Production of Phytotoxic Metabolites by Botryosphaeriaceae in Naturally Infected and Artificially Inoculated Grapevines
Abstract
1. Introduction
2. Results
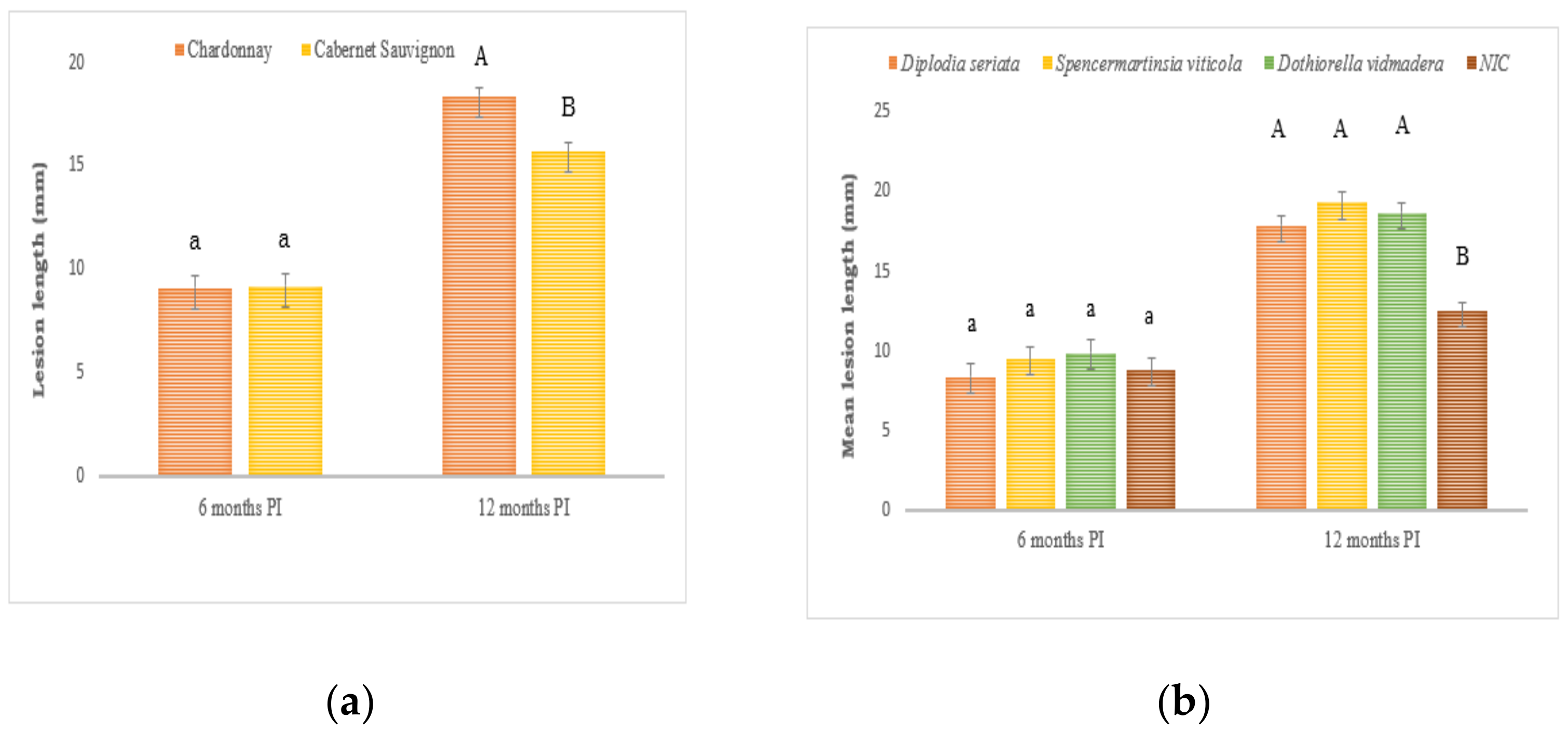
2.1. Artificially Inoculated Vines
2.1.1. Wood Symptoms
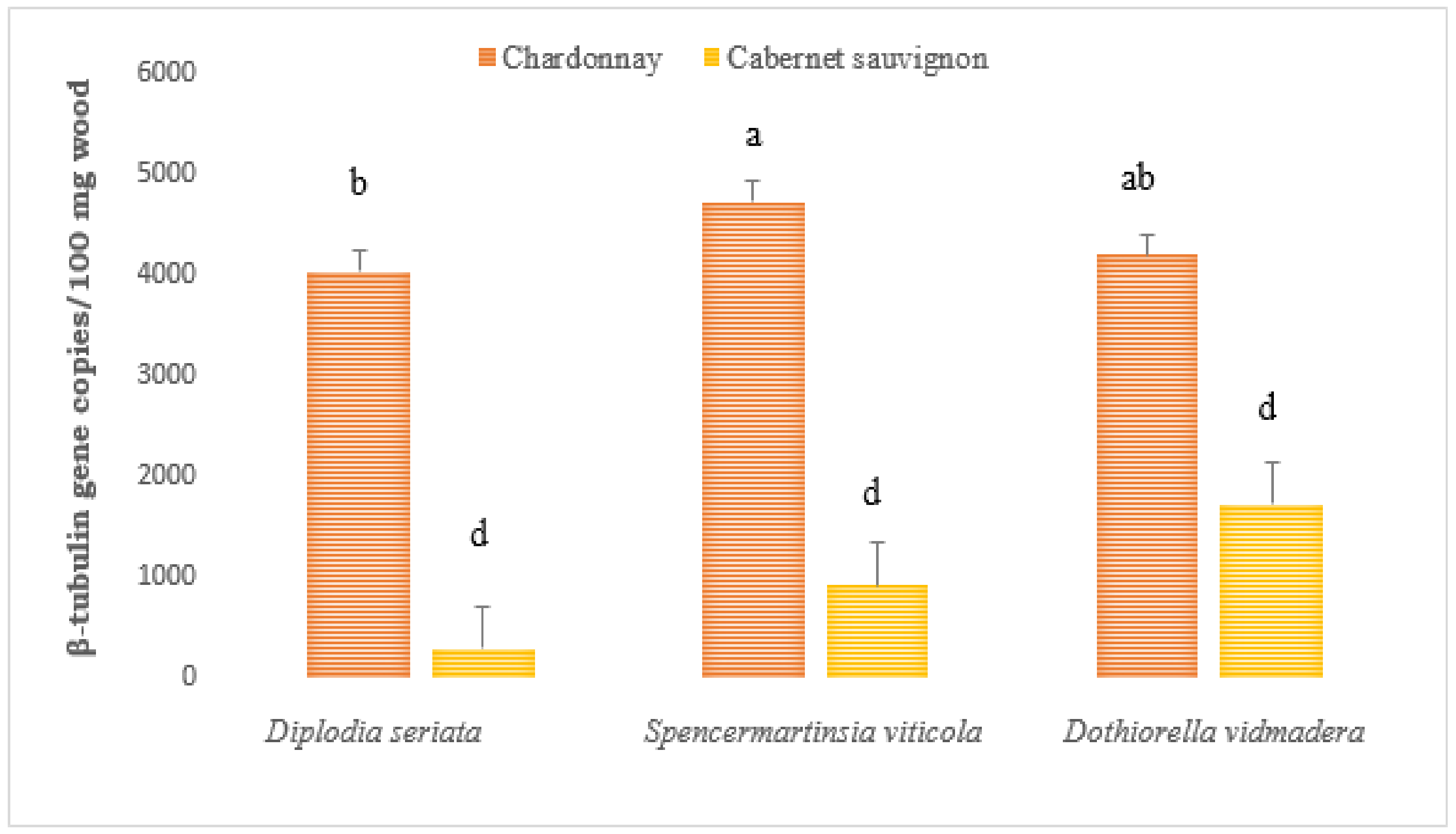
2.1.2. Botryosphaeriaceae DNA in Wood Tissues of Artificially Inoculated Vines
2.2. Naturally Infected Vines
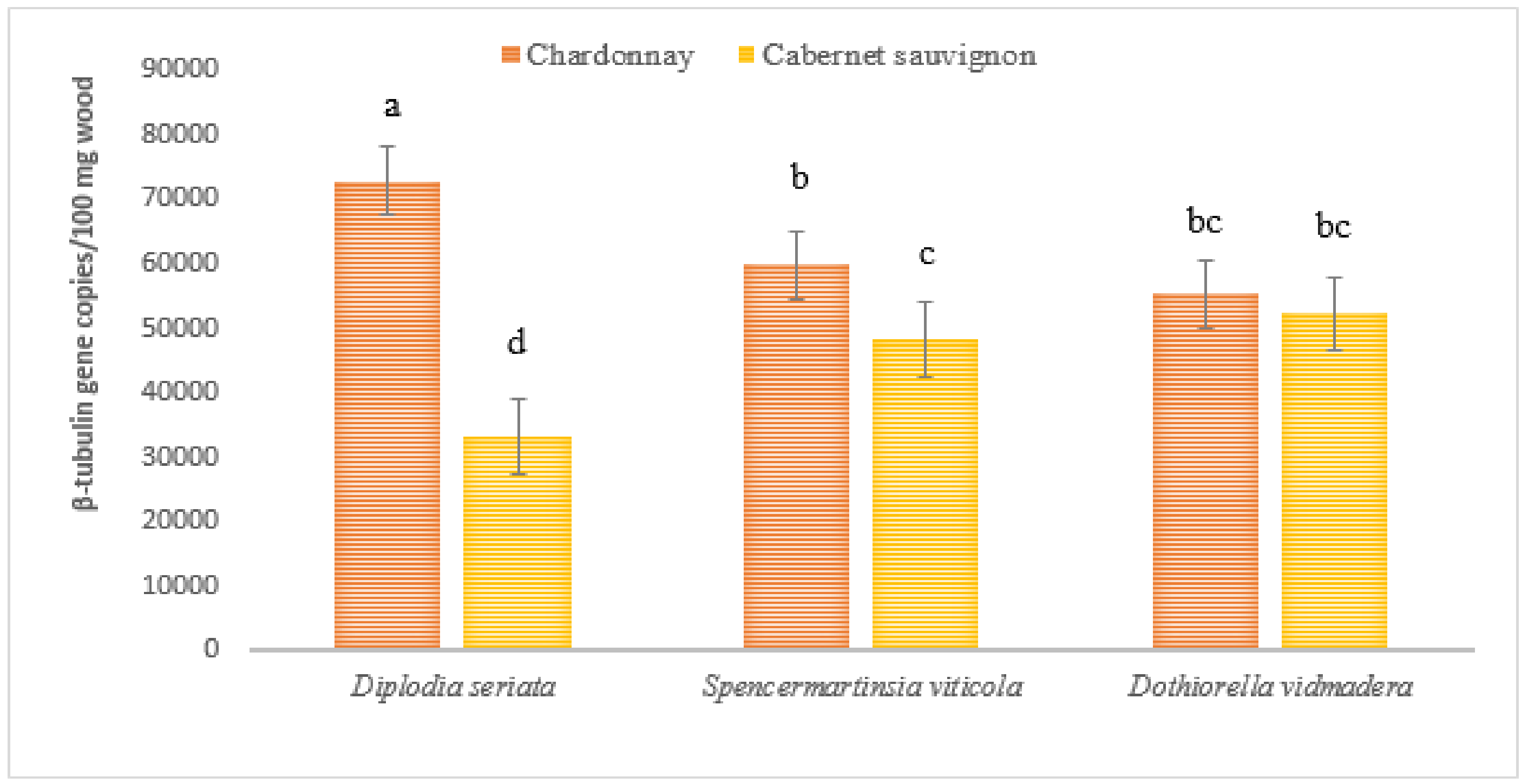
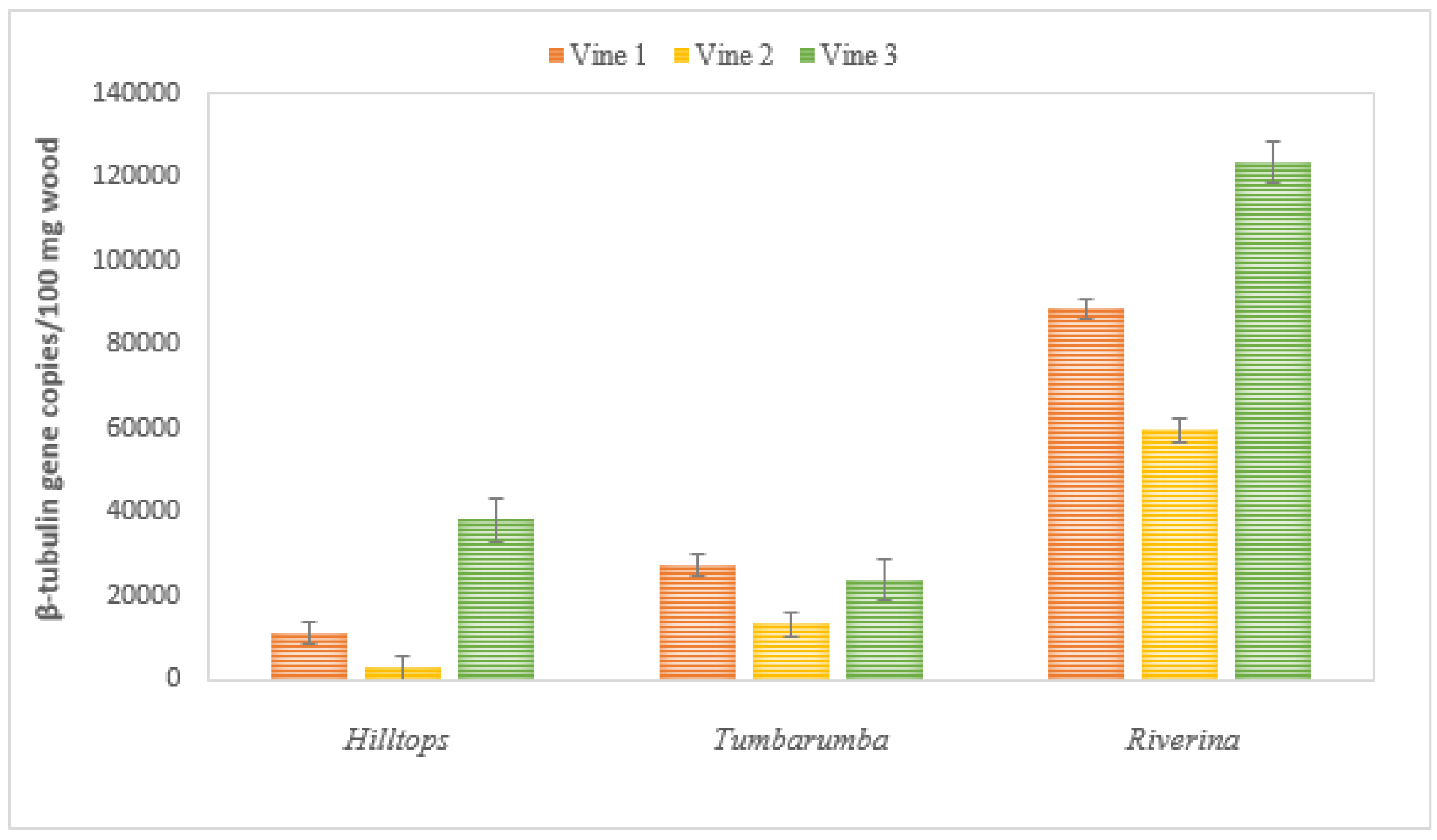
2.3. Botryosphaeriaceae DNA in Wood Tissues of Naturally Infected Vines
2.4. Selection of Protocol for Extraction of PMs from Wood
2.5. PMs in Wood Tissues of Naturally Infected Vines
2.6. PMs in Wood Tissues of Artificially Inoculated Vines
3. Discussion
4. Materials and Methods
4.1. Artificially Inoculated Vines
4.1.1. Planting Materials
4.1.2. Fungal Isolates
4.1.3. Sampling of Artificially Inoculated Vines
4.1.4. Fungal Isolation from Artificially Inoculated Vines
4.2. Fungal Isolation from Naturally Infected Vines
4.3. DNA Extractions from Fungal Mycelia
4.4. DNA Extraction from Grapevine Wood
4.5. Identification of Isolated Botryosphaeriaceae by PCR
4.6. Quantification of Botryosphaeriaceae spp. from Wood Samples by qPCR
4.7. Statistical Analysis
4.8. Chemicals and Standards for LC-MS/MS
4.9. Testing of Protocols for Extraction of PMs from Wood
4.10. LC-MS/MS Analysis of Targeted PMs from Wood
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popa, E.O.; Roşca, I. Main trends of the pests management in agroecosystems of grapevine plantations. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2011, 11, 146–150. [Google Scholar]
- Halleen, F.; Fourie, P.H.; Crous, P.W. A review of black foot disease of grapevine. Phytopathol. Med. 2006, 45, 55–67. [Google Scholar]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Med. 2011, 50, 3–44. [Google Scholar]
- Úrbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Med. 2011, 50, 5–45. [Google Scholar]
- Fontaine, F.; Pinto, C.; Vallet, J.; Clément, C.; Gomes, A.C.; Spagnolo, A. The effects of grapevine trunk diseases (GTDs) on vine physiology. Eur. J. Plant Pathol. 2016, 144, 707–721. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Baránek, M.; Armengol, J.; Holleinová, V.; Pečenka, J.; Calzarano, F.; Peňázová, E.; Vachůn, M.; Eichmeier, A. Incidence of symptoms and fungal pathogens associated with grapevine trunk diseases in Czech vineyards: First example from a north-eastern European grape-growing region. Phytopathol. Mediterr. 2018, 57, 449–458. [Google Scholar]
- Calzarano, F.; D’Agostino, V.; Pepe, A.; Osti, F.; Della Pelle, F.; De Rosso, M.; Flamini, R.; Di Marco, S. Patterns of phytoalexins in the grapevine leaf stripe disease (esca complex)/grapevine pathosystem. Phytopathol. Mediterr. 2016, 55, 410–426. [Google Scholar]
- Calzarano, F.; Di Marco, S. Further evidence that calcium, magnesium and seaweed mixtures reduce grapevine leaf stripe symptoms and increase grape yield. Phytopathol. Mediterr. 2018, 57, 459–471. [Google Scholar]
- Claverie, M.; Notaro, M.; Fontaine, F.; Wéry, J. Current knowledge on grapevine trunk diseases with complex etiology: A systemic approach. Phytopathol. Med. 2020, 59, 29–53. [Google Scholar]
- Hrycan, J.; Hart, M.; Bowen, P.; Forge, T.; Úrbez-Torres, J.R. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Med. 2020, 59, 395–424. [Google Scholar]
- Bruez, E.; Vallance, J.; Gautier, A.; Laval, V.; Compant, S.; Maurer, W.; Sessitsch, A.; Lebrun, M.H.; Rey, P. Major changes in grapevine wood microbiota are associated with the onset of esca, a devastating trunk disease. Environ. Microbiol. 2020, 22, 5189–5206. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Ayres, M.; Scott, E. The influence of water deficit stress on the grapevine trunk disease pathogens Eutypa lata and Diplodia seriata. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Billones-Baaijens, R.; Savocchia, S. A review of Botryosphaeriaceae species associated with grapevine trunk diseases in Australia and New Zealand. Austral. Plant Pathol. 2019, 48, 3–18. [Google Scholar] [CrossRef]
- Guerin-Dubrana, L.; Fontaine, F.; Mugnai, L. Grapevine trunk disease in European and Mediterranean vineyards: Occurrence, distribution and associated disease-affecting cultural factors. Phytopathol. Med. 2019, 58, 49–71. [Google Scholar]
- Larignon, P.; Dubos, B.; Cere, L.; Fulchic, R. Observation on black dead arm in French vineyards. Phytopathol. Med. 2001, 40, 336–342. [Google Scholar]
- Pitt, W.M.; Huang, R.; Steel, C.; Savocchia, S. Pathogenicity and epidemiology of Botryosphaeriaceae species isolated from grapevines in Australia. Austral. Plant Pathol. 2013, 42, 573–582. [Google Scholar] [CrossRef]
- Carter, M. The Status of Eutypa lata as a Pathogen. In Monograph–Phytopathological Paper No. 32; International Mycological Institute: Surrey, UK, 1991. [Google Scholar]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef]
- Surico, G.; Mugnai, L.; Marchi, G. The esca disease complex. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Springer: Berlin/Heidelberg, Germany, 2008; pp. 119–136. [Google Scholar]
- Sosnowski, M.; Shtienberg, D.; Creaser, M.; Wicks, T.; Lardner, R.; Scott, E. The influence of climate on foliar symptoms of Eutypa dieback in grapevines. Phytopathology 2007, 97, 1284–1289. [Google Scholar] [CrossRef]
- Reis, P.; Magnin-Robert, M.; Nascimento, T.; Spagnolo, A.; Abou-Mansour, E.; Fioretti, C.; Clement, C.; Rego, C.; Fontaine, F. Reproducing Botryosphaeria dieback foliar symptoms in a simple model system. Plant Dis. 2016, 100, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Larignon, P.; Dubos, B. The villainy of black dead arm. Wines Vines 2001, 82, 86–89. [Google Scholar]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on fungal phytotoxins and their role in grapevine trunk diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef]
- Tey-Rulh, P.; Philippe, I.; Renaud, J.-M.; Tsoupras, G.; de Angelis, P.; Fallot, J.; Tabacchi, R. Eutypine, a phytotoxin produced by Eutypa lata the causal agent of dying-arm disease of grapevine. Phytochemistry 1991, 30, 471–473. [Google Scholar] [CrossRef]
- Mahoney, N.; Molyneux, R.J.; Smith, L.R.; Schoch, T.K.; Rolshausen, P.E.; Gubler, W.D. Dying-arm disease in grapevines: Diagnosis of infection with Eutypa lata by metabolite analysis. J. Agric. Food Chem. 2005, 53, 8148–8155. [Google Scholar] [CrossRef] [PubMed]
- Lardner, R.; Mahoney, N.; Zanker, T.P.; Molyneux, R.J.; Scott, E.S. Secondary metabolite production by the fungal pathogen Eutypa lata: Analysis of extracts from grapevine cultures and detection of those metabolites in planta. Aust. J. Grape Wine Res. 2006, 12, 107. [Google Scholar] [CrossRef]
- Rolshausen, P.; Greve, L.; Labavitch, J.; Mahoney, N.; Molyneux, R.; Gubler, W. Pathogenesis of Eutypa lata in grapevine: Identification of virulence factors and biochemical characterization of cordon dieback. Phytopathology 2008, 98, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef]
- Martos, S.; Andolfi, A.; Luque, J.; Mugnai, L.; Surico, G.; Evidente, A. Production of phytotoxic metabolites by five species of Botryosphaeriaceae causing decline on grapevines, with special interest in the species Neofusicoccum luteum and N. parvum. Eur. J. Plant Pathol. 2008, 121, 451–461. [Google Scholar] [CrossRef]
- Djoukeng, J.D.; Polli, S.; Larignon, P.; Abou-Mansour, E. Identification of phytotoxins from Botryosphaeria obtusa, a pathogen of black dead arm disease of grapevine. Eur. J. Plant Pathol. 2009, 124, 303–308. [Google Scholar] [CrossRef]
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic phytotoxins produced by Neofusicoccum parvum, a grapevine canker agent. Phytopathol. Med. 2010, 49, 74–79. [Google Scholar]
- Abou-Mansour, E.; Débieux, J.-L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Cimmino, A.; Evidente, A. Phytotoxic metabolites by nine species of Botryosphaeriaceae involved in grapevine dieback in Australia and identification of those produced by Diplodia mutila, Diplodia seriata, Neofusicoccum australe and Neofusicoccum luteum. Nat. Prod. Res. 2019, 33, 2223–2229. [Google Scholar] [CrossRef]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Cimmino, A.; Evidente, A. Isolation of phytotoxic phenols and characterization of a new 5-hydroxymethyl-2-isopropoxyphenol from Dothiorella vidmadera, a causal agent of grapevine trunk disease. J. Agric. Food Chem. 2018, 66, 1760–1764. [Google Scholar] [CrossRef]
- Masi, M.; Reveglia, P.; Baaijens-Billones, R.; Górecki, M.; Pescitelli, G.; Savocchia, S.; Evidente, A. Phytotoxic metabolites from three Neofusicoccum species causal agents of Botryosphaeria dieback in Australia, luteopyroxin, neoanthraquinone, and luteoxepinone, a disubstituted furo-α-pyrone, a hexasubstituted anthraquinone, and a trisubstituted oxepi-2-one from Neofusicoccum luteum. J. Nat. Prod. 2020, 83, 453–460. [Google Scholar]
- Masi, M.; Reveglia, P.; Femina, G.; Baaijens-Billones, R.; Savocchia, S.; Evidente, A. Luteoethanones A and B, two phytotoxic 1-substituted ethanones produced by Neofusicoccum luteum, a causal agent of Botryosphaeria dieback on grapevine. Nat. Prod. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Evidente, A. Spencertoxin and spencer acid, new phytotoxic derivatives of diacrylic acid and dipyridinbutan-1,4-diol produced by Spencermartinsia viticola, a causal agent of grapevine Botryosphaeria dieback in Australia. Arab. J. Chem. 2020, 13, 1803–1808. [Google Scholar] [CrossRef]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Cimmino, A.; Evidente, A. Diploquinones A and B, two new phytotoxic tetrasubstituted 1, 4-naphthoquinones from Diplodia mutila, a causal agent of grapevine trunk disease. J. Agric. Food Chem. 2018, 66, 11968–11973. [Google Scholar] [CrossRef]
- Saviano, G.; Paris, D.; Melck, D.; Falasca, A.; Trupiano, D.; Iorizzi, M.; Scippa, G.S.; Motta, A. Monitoring spatial and temporal metabolic dynamics of woody poplar root under mechanical stress conditions by NMR-based metabolomics. Metabolomics 2016, 12, 65. [Google Scholar] [CrossRef]
- Deighton, N.; Muckenschnabel, I.; Colmenares, A.J.; Collado, I.G.; Williamson, B. Botrydial is produced in plant tissues infected by Botrytis cinerea. Phytochemistry 2001, 57, 689–692. [Google Scholar] [CrossRef]
- Zhao, Y.; Jones, W.; Sutherland, P.; Palmer, D.; Mitchell, R.; Reynolds, P.; Damicone, J.; Bender, C. Detection of the phytotoxin coronatine by ELISA and localization in infected plant tissue. Physiol. Mol. Plant. Path. 2001, 58, 247–258. [Google Scholar] [CrossRef]
- Ghosh, M.; Amudha, R.; Jayachandran, S.; Sakthivel, N. Detection and quantification of phytotoxic metabolites of Sarocladium oryzae in sheath rot-infected grains of rice. Lett. Appl. Microbiol. 2002, 34, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Buckel, I.; Andernach, L.; Schüffler, A.; Piepenbring, M.; Opatz, T.; Thines, E. Phytotoxic dioxolanones are potential virulence factors in the infection process of Guignardia bidwellii. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Billones-Baaijens, R.; Úrbez-Torres, J.R.; Liu, M.; Ayres, M.; Sosnowski, M.; Savocchia, S. Molecular methods to detect and quantify Botryosphaeriaceae inocula associated with grapevine dieback in Australia. Plant Dis. 2018, 102, 1489–1499. [Google Scholar] [CrossRef]
- Billones-Baaijens, R.; Jones, E.; Ridgway, H.; Jaspers, M. Virulence affected by assay parameters during grapevine pathogenicity studies with Botryosphaeriaceae nursery isolates. Plant Pathol. 2013, 62, 1214–1225. [Google Scholar] [CrossRef]
- Del Río, J.A.; Gómez, P.; Báidez, A.; Fuster, M.D.; Ortuño, A.; Frías, V. Phenolic compounds have a role in the defence mechanism protecting grapevine against the fungi involved in Petri disease. Phytopathol. Med. 2004, 43, 87–94. [Google Scholar]
- Lambert, C.; Bisson, J.; Waffo-Téguo, P.; Papastamoulis, Y.; Richard, T.; Corio-Costet, M.-F.; Mérillon, J.-M.; Cluzet, S.P. Phenolics and their antifungal role in grapevine wood decay: Focus on the Botryosphaeriaceae family. J. Agric. Food Chem. 2012, 60, 11859–11868. [Google Scholar] [CrossRef]
- Magnin-Robert, M.; Spagnolo, A.; Boulanger, A.; Joyeux, C.; Clément, C.; Abou-Mansour, E.; Fontaine, F. Changes in plant metabolism and accumulation of fungal metabolites in response to esca proper and apoplexy expression in the whole grapevine. Phytopathology 2016, 106, 541–553. [Google Scholar] [CrossRef]
- Ramírez-Suero, M.; Bénard-Gellon, M.; Chong, J.; Laloue, H.; Stempien, E.; Abou-Mansour, E.; Fontaine, F.; Larignon, P.; Mazet-Kieffer, F.; Farine, S. Extracellular compounds produced by fungi associated with Botryosphaeria dieback induce differential defence gene expression patterns and necrosis in Vitis vinifera cv. Chardonnay cells. Protoplasma 2014, 251, 1417–1426. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Abou-Mansour, E.; Courteaux, B.; Rabenoelina, F.; Clément, C.; Fontaine, F.; Aziz, A. Bacillus subtilis PTA-271 counteracts Botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Front. Plant. Sci. 2019, 10, 25. [Google Scholar] [CrossRef]
- Larignon, P.; Fontaine, F.; Farine, S.; Clement, C.; Bertsch, C. Esca and black dead arm: Two major actors of grapevine trunk diseases. Compt. Rendus Biol. 2009, 332, 765–783. [Google Scholar] [CrossRef]
- Calzarano, F.; Osti, F.; Baranek, M.; Di Marco, S. Rainfall and temperature influence expression of foliar symptoms of grapevine leaf stripe disease (esca complex) in vineyards. Phytopathol. Med. 2018, 57, 488–505. [Google Scholar]
- Serra, S.; Ligios, V.; Schianchi, N.; Prota, V.A.; Scanu, B. Expression of grapevine leaf stripe disease foliar symptoms in four cultivars in relation to grapevine phenology and climatic conditions. Phytopathol. Med. 2019, 57, 557–568. [Google Scholar]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef]
- Möbius, N.; Hertweck, C. Fungal phytotoxins as mediators of virulence. Curr. Opin. Plant Biol. 2009, 12, 390–398. [Google Scholar] [CrossRef]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in plant stress physiology. In Plant Genetics and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 187–236. [Google Scholar]
- Poulin, R.X.; Pohnert, G. Simplifying the complex: Metabolomics approaches in chemical ecology. Anal. Bioanal. Chem. 2019, 411, 13–19. [Google Scholar] [CrossRef]
- Labois, C.; Wilhelm, K.; Laloue, H.; Tarnus, C.; Bertsch, C.; Goddard, M.-L.; Chong, J. Wood metabolomic responses of wild and cultivated grapevine to infection with Neofusicoccum parvum, a trunk disease pathogen. Metabolites 2020, 10, 232. [Google Scholar] [CrossRef]
- WineAustralia. Wine Australia Annual Report; WineAustralia: Kent Town, Australia, 2018. [Google Scholar]
- WineAustralia.com. Available online: https://www.wineaustralia.com/growing-making/environment-and-climate (accessed on 31 March 2020).
- Pouzoulet, J.; Mailhac, N.; Couderc, C.; Besson, X.; Daydé, J.; Lummerzheim, M.; Jacques, A. A method to detect and quantify Phaeomoniella chlamydospora and Phaeoacremonium aleophilum DNA in grapevine-wood samples. Appl. Microbiol. Biotechnol. 2013, 97, 10163–10175. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bul. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]


| Location | Variety | Vine Sample | Botryosphaeriaceae Species |
|---|---|---|---|
| Hilltops | Chardonnay | 1 | Neofusicoccum parvum |
| 2 | Diplodia seriata | ||
| 3 | Diplodia mutila | ||
| Tumbarumba | Chardonnay | 1 | D.seriata |
| 2 | D. seriata, N. parvum | ||
| 3 | D. seriata | ||
| Riverina | Shiraz | 1 | D. seriata |
| 2 | Botryosphaeria dothidea | ||
| 3 | D. seriata |
| Toxin | Precursor Ion m/z | Fragment Ion m/z | Fragmentor Voltage * | CV ** | Retention Time (Min) |
|---|---|---|---|---|---|
(R)-mellein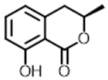 | 179.1 [M + H]+ | 161.0 133.0 105.0 | 90 | 12 16 24 | 27.44 |
| Protocatechuic alcohol 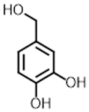 | 123.1 [M-H2O + H]+ | 67.1 55.1 51.1 | 90 | 16 24 40 | 6.94 |
Spencertoxin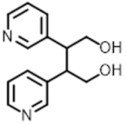 | 283.1 [M + K]+ | 177.8 118 | 90 | 44 50 | 18.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reveglia, P.; Billones-Baaijens, R.; Millera Niem, J.; Masi, M.; Cimmino, A.; Evidente, A.; Savocchia, S. Production of Phytotoxic Metabolites by Botryosphaeriaceae in Naturally Infected and Artificially Inoculated Grapevines. Plants 2021, 10, 802. https://doi.org/10.3390/plants10040802
Reveglia P, Billones-Baaijens R, Millera Niem J, Masi M, Cimmino A, Evidente A, Savocchia S. Production of Phytotoxic Metabolites by Botryosphaeriaceae in Naturally Infected and Artificially Inoculated Grapevines. Plants. 2021; 10(4):802. https://doi.org/10.3390/plants10040802
Chicago/Turabian StyleReveglia, Pierluigi, Regina Billones-Baaijens, Jennifer Millera Niem, Marco Masi, Alessio Cimmino, Antonio Evidente, and Sandra Savocchia. 2021. "Production of Phytotoxic Metabolites by Botryosphaeriaceae in Naturally Infected and Artificially Inoculated Grapevines" Plants 10, no. 4: 802. https://doi.org/10.3390/plants10040802
APA StyleReveglia, P., Billones-Baaijens, R., Millera Niem, J., Masi, M., Cimmino, A., Evidente, A., & Savocchia, S. (2021). Production of Phytotoxic Metabolites by Botryosphaeriaceae in Naturally Infected and Artificially Inoculated Grapevines. Plants, 10(4), 802. https://doi.org/10.3390/plants10040802









