Early Season Symptoms on Stem, Inflorescences and Flowers of Grapevine Associated with Botryosphaeriaceae Species
Abstract
1. Introduction
2. Results
2.1. Sampling and Fungal Isolation
2.2. Morphological Characterization and DNA Analysis for Fungal Identification
2.3. Pathogenicity Tests
2.3.1. Pathogenicity Tests on Tendrils and Leaves
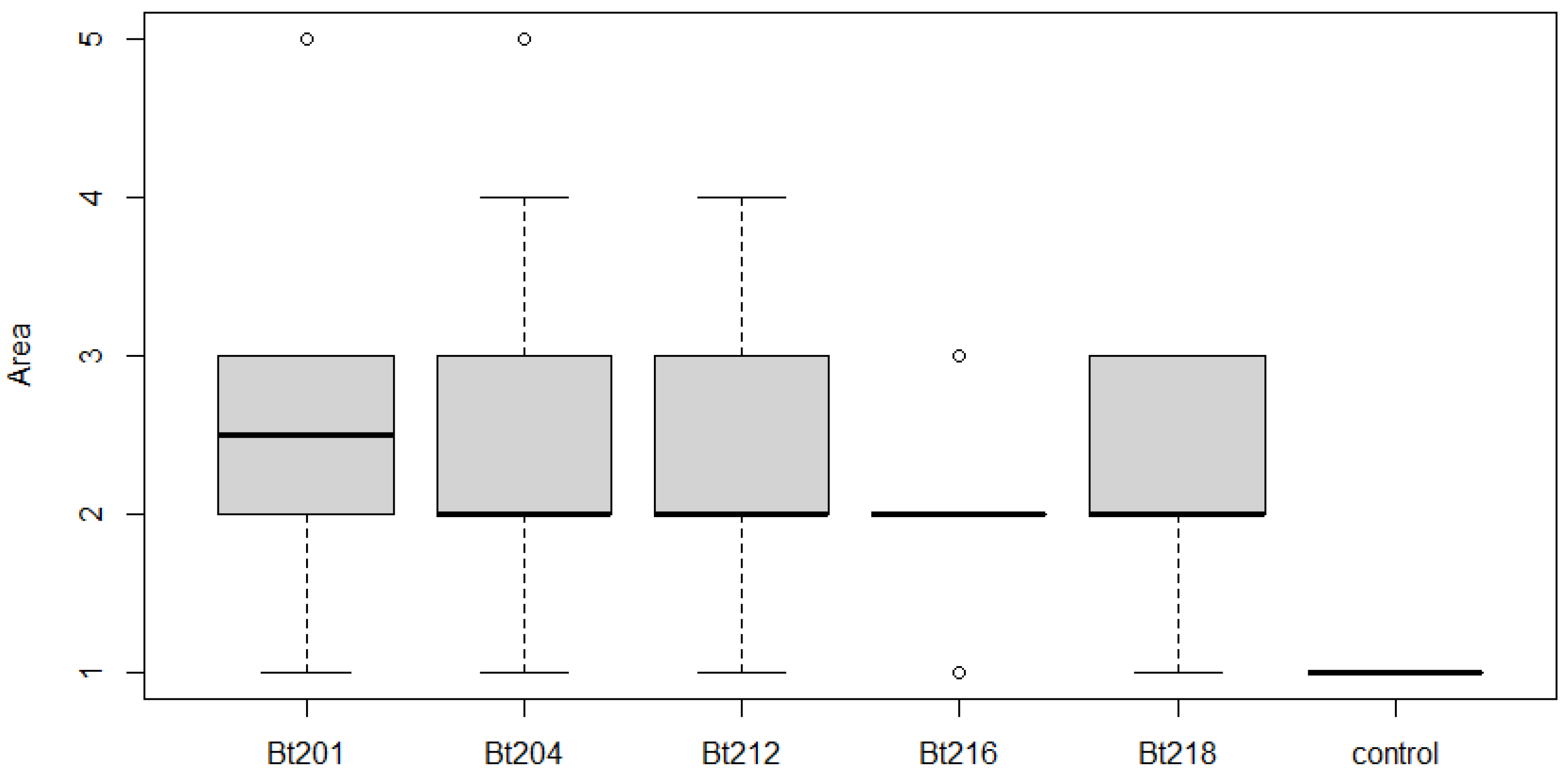
2.3.2. Pathogenicity Tests on Green Stems
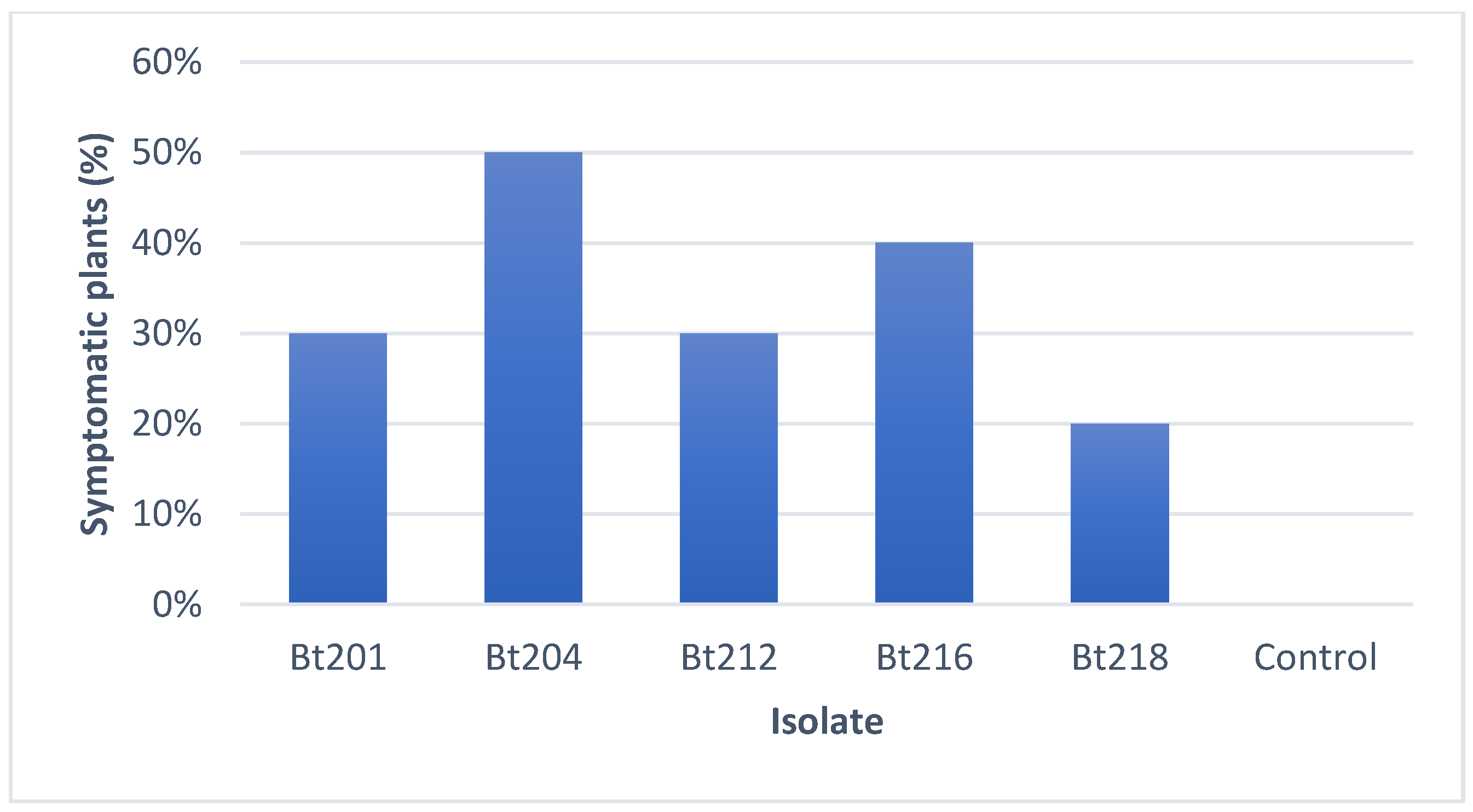
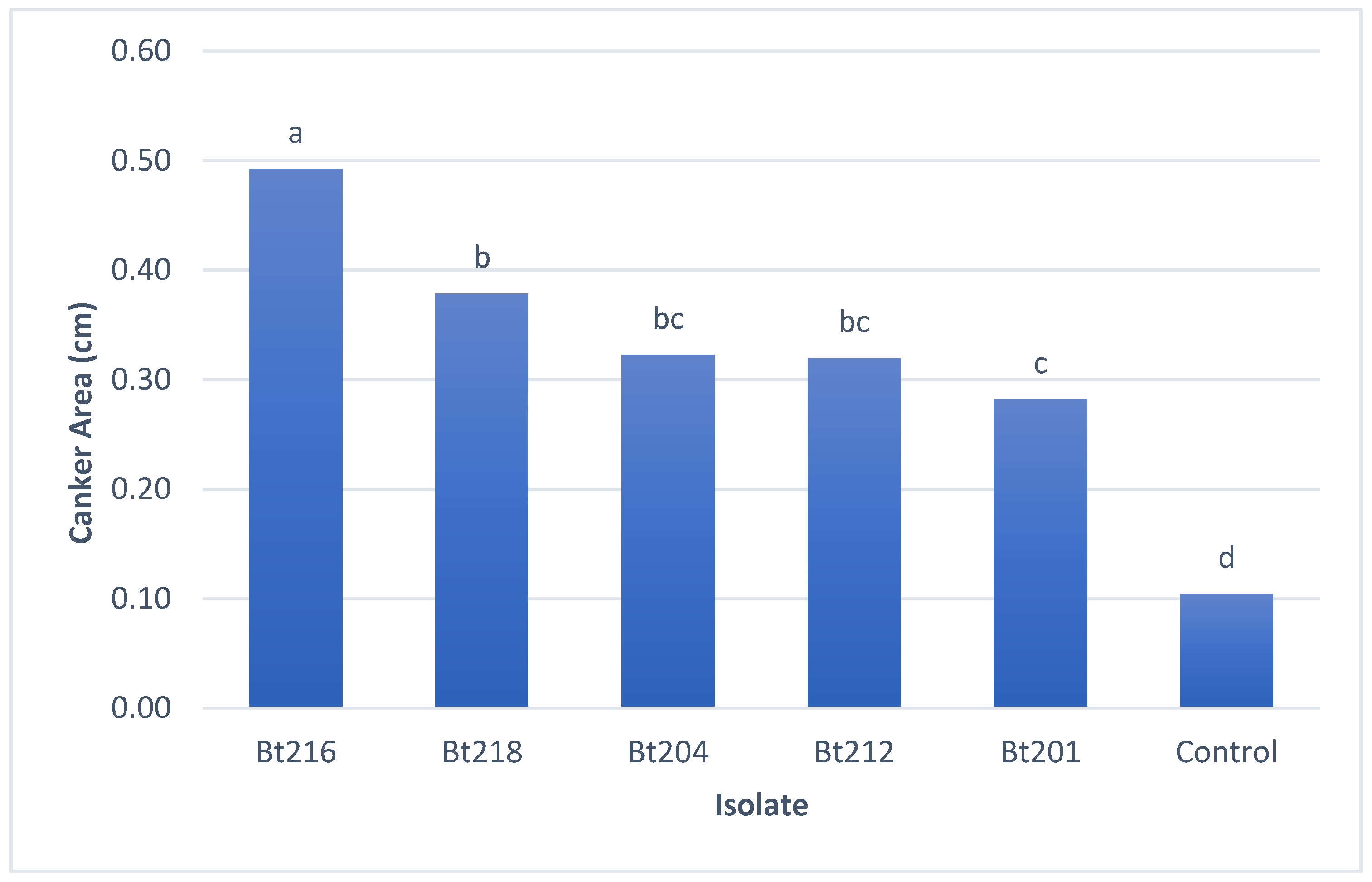
2.3.3. Field Pathogenicity Tests on Clusters
3. Discussion
4. Materials and Methods
4.1. Sampling and Fungal Isolation
4.2. Morphological Characterization and DNA Analysis for Fungal Identification
4.2.1. Morphological Characterization
4.2.2. DNA Analysis
4.3. Pathogenicity Tests
4.3.1. Pathogenicity tests on Tendrils and Leaves
4.3.2. Pathogenicity Tests on Green Stems
4.3.3. Field Pathogenicity Tests on Clusters
Author Contributions
Funding
Conflicts of Interest
References
- Rubio, J.J.; Garzón, E. Las enfermedades de madera de vid como amenaza del sector vitícola. Rev. Winetech 2011, 11, 18–21. [Google Scholar]
- De la Fuente, M.; Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Rego, C.; Corio-Costet, M. Grapevine Trunk Diseases. A Review, 1st ed.; OIV Publications: Paris, France, 2016. [Google Scholar]
- Ridgway, H.J.; Baskarathevan, J.; Amponsah, N.; Jaspers, M.V.; Jones, E.E. The identity, distribution and diversity of botryosphaeriaceous species in New Zealand vineyards—A national perspective. Phytopathol. Mediterr. 2014, 53, 565. [Google Scholar]
- Bertsch, C.; Ramirez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Trunk diseases of grapevine: Complex and still poorly understood syndromes described as eutypa dieback, esca and black dead arm. Plant Pathol. 2012, 62, 243–265. [Google Scholar] [CrossRef]
- Bruez, E.; Lecomte, P.; Grosman, J.; Doublet, B.; Bertsch, C.; Fontaine, F.; Da Costa, J.P.; Ugaglia, A.; Teissedre, P.L.; Guerin-Dubrana, L.; et al. Overview of grapevine trunk diseases in France in the early 2000s. Phytopathol. Mediterr. 2013, 52, 262–275. [Google Scholar]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Smith, H.; Wingfield, M.J.; Crous, P.W.; Coutinho, T.A. Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. in South Africa. S. Afr. J. Bot. 1996, 62, 86–88. [Google Scholar] [CrossRef]
- Smith, H.; Wingfield, M.J.; Petrini, O. Botryosphaeria dothidea endophytic in Eucalyptus grandis and Eucalyptus nitens in South Africa. Forest. Ecol. Manag. 1996, 89, 189–195. [Google Scholar] [CrossRef]
- Van Niekerk, J.M.; Fourie, P.H.; Halleen, F.; Crous, P.W. Botryosphaeria spp. as grapevine trunk disease pathogens. Phytopathol. Mediterr. 2006, 45, S43–S54. [Google Scholar]
- Bellé, A.; Comont, G.; Nivault, A.; Abou-Mansour, E.; Coppin, C.; Dufour, M.C.; Corio-Costet, M.F. Life traits of four Botryosphaeriaceae species and molecular responses of different grapevine cultivars or hybrids. Plant Pathol. 2017, 66, 763–776. [Google Scholar] [CrossRef]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowsky, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef]
- Pitt, W.M.; Huang, R.; Steel, C.C.; Savocchia, S. Pathogenicity and epidemiology of Botryosphaeriaceae species isolated from grapevines in Australia. Australas. Plant Pathol. 2013, 42, 573–582. [Google Scholar] [CrossRef]
- Pitt, D.G.; Lanteigne, L.; Hoepting, M.K.; Plamondon, J. Effects of precommercial thinning on the forest value chain in north-western New Brunswick: Part 1—Roundwood production and stumpage value. For. Chron. 2013, 89, 446–457. [Google Scholar] [CrossRef]
- Pitt, W.M.; Úrbez-Torres, J.R.; Trouillas, F.P. Dothiorella vidmadera, a novel species from grapevines in Australia and notes on Spencermartinsia. Fungal Divers. 2015, 61, 209–219. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Akgül, D.S.; Perez, R.; Eskalen, A.; Gispert, C. First report of wood canker caused by Neoscytalidium dimidiatum on grapevine in California. Plant Dis. 2013, 97, 1511. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar]
- Yang, T.; Groenewald, J.Z.; Cheewangkoon, R.; Jami, F.; Abdollahzadeh, J.; Lombard, L.; Crous, P.W. Families, genera and species of Botryosphaeriales. Fungal Biol. 2017, 121, 322–346. [Google Scholar] [CrossRef]
- Cristinzo, G. Gravil attachi di Botryosphaeria obtusa su vite provincial di Insernia. Inf. Fitopatol. 1978, 6, 21–23. [Google Scholar]
- Castillo-Pando, M.; Sommers, A.; Green, C.D.; Priest, M.; Sriskanthades, M. Fungi associated with dieback of Semillon grapevines in the Hunter Valley of New South Wales. Australas. Plant Pathol. 2001, 30, 59–63. [Google Scholar] [CrossRef]
- Larignon, P.; Fulchic, R.; Laurent, C.; Dubos, B. Observation of black dead arm in French vineyards. Phytopathol. Mediterr. 2001, 40, S336–S342. [Google Scholar]
- Phillips, A.J.L.; Crous, P.W.; Alves, A. Diplodia seriata, the anamorph of “Botryosphaeria” obtusa. Fungal Divers. 2007, 25, 141–155. [Google Scholar]
- Rovesti, L.; Montermini, A. Un deprimento della vitte causato de Sphaeropsis malorum in provincia di Reggio Emilia. Inf. Fitopatol. 1987, 1, 59–61. [Google Scholar]
- Savocchia, S.; Steel, C.C.; Stodart, B.J.; Somers, A. Pathogenicity of Botryosphaeria species from declining grapevines in sub-tropical of Eastern Australia. Vitis 2007, 46, 27–32. [Google Scholar]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Guerrero, J.C.; Guevara, J.; Gubler, W.D. Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of grapevines in Mexico. Plant Dis. 2008, 92, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Lehoczky, J. Black dead arm disease of grapevine caused by Botryosphaeria stevensii infection. Acta Phytopathol. Acad. Sci. Hung. 1974, 9, 319–327. [Google Scholar]
- Taylor, A.; Hardy, G.E.; St, J.; Wood, P.; Burgess, T. Identification and pathogenicity of Botryosphaeria species associated with grapevine decline in Western Australia. Australas. Plant Pathol. 2005, 34, 187–195. [Google Scholar] [CrossRef]
- Crous, P.W.; Slippers, M.J.; Rheeder, J.; Marasas, W.F.O.; Phillips, A.J.L.; Alves, A.; Burguess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.L. Botryosphaeria species associated with diseases of grapevines in Portugal. Phytopathol. Mediterr. 2002, 41, 3–18. [Google Scholar]
- Luque, J.; Martos, S.; Aroca, A.; Raposo, R.; Garcia-Figueres, F. Symptoms and fungi associated with mature grapevine plants in Northeast Spain. J. Plant Pathol. 2009, 91, 381–390. [Google Scholar]
- Rodríguez-Gálvez, E.; Maldonado, E.; Alves, A. Identification and pathogenicity of Lasiodiplodia theobromae causing dieback of table grapes in Peru. Eur. J. Plant Pathol. 2015, 141, 477–489. [Google Scholar] [CrossRef]
- Van Niekerk, J.M.; Crous, P.W.; Groenewald, J.Z.; Fourie, P.H.; Halleen, F. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 2004, 96, 781–798. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B. The villainy of Black Dead Arm. Wines Vines 2001, 82, 86–89. [Google Scholar]
- Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Francheschini, A.; Serra, S.; Berraf-Tebbal, A.; Zouaoui Boutiti, M.; Ben Jamâa, M.L.; Phillips, A.J.L. Diversity of Botryosphaeriaceae species associated with grapevine and other woody hosts in Italy, Algeria and Tunisia, with descriptions of Lasiodiplodia exigua and Lasiodiplodia mediterranea sp. Nov. Fungal Divers. 2015, 71, 201–214. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Voegel, T.; Gubler, W.D. Identification and distribution of Botryosphaeria species associated with grapevine cankers in California. Plant Dis. 2006, 90, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Magnin-Robert, M.; Nascimento, T.; Spagnolo, A.; Abou-Mansour, E.; Fioretti, C.; Clément, C.; Rego, C.; Fontaine, F. Reproducing Botryosphaeria Dieback Foliar Symptoms in a Simple Model System. Plant Dis. 2016, 100, 1071–1079. [Google Scholar] [CrossRef]
- Reis, P.; Pierron, P.; Larignon, L.; Lecomte, P.; Abou-Mansour, E.; Farine, S.; Bertsch, C.; Jaques, A.; Trotel-Aziz, P.; Fontaine, F. Vitis methods to understand and develop strategies for diagnosis and sustainable control of grapevine trunk diseases. Phytopathology 2019, 109, 916–931. [Google Scholar] [CrossRef]
- Spagnolo, A.; Larignon, P.; Magnin-Robert, M.; Hovasse, A.; Cilindre, C.; Van Dorsselaer, A.; Clément, C.; Schaffer-Reiss, C.; Fontaine, F. Flowering as the most highly sensitive period of grapevine (Vitis vinifera cv. Mourvèdre) to the botryosphaeria dieback agents of Neofusicoccum parvum and Diplodia seriata infection. Int. J. Mol. Sci. 2014, 15, 9644–9669. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenwald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Alves, A.; Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Phillips, A.J.L. The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Divers. 2014, 67, 143–156. [Google Scholar] [CrossRef]
- Auger, J.; Esterio, M.; Ricke, G.; Pérez, I. Black dead arm and basal canker of Vitis vinifera cv. Red Globe caused by Botryosphaeria obtusa in Chile. Plant Dis. 2004, 88, 1286. [Google Scholar] [CrossRef]
- Carlucci, A.; Cibelli, F.; Lops, F.; Raimondo, M.L. Characterization of Botryosphaeriaceae species as casual agents of trunk diseases on grapevines. Plant Dis. 2015, 99, 1678–1688. [Google Scholar] [CrossRef]
- Von Arx, J.A.; Muller, E. Die Gattungen der amerosporen Pyrenomyceten. Beriage Kryptogamenflora Schweiz 1954, 11, 1–434. [Google Scholar]
- Smith, H.; Kemp, G.H.J.; Wingfield, M.J. Canker and die-back of Eucalyptus in South Africa caused by Botryosphaeria dothidea. Plant Pathol. 1994, 43, 1031–1034. [Google Scholar] [CrossRef]
- Kim, K.W.; Park, E.W.; Ahn, K.-K. Pre-penetration behaviour of Botryosphaeria dothidea on apple fruits. Plant Pathol. J. 1999, 15, 223–227. [Google Scholar]
- Michailides, K. Pathogenicity, distribution, sources of inoculum, and infection courts of Botryosphaeria dothidea on pistachio. Phytopathology 1991, 81, 566–573. [Google Scholar] [CrossRef]
- Smith, R.J.; Verdegaal, P.; Gubler, W.D. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 2010, 94, 717–724. [Google Scholar]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Shafi, A.; Ridgway, H.; Jaspers, M.; Jones, E. Saprophytic colonization of the bark by Neofusicoccum species mediates subsequent infection of grapevines through wounds. Phytopathol. Mediterr. 2019, 58, 395–449. [Google Scholar]
- Elena, G.; Luque, J. Pruning debris of grapevine as a potential inoculum source of Diplodia seriata, causal agent of Botryospaheria dieback. Eur. J. Plant Pathol. 2016, 144, 803–810. [Google Scholar] [CrossRef]
- Hewitt, W.B. Diplodia cane dieback and bunch rot. In Compendium of Grape Diseases; Pearson, R.C., Goheen, A.C., Eds.; APS Press: St. Paul, MN, USA, 1988; pp. 25–26. [Google Scholar]
- Van Niekerk, J.M.; Calitz, F.J.; Halleen, F.; Fourie, P.H. Temporal spore dispersal patterns of grapevine trunk pathogens in South Africa. Eur. J. Plant Pathol. 2010, 127, 375–390. [Google Scholar] [CrossRef]
- Kuntzmann, P.; Villaume, S.; Bertsch, C. Conidial dispersal on Diplodia species in a French vineyard. Phytopathol. Mediterr. 2009, 48, 150–154. [Google Scholar]
- Spagnolo, A.; Mondello, V.; Larignon, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Fontaine, F. Defense responses in grapevine (cv. Mourvèdre) after inoculation with the Botryosphaeria dieback pathogens Neofusicoccum parvum and Diplodia seriata and their relationship with flowering. Int. J. Mol. Sci. 2017, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Peighami Ashnaei, S. Grapevine, esca complex, and environment: The disease triangle. Phytopathol. Mediterr. 2019, 58, 17–37. [Google Scholar]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef]
- Dry, P.; Coombe, B. Grapevine growth stages—The modified E-L system. In Viticulture 1—Resources, 2nd ed.; Winetitles: Broadview, Australia, 2004. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplified and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- OEPP/EPPO. EPPO Standards PP1, Efficacy Evaluation of Plant Protection Products, Vol. 2, Fungicides & Bactericides; OEPP/EPPO: Paris, France, 2004; pp. 22–24. [Google Scholar]


| Host | Origin | GenBank Accession Number | Percent Identity * | |||||
|---|---|---|---|---|---|---|---|---|
| Isolate Number | (V. vinifera cvs.) | Isolation Region | Species | ITS | Tef1-α | ITS | Tef1-α | |
| Bt201 | Seara Nova | Apex of the shoot | Vineyard 1 | Diplodia seriata | MT786219 | MW018672 | 100% | 99% |
| Bt202 | Alicante | Base of the shoot | Vineyard 2 | Diplodia seriata | MT786220 | MW018673 | 99% | 99% |
| Bt203 | Castelão | Rachis | Vineyard 3 | Diplodia seriata | MT786221 | MW018674 | 99% | 100% |
| Bt204 | Syrah | Base of the shoot | Vineyard 4 | Diplodia seriata | MT786222 | MW018675 | 100% | 99% |
| Bt205 | Castelão | Cluster | Vineyard 5 | Neofusicoccum parvum | MT786223 | MW018676 | 100% | 99% |
| Bt206 | Castelão | Leaf stem | Vineyard 5 | Diplodia seriata | MT786224 | MW018677 | 98% | 99% |
| Bt207 | Castelão | Base of the shoot | Vineyard 6 | Diplodia seriata | MT786225 | MW018678 | 100% | 100% |
| Bt208 | Aragonez | Apex of the shoot | Vineyard 7 | Diplodia seriata | MT786226 | MW018679 | 100% | 99% |
| Bt209 | Aragonez | Rachis | Vineyard 8 | Diplodia seriata | MT786227 | MW018680 | 100% | 100% |
| Bt210 | Castelão | Apex of the shoot | Vineyard 5 | Diplodia seriata | MT786228 | MW018681 | 99% | 99% |
| Bt211 | Arinto | Base of the shoot | Vineyard 9 | Neofusicoccum parvum | MT786229 | MW018682 | 99% | 99% |
| Bt212 | Castelão | Base of the shoot | Vineyard 10 | Diplodia seriata | MT786230 | MW018683 | 100% | 100% |
| Bt213 | Seara Nova | Apex of the shoot | Vineyard 1 | Diplodia seriata | MT786231 | MW018684 | 100% | 99% |
| Bt214 | Castelão | Apex of the shoot | Vineyard 11 | Diplodia seriata | MT786232 | MW018685 | 100% | 100% |
| Bt215 | Seara Nova | Rachis | Vineyard 12 | Diplodia seriata | MT786233 | MW018686 | 98% | 100% |
| Bt216 | Alicante | Apex of the shoot | Vineyard 13 | Neofusicoccum parvum | MT786234 | MW018687 | 100% | 98% |
| Bt217 | Aragonez | Apex of the shoot | Vineyard 14 | Neofusicoccum parvum | MT786235 | MW018688 | 100% | 98% |
| Bt218 | Aragonez | Base of the shoot | Vineyard 15 | Diplodia mutila | MT786236 | MW018689 | 100% | 100% |
| Bt219 | Alicante | Cluster | Vineyard 2 | Neofusicoccum parvum | MT786237 | MW018690 | 99% | 100% |
| Bt220 | Seara Nova | Base of the shoot | Vineyard 1 | Diplodia seriata | MT786238 | MW018691 | 99% | 99% |
| Bt221 | Seara Nova | Apex of the shoot | Vineyard 1 | Diplodia seriata | MT786239 | MW018692 | 100% | 99% |
| Bt222 | Castelão | Apex of the shoot | Vineyard 16 | Diplodia seriata | MT786240 | MW018693 | 100% | 100% |
| Bt223 | Alicante | Rachis | Vineyard 17 | Diplodia seriata | MT786241 | MW018694 | 99% | 99% |
| Species/Isolate | Conidial dimensions | |
|---|---|---|
| Length (μm) | Width (μm) | |
| D. seriata | ||
| Bt201 | (21.27–) 25.22 ± 1.89 (–28.69) | (8.33–) 9.91 ± 0.88 (–11.75) |
| Bt202 | * | |
| Bt203 | (20.75–) 22.50 ± 1.12 (–24.23) | (6.71–) 8.17 ± 0.57 (–9.16) |
| Bt204 | (20.43–) 22.65 ± 1.56 (–26.20) | (7.83–) 9.06 ± 0.76 (–10.93) |
| Bt206 | (20.99–) 23.84 ± 1.52 (–26.36) | (7.71–) 9.23 ± 0.95 (–10.77) |
| Bt207 | (19.61–) 23.58 ± 1.95 (–27.30) | (7.49–) 9.29 ± 0.81 (–11.26) |
| Bt208 | (21.72–) 25.26 ± 1.41 (–28.73) | (9.53–) 11.13 ± 1.01 (–13.37) |
| Bt209 | (19.61–) 22.17 ± 1.52 (24.53) | (7.20–) 9.07 ± 0.78 (–10.55) |
| Bt210 | ||
| Bt212 | (21.27–) 23.91 ± 1.36 (–26.22) | (7.34–) 8.34 ± 0.60 (–9.36) |
| Bt213 | (23.28–) 25.99 ± 1.58 (–29.18) | (7.73–) 9.03 ± 0.56 (–9.84) |
| Bt214 | ||
| Bt215 | (21.02–) 24.58 ± 1.47 (–26.99) | (10.14–) 11.21 ± 0.66 (–12.83) |
| Bt220 | ||
| Bt221 | (20.80–) 23.28 ± 1.35 (–25,53) | (8.59–) 9.99 ± 0.60 (–11.10) |
| Bt222 | ||
| Bt223 | (21.42–) 22.94 ± 1.34 (–26.37) | (8.97–) 9.72 ± 0.66 (–11.40) |
| N. parvum | ||
| Bt205 | (15.51–) 17.18 ± 1.02 (–19.92) | (4.56–) 5.22 ± 0.43 (–6.11) |
| Bt211 | (15,.2–) 18.28 ± 1.75 (–22,.4) | (4.73–) 5.33 ± 0.31 (–5.94) |
| Bt216 | (11.39–) 13.71 ± 1.28 (–16.13) | (4.20–) 5.02 ± 0.45 (–6.02) |
| Bt217 | ||
| Bt219 | ||
| D. mutila | (20.80–) 23.50 ± 1.41 (–25.05) | (9.47–) 11.15 ± 1.57 (–16.25) |
| Bt218 | (21.27–) 25.22 ± 1.89 (–28.69) | (8.33–) 9.91 ± 0.88 (–11.75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, P.; Gaspar, A.; Alves, A.; Fontaine, F.; Lourenço, I.; Saramago, J.; Mota, M.; Rego, C. Early Season Symptoms on Stem, Inflorescences and Flowers of Grapevine Associated with Botryosphaeriaceae Species. Plants 2020, 9, 1427. https://doi.org/10.3390/plants9111427
Reis P, Gaspar A, Alves A, Fontaine F, Lourenço I, Saramago J, Mota M, Rego C. Early Season Symptoms on Stem, Inflorescences and Flowers of Grapevine Associated with Botryosphaeriaceae Species. Plants. 2020; 9(11):1427. https://doi.org/10.3390/plants9111427
Chicago/Turabian StyleReis, Pedro, Ana Gaspar, Artur Alves, Florence Fontaine, Inês Lourenço, José Saramago, Mariana Mota, and Cecília Rego. 2020. "Early Season Symptoms on Stem, Inflorescences and Flowers of Grapevine Associated with Botryosphaeriaceae Species" Plants 9, no. 11: 1427. https://doi.org/10.3390/plants9111427
APA StyleReis, P., Gaspar, A., Alves, A., Fontaine, F., Lourenço, I., Saramago, J., Mota, M., & Rego, C. (2020). Early Season Symptoms on Stem, Inflorescences and Flowers of Grapevine Associated with Botryosphaeriaceae Species. Plants, 9(11), 1427. https://doi.org/10.3390/plants9111427









