Ann. Bot. 2010, 106, 277–284. [Google Scholar] [CrossRef]
- Thomas, A.L.; Guerreiro, S.M.C.; Sodek, L. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann. Bot. 2005, 96, 1191–1198. [Google Scholar] [CrossRef]
- Sou, H.D.; Masumori, M.; Kurokochi, H.; Tange, T. Histological observation of primary and secondary aerenchyma formation in adventitious roots of Syzygium kunstleri (King) Bahadur and R.C. Gaur Grown in hypoxic medium. Forests 2019, 10, 137. [Google Scholar] [CrossRef]
- Stevens, K.J.; Peterson, R.L.; Reader, R.J. The aerenchymatous phellem of Lythrum salicaria (L.): A pathway for gas transport and its role in flood tolerance. Ann. Bot. 2002, 89, 621–625. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Liu, J.; Li, S.; van Bodegom, P.M.; Cornelissen, J.H.C. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Masumori, M.; Yamanoshita, T.; Tange, T. Morphological and anatomical changes of Melaleuca cajuputi under submergence. Trees 2011, 25, 695–704. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sou, H.-D.; Masumori, M.; Ezaki, G.; Tange, T. Source of Oxygen Fed to Adventitious Roots of Syzygium kunstleri (King) Bahadur and R.C. Gaur Grown in Hypoxic Conditions. Plants 2020, 9, 1433. https://doi.org/10.3390/plants9111433
Sou H-D, Masumori M, Ezaki G, Tange T. Source of Oxygen Fed to Adventitious Roots of Syzygium kunstleri (King) Bahadur and R.C. Gaur Grown in Hypoxic Conditions. Plants. 2020; 9(11):1433. https://doi.org/10.3390/plants9111433
Chicago/Turabian StyleSou, Hong-Duck, Masaya Masumori, Goro Ezaki, and Takeshi Tange. 2020. "Source of Oxygen Fed to Adventitious Roots of Syzygium kunstleri (King) Bahadur and R.C. Gaur Grown in Hypoxic Conditions" Plants 9, no. 11: 1433. https://doi.org/10.3390/plants9111433
APA StyleSou, H.-D., Masumori, M., Ezaki, G., & Tange, T. (2020). Source of Oxygen Fed to Adventitious Roots of Syzygium kunstleri (King) Bahadur and R.C. Gaur Grown in Hypoxic Conditions. Plants, 9(11), 1433. https://doi.org/10.3390/plants9111433