Abstract
This study investigated the effect of water stress, simulated by the polyethylene glycol (PEG-6000) method, on nitrogen (N) metabolism in leaves and roots of hydroponically grown sweet potato seedlings, Xushu 32 (X32) and Ningzishu 1 (N1). The concentrations of PEG-6000 treatments were 0%, 5% and 10% (m/v). The results showed that the drought-treated plants showed a decline leaf relative water content, and revealed severe growth inhibition, compared with the 0% treatment. Under drought stress, the decline in biomass of the leaf and stem was more noticeable than in root biomass for X32, leading to a higher root to shoot ratio. Drought stress increased the nitrate nitrogen (NO3−-N) and protein in leaves, but reduced all the activities of N-metabolism enzymes and the transcriptional levels of nitrate reductase (NR), glutamine synthetase (GS) and glutamate synthase (GOGAT); in roots, NO3−-N and NR had opposite trends. The leaf ammonium nitrogen (NH4+-N), GS and amino acid had different trends between X32 and N1 under drought stress. Furthermore, the transcriptional level of nitrate transporter genes NRT1.1 in leaves and roots were upregulated under drought stress, except in N1 roots. In conclusion, NR determined the different response to drought in leaves for X32 and N1, and GS and GOGAT determined the response to drought in roots, respectively.
1. Introduction
Sweet potato (Ipomoea batatas (L.) Lam.) is a vital grain and forage crop in China. It has strong drought resistance and wide adaptability, and is often used as a drought relief crop [1]; however, during growth and the development of maturity the plants are susceptible to drought [2]. Drought has become a major environmental factor limiting global crop yields in various abiotic stresses [3]. In China, more than 70% of sweet potato is planted in hilly and mountainous areas. In these areas, the annual rainfall of the sweet potato growth season is uneven, and drought is frequent, resulting in the serious decline in the tuberous yield of the sweet potato. Rapid root formation after planting is the basis to ensure the survival rate of sweet potato seedlings and root tuber formation [4]. The sweet potato is very sensitive to soil moisture at the seedling stage. It is suitable for soil moisture to be 60–75% of the maximum water holding capacity in the field for the sweet potato, and irrigation should be carried out when the soil relative moisture content is less than 60% [5], and less than 50% of the soil relative water content (RWC) will directly affect the formation, growth and differentiation of adventitious roots. While at lower soil moisture, more soil resistance restricted growth and tuber development, at the same time less labile N and K were transferred from the soil reducing tuber dry matter production and tuber yield [6].
As one of the three major nutrients, nitrogen (N) greatly contributes to the increased yield of major food crops [7]. Generally, nitrate nitrogen (NO3−-N) and ammonium nitrogen (NH4+-N) are mainly taken up in plants, rather than amino acids (AAs) or other N organic forms. Moreover, NO3−-N is also the regulatory factor in response to N metabolism, thus playing a critical role in regulating carbon absorption, the carbon and N absorption ratio and light cooperation efficiency [8]. NH4+-N plays a crucial role in the N acquisition regulation by influencing the transcription and/or activity of root NO3− transport systems [9]. In sugarcane, drought stress reduced NO3− and NH4+ uptake [8]. Additionally, the nitrate transporter family (NRT1:1 and NRT2.1) may play crucial roles in NH4+ and NO3− uptake in winter wheat (Triticum aestivum L.) and Populus [10,11]. Nitrate reductase (NR) is a key rate-limiting enzyme that regulates nitrogen metabolism and assimilation and is sensitive to environmental conditions [12]. Glutamine synthetase (GS), key enzyme in the NH4+ assimilation, is encoded by genes expressed in the cytoplasm or root plastid [13], which regulation is determined by a vast number of biotic and abiotic factors. Under water stress, NH4+ produced through NR and nitrite reductase-mediated (NiR) reduction of NO3− was reduced as well as the activities of GS and glutamate synthase (GOGAT). These results led to ammonium accumulation and toxic effects on plants [14]. Glutamate dehydrogenase (GDH) also plays a major role in the NH4+ assimilation and is determined by stress factors [13,15]. Under water stress, NR and nitrite reductase, mediated by NO3− reduction, was reduced as well as the activities of GS and GOGAT, leading to NH4+ accumulation and toxic effects on plants [16]. There are also studies demonstrating that drought stress increased NR activity, and produced sufficient nitric oxide, resulting in higher nitric oxide synthase activity in adventitious roots [17], and the activities of GS, GOGAT and GDH was increased for dwarf bamboo [18]. Additionally, in maize, drought stress has also enhanced the activities of hydrolytic enzymes, soluble protein (SP) content, some osmotic regulators containing nitrogen, such as AAs and amides, which can reflect the degree of water stress [19]. Therefore, the decrease of crop yield under soil drought can be partly attributed to the hindrance of N absorption and utilization, and different crops respond differently to drought stress [20,21].
In recent years, previous studies mainly focused on the root morphological structure, the production of reactive oxygen species (ROS) and the relationship between physiological and biochemical indexes such as photosynthetic capacity, endogenous hormones and antioxidant enzyme activity and drought resistance of sweet potato varieties [22,23,24,25,26]. Under drought stress, the net photosynthetic rate, stomatal conductance, water use efficiency, maximum photochemical efficiency of PS II, quantum yield and photochemical quenching coefficient of sweet potato plants were reduced, and the transpiration rate, non-photochemical quenching coefficient and stomatal limit value of sweet potato leaves were increased [27]. Additionally, under drought stress, ABA, proline content was significantly increased, whereas malonaldehyde (MDA) and H2O2 content was significantly decreased in the transgenic sweet potato plants [3]. Moreover, drought stress significantly reduced the average diameter and root volume [28]. However, few articles on N metabolism in sweet potato have been reported, only one about the changes of ion homeostasis and N metabolism of the sweet potato in response to salt stress [29]. N metabolism in response to drought stress for the sweet potato has, to our knowledge, not been well studied.
In the present study, drought-induced changes of inorganic N, activities of the main N-metabolism enzymes and the related genes transcription in sweet potato seedlings were investigated. The purpose of this study was (1) to evaluate the N metabolism to the overall drought tolerance at the adventitious root stage, (2) to analyze relationship between leaf RWC and N metabolism in sweet potato seedlings and (3) to study the relationship of N transporter genes with NO3−. The changes induced by drought stress in N metabolism in sweet potato seedlings may be considered to implement water management strategies to cope with the damage caused by drought.
2. Results
2.1. Leaf RWC and Membrane Stability Index (MSI)
Leaf RWC can truly reflect the degree of water deficit in plants under drought stress. In this study, the leaf RWC of sweet potato seedlings showed a decreasing trend after drought stress, and the difference increased with increasing PEG concentration (Figure 1). Leaf membrane stability index (MSI) had a similar trend to leaf RWC. When the drought treatment lasted for five days, compared to the 0% treatment, the leaf RWC and MSI decreased by 8–23% and 15–22% under drought stress, respectively. In addition, leaf RWC was significantly positively correlated with MSI (Figure 2).
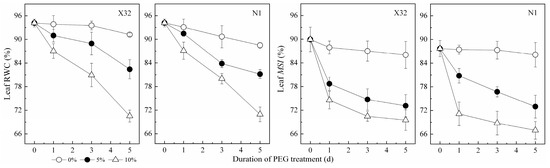
Figure 1.
Effects of drought stress on relative water content (RWC) and the membrane stability index (MSI) in sweet potato leaves. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively.
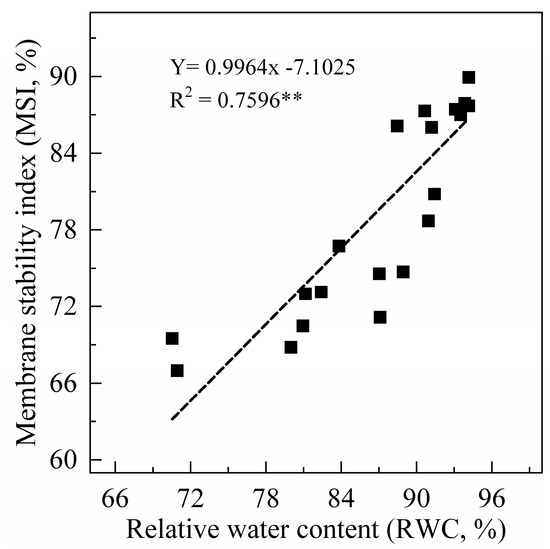
Figure 2.
Correlation analysis between relative water content (RWC) and membrane stability index (MSI) in sweet potato leaves. n = 24, R20.05 = 0.164, and R20.01 = 0.265.
2.2. Growth Parameters in the Sweet Potato
After 5 days of the drought treatment, serious growth inhibition was observed in the PEG-treated plants (Figure 3), leading to a decline in biomass compared with the 0% PEG-induced treatment (the controls, Figure 4, main part). Drought stress obviously reduced the stem biomass, leaf biomass and total plant biomass (Figure 4, main part). Compared to Ningzishu 1 (N1), drought stress induced an even higher reduction for the three indices in Xushu 32 (X32). The 5% and 10% PEG-induced drought reduced stem, leaf and total biomass by 44–67%, 37–80% and 40–69% in X32, respectively, and by 26–37%, 23–48% and 33–49% in N1. However, there was an opposite trend for the root biomass.
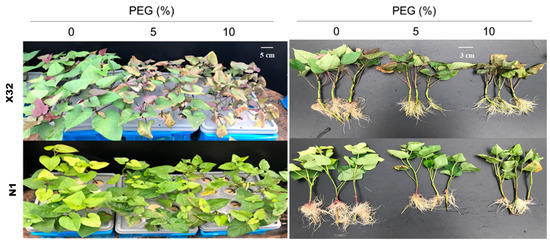
Figure 3.
Effect of PEG-induced drought stress on plant growth and development in the sweet potato. Each treatment consisted of five pots, each containing twelve plants. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively.
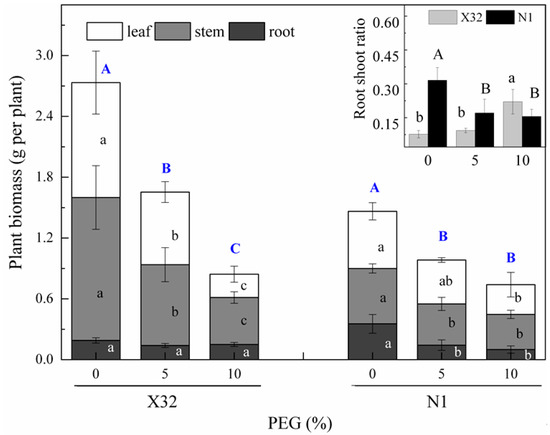
Figure 4.
Growth parameters of sweet potato seedlings as affected by the different PEG concentrations. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. Values followed by the different lowercase letters within the same cultivar in the main part are significantly different (p < 0.05) among the three PEG levels for leaf, stem and root biomass, and by the different big letters within the same cultivar in the main part are significantly different for plant biomass. Values followed by different small letters within the same cultivar in the input part are significantly different (p < 0.05) among the three PEG levels for X32, and by different capital letters for N1.
Moreover, drought had a significant effect on the root to shoot ratio (R/S, Figure 4, inset). With increasing PEG concentration, the R/S significantly increased in X32, while it significantly decreased in N1.
2.3. Chlorophyll Content in Leaves
Drought markedly reduced chlorophyll (Chl) content (Figure 5A–C). As for sweet potato in the 5% and 10% PEG treatment, the content of Chl a was increased by 25–35% for X32 and by 20–29% for N1, respectively. A significant decrease in the content of Chl b (58–90% for X32 and 42–79% for N1) and total Chl (46–80% for X32 and 32–65% for N1) were also observed. Moreover, X32 had higher Chl content than N1.
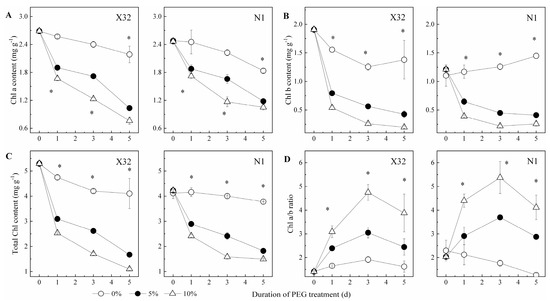
Figure 5.
Effects of drought stress on chlorophyll content (A: Chl a content; B: Chl b content; C: Total Chl content; D: Chl a/b ratio) in sweet potato leaves. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. The stages labeled with an asterisk (*) indicate significant differences (p < 0.05) among the three PEG levels in the figure.
The Chl a/b ratio increased with the extension of the drought time and the aggravation of the drought degree (Figure 5D). It was thus indicated that Chl a content fell more slowly than Chl b under drought stress. Furthermore, the increased degrees of X32 among different drought treatments were higher than N1.
2.4. NO3−-N and NH4+-N, AA and SP Content in Leaves and Roots
A sharp NO3−-N increase was found after PEG-induced drought stress in leaves for the two cultivars (Figure 6A). Contrary to leaves, NO3−-N content was remarkably reduced by drought stress in roots (Figure 6B). However, NH4+-N content remained constant in leaves of PEG-treated X32 and N1 (p > 0.05, Figure 6C). Drought stress quickly reduced NO3−-N in X32 roots, whereas an increased NO3−-N in roots were observed for N1 (Figure 6D). Additionally, drought stress reduced the NH4+ to NO3− ratio in leaves and roots, except in N1 roots with an increasing trend (Figure 7).
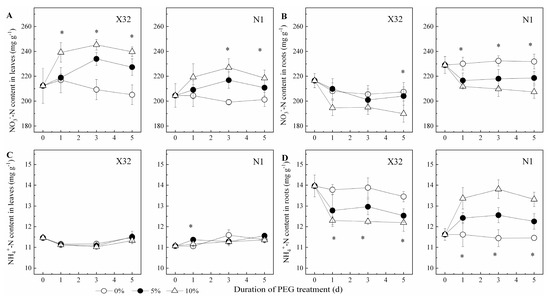
Figure 6.
Effects of drought stress on NO3−-N and NH4+-N content in leaves (A,C) and roots (B,D) of the sweet potato. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. The stages labeled with an asterisk (*) indicate significant differences (p < 0.05) among the three PEG levels in the figure.
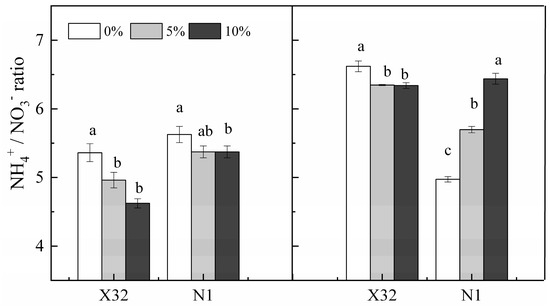
Figure 7.
Effects of drought stress on the NH4+/NO3− ratio in leaves and roots of the sweet potato. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. Different letters in the same cultivar indicate significant differences (p < 0.05) among the three PEG levels in the figure.
Drought stress significantly increased AA in leaves and roots of X32, but an opposite trend was observed upon drought induction in N1 (Figure 8A,B). Drought-stressed plants showed 21–48% higher AA content in leaves and 10–25% higher in roots as compared to 0% PEG-treated X32, whereas a 10–26% reduction in leaves and 11–28% in roots were observed as compared to 0% PEG-treated N1. Leaf SP content was significantly enriched under drought stress (Figure 8C). Contrary to leaves, SP content was remarkably reduced by drought stress in roots (Figure 8D).
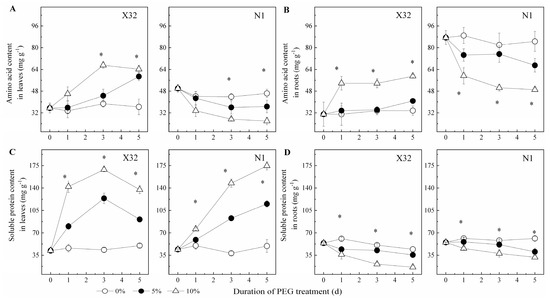
Figure 8.
Effects of drought stress on amino acid and soluble protein content in leaves (A,C) and roots (B,D) of sweet potato. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. The stages labeled with an asterisk (*) indicate significant differences (p < 0.05) among the three PEG levels in the figure.
Furthermore, the relationship of NO3—N, NH4+-N, AA and SP with leaf RWC was analyzed (Figure 9). Leaf NO3−-N was significantly negatively correlated with leaf RWC for both cultivars (Figure 9A), with the slope of the fitted line in X32 being higher than that of N1. However, there was non-significant correlation between leaf NH4+-N and leaf RWC for the two cultivars (p > 0.05, Figure 9B). Root NO3−-N and NH4+-N was also significantly linearly correlated with leaf RWC (Figure 9E,F), with the slope of the fitted line in X32 also being higher than that in N1. There was a significant negative correlation between leaf AA for X32, SP for the two sweet potato and leaf RWC (p < 0.05, Figure 9C,D), and a significant negative correlation between leaf AA and leaf RWC for N1 (p < 0.05). Whereas, the content of AA and SP in roots showed an opposite correlation with a linear response to leaf RWC (p < 0.05, Figure 9G,H).
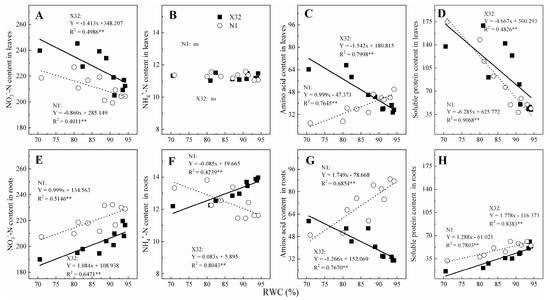
Figure 9.
Relationship between NO3−-N, NH4+-N, amino acid or the soluble protein content with leaf relative water content in leaves (A–D) and roots (E–H) for the two sweet potatoes. The solid and dotted lines represent X32 and N1, respectively.
2.5. Changes in N Metabolism Enzymes in Leaves and Roots
NR activity increased in leaves, but showed an opposite trend in roots (Figure 10A,B). After exposure to drought stress, the drought-treated sweet potato showed a significantly lower GS activity in both leaves and roots, except in N1 roots (Figure 10C,D). The activity of GOGAT and GDH was remarkably reduced in roots and leaves of drought-treated plants (Figure 11). In X32 leaves, NR, GS, GOGAT and GDH activities was reduced by 20–31%, 14–25%, 12–29% and 18–25% under 5% and 10% PEG-treated conditions, respectively, as compared to 0% PEG-treated plants. Additionally, N1 leaves showed a higher response to drought in NR and GS. Compared to leaves, cultivar differences had an opposite response in roots. In addition, protease activity varied little among the three PEG treatments in leaves and roots (data not shown).
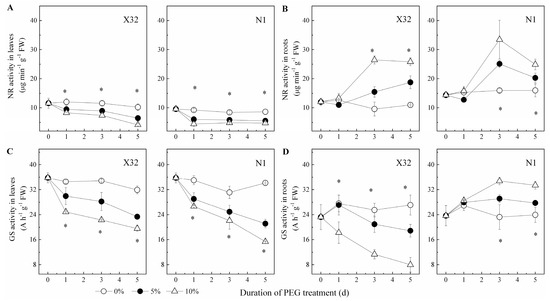
Figure 10.
Effects of drought stress on NR activity and GS activity in leaves (A,C) and roots (B,D) of the sweet potato. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. The stages labeled with an asterisk (*) indicate significant differences (p < 0.05) among the three PEG levels in the figure.
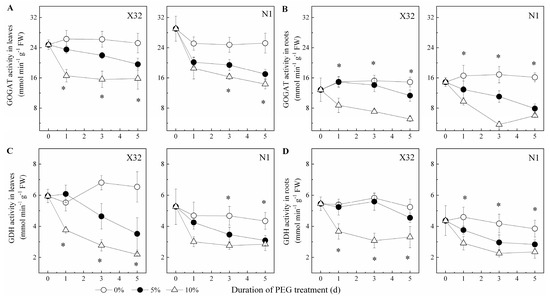
Figure 11.
Effects of drought stress on GOGAT activity and GDH activity in leaves (A,C) and roots (B,D) of the sweet potato. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. The stages labeled with an asterisk (*) indicate significant differences (p < 0.05) among the three PEG levels in the figure.
Furthermore, the relationship between enzyme activity and leaf RWC was investigated (Figure 12). The activity of NR, GS, GOGAT and GDH in leaves and roots could all be related to leaf RWC using a linear equation for both cultivars (R2 = 0.4886–0.9234**). There was a positive correlation between NR, GS, GOGAT and GDH with leaf RWC, except NR activity for the two cultivars roots and GS activity for N1 roots, with a negatively correlation with leaf RWC.
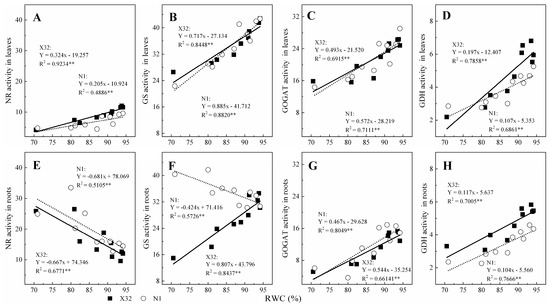
Figure 12.
Relationship between NR, GS, GOGAT or GDH activities with the leaf relative water content in leaves (A–D) and roots (E–H) for the two sweet potato. The solid and dotted lines represent X32 and N1, respectively.
2.6. Transcript Abundance Related to N Metabolism Exposed to Drought Stress
The transcript level of NR2, GS2 and GOGAT significantly reduced in leaves and roots, while genes GS2 and GOGAT in X32 roots were not affected by drought stress. Drought induced a 60–96% decrease in the transcript abundance of NR2, GS2 and GOGAT in leaves, and a 23% to 1-fold decrease in the three genes transcript abundance in roots. However, there was an opposite regulation in genes NRT1 involved in nitrate transport in leaves and roots under drought stress, except in X32 roots (Figure 13).
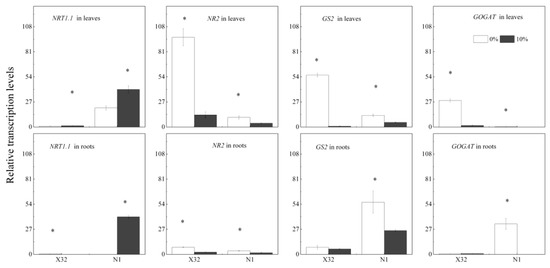
Figure 13.
Effects of drought stress on the transcription of N metabolism-related genes in leaves and roots of two sweet potato cultivars. Sweet potato seedlings were subjected to 5 days of drought stress (10% PEG); total RNA was isolated from leaves and roots for real-time PCR analysis. X32 and N1 represent Xushu 32 and Ningzishu 1, respectively. The stages labeled with an asterisk (*) indicate significant differences (p < 0.05) between the 0% and 10% PEG levels in the figure.
3. Discussion
3.1. Changes of Biomass, Chl and Inorganic Nitrogen Exposed to Drought Stress
Drought represents an excellent example of environmental stresses that occur in the field. It is often reported that drought stress leads to the inhibition of plant growth and the changes of physiologic parameters. In the present study, drought stress significantly reduced RWC and MSI, and plant growth was inhibited (Figure 1 and Figure 3). In accordance with previous studies [10,16], PEG-treated plants showed lower stem, root and total biomass (Figure 4). The decrease in leaf RWC and MSI may help the leaves to absorb more water from the solution under hydroponic conditions [30]. However, the effect of drought stress on nitrate reduction and N assimilation in the sweet potato were not fully investigated. In the present study, Chl, NO3−-N, NH4+-N and related gene expression involved in N metabolism were obviously influenced by drought stress. In X32, we found that drought inhibited biomass accumulation in leaves and stems more than in roots, resulting in higher R/S in the PEG-treated sweet potato (Figure 1 and Figure 3, as shown in Figure 14), consistent with reports in rice under drought stress [16]. Increased R/S in X32 can be an adaptation trait for crops to increase drought tolerance, because maintaining an effective root system can help to increase water uptake and reduce water loss to cope with drought stress [31]. In our previous study, it was also observed that X32 could make up for part of the loss of yield reduction through its own variety attribute (high yield) under drought stress, even though X32 and N1 belonged to low-resistant varieties [32].
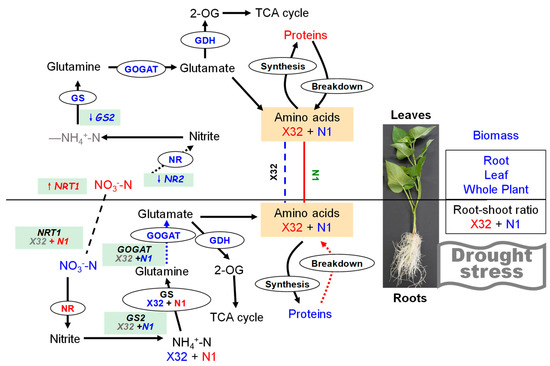
Figure 14.
A model of the N regulation in sweet potato seedlings in response to drought stress. Here, drought stress stimulates a N-mediated tandem reaction, which improves the sweet potato drought tolerance. Reducing leaf RWC might alter the expression levels of key regulatory metabolic genes and the activities of N metabolism enzymes, which in turn modulates N accumulation, activates the transcription of NO3− transporters to regulate sugar allocation to adapt to environmental stress. Upregulated items under drought stress are marked with red, downregulated items under PEG-induced drought stress are marked with blue, and items that do not change significantly under PEG-induced drought stress are marked with grey.
Similar to growth, photosynthetic pigments were damaged in drought-stressed plants, which could directly influence the carbon flux of plants under stress [33]. One possible reason may be that the reduced N metabolism under drought stress led to the decline in Chl content. After all, higher Chl content in plants is an indication of higher leaf N status, which proved that reduced N metabolism may be one reason for lower leaf Chl content in drought-induced sweet potato (Figure 5), as similar results reported in cotton leaves [33]. Under drought stress, the AA content in X32 leaves showed an increasing trend, while that of N1 had an opposite trend. The SP content for X32 and N1 leaves were both increased. Previous studies have reported that exogenous AAs reduced the activity of NO3− and NH4+ absorption and assimilation [8].
3.2. Effects of Drought Stress on N Assimilation and Recycling
Generally, plant biomass under stress was reduced, commonly together with the downregulation of N uptake genes [34], such as NRT1, one of the genes mediated NO3− transport. The transcriptional level of NRT1 could reflect N absorption and utilization [29]. In the present study, the transcriptional level of NRT1 was induced by drought stress in sweet potato leaves and roots, except that in X32 roots (Figure 13). It was confirmed that the transcriptional level of NRT1.1 in a N-efficient cultivar was mainly repressed by drought stress, but its transcriptional level in the N-inefficient cultivar was induced by drought stress [11]. The transcriptional difference of NRT1 gene in drought-treated plants, to a large extent, reflects the differences in crop species, drought conditions and growth stage.
As a key inorganic N, NO3− also plays a critical role in regulating plant growth, osmotic regulation and adaptation to abiotic stress [16]. In the present study, it was observed that drought remarkably declined root NO3−-N content, but improved leaf NO3−-N (Figure 6). The variation in NO3−-N accumulation for leaves and roots was in accord with the trends of root NO3−-N uptake rate and assimilation. Leaf NO3−-N plays a critical role in controlling stomatal closing by affecting guard cells depolarization [35]. The reduction of leaf NR activity could be related to a decrease in the photosynthesis rate as a result of stomatal closure, thus inhibiting growth (Table S2). It was confirmed that the reduction of leaf NO3−-N could utilize the residual energy produced by the photosynthetic apparatus [36]. Hence, the elevated leaf NO3−-N and lower NR activity revealed that NO3− reduction and assimilation were remarkably declined. This phenomenon was unhelpful for avoiding ROS accumulation due to the effects of drought stress, in agreement with previous studies [37]. In addition, reduced NR activity in drought-stressed plants is also largely due to an improved NO3− content [15]. Moreover, the mismatched variation in NR activity and NO3− content in sweet potato leaves may be attributable to leaf NO3− assimilation compartmentalization, as NO3− is isolated from cytoplasmic NR, mainly stored in vacuoles [38].
Furthermore, the unaffected NH4+-N assimilation in sweet potato leaves was probably due to the improved NO3− uptake and reduced NO3−-N pathway in drought-treated leaves (Figure 6C). Nevertheless, the NH4+ to NO3− ratio in X32 and N1 leaves was reduced under drought stress (Figure 7). These results indicate that more of the absorbed NO3− was stored under drought stress. Therefore, the resistance of NR activity to drought in sweet potato leaves could be a main factor that ameliorates the sweet potato growth under drought stress, in agreement with Salicornia europaeae under abiotic stress conditions, revealing improved NO3− uptake [39]. Another reason for increased NO3− content in leaves under drought stress was possibly closely related to K; K+ is transported with NO3− in the xylem from roots to aerial plant parts, as a supplementary cation [10]. In the next studies, the experiments on the interaction between drought and K fertilizer should be arranged.
NR and NiR can convert NO3− to NH4+; thus GS and GOGAT can assimilate glutamine and glutamate to NH4+. NH4+-N is necessary for N metabolism in plants, and its efflux maybe depletes NH4+ in cellular and disrupts the assimilation of NH4+ [9]. In the present study, both NH4+-N content, GS and GOGAT activity inX32 roots was reduced under drought stress, whereas NR activity remained increased by drought stress, consistent with NO3−-N content in roots. These results indicated that the low NH4+ to NO3− ratio in X32 roots was mainly attributed to NH4+ uptake reductions instead of NO3− uptake (Figure 7). However, the prolonged drought time did not decline NH4+ content and GS activity in N1 roots (Figure 6D and Figure 10D), but drought stress obviously reduced the transcriptional levels of genes GS2 and GOGAT in N1 roots (Figure 13). Indeed, drought stress enhanced the root NH4+ to NO3− ratio in N1. The possibly reason was that NH4+ has a lower energy requirement compared with NO3−. Moreover, NH4+ could stimulate root growth in plants [40].
4. Materials and Methods
4.1. Plant Materials and Drought Treatment
Sweet potato (Ipomoea batatas L., Xushu 32 (X32) and Ningzishu 1 (N1)) seedlings were used in this study. The shoots of X32 were obtained from the laboratory of Zhonghou Tang, Xuzhou Academy of Agricultural Sciences, and the shoots of N1 were obtained from the laboratory of Yizhi Xie in Jiangsu Academy of Agricultural Sciences. Sweet potato seedlings with five leaves were collected from tuberous root (20 days) and to initiate adventitious root growth in 40 cm × 30 cm × 23 cm transfer pots containing 2.5 L quarter-strength modified Hoagland’s solution. The composition of the solution was as following: 1 mM MgSO4, 2.5 mM Ca (NO3)2, 0.2 μM CuSO4, 1 μM ZnSO4, 0.5 mM (NH4)2H2PO4, 1 μM MnSO4, 0.1 mM Fe Na EDTA, 20 μM H3BO3, 5 pM (NH4)6Mo7O24 and 2.5 mM K2SO4. Twelve seedlings were grown in each pot in the greenhouse. The solution was renewed every 48 h. The temperature in the greenhouse ranged from 20 to 25 °C with a photoperiod of 16 h and photosynthetically active radiation of 150 mmol m−2 s−1. After seven days, uniform seedlings were chosen to subject to different drought stress treatments produced by adding, in a single step, 0%, 5% and 10% PEG-6000 to the nutrient solution for 5 days. Every pot was repeated, and five pots were grown for each treatment.
At corresponding time points (0, 1, 3 and 5 days after drought stress), three sweet potato seedlings with uniform growth were collected and divided into roots, shoots and leaves. Thereafter, the samples were oven-dried, first at 105 °C for 30 min and then at 65 °C to a constant weight. In the meantime, fine roots or leaves (the 3rd fully expanded main-stem leaf from the top) were collected. In the laboratory, after rinsing with water, the samples were divided into two equal parts. The first half was stored in a refrigerator (−80 °C) for enzyme activities and gene expression analysis, while the second half was dried in an oven follow the above drying method for N assimilation compound analysis. The samples had three biological duplicates.
4.2. Leaf RWC and MSI
Leaf fresh sample (FW, 0.3 g) was placed into the distilled water for 4 h, and the weight was recorded as TW. Additionally, then the leaf was oven-dried at 80 °C to a constant dry weight (DW). Leaf RWC was calculated by:
RWC = (FW − DW)/(TW − DW) × 100%
Two leaf fresh samples (0.2 g) were mixed with distilled water (10 mL). One sample was placed at 40 °C for 30 min, and the solution conductivity (Ca) was determined after removal. The other blade was boiled in water for 10 min, and the conductivity (Cb) was determined. MSI was calculated by [41]:
MSI = (1 − Ca/Cb) × 100%
4.3. Chlorophyll, NO3−-N, NH4+-N and AA Content
Chlorophyll (Chl) was extracted from fresh leaf tissue collected using 50% acetone, mixed in ethanol (25 mL) for 1 day under dark conditions. Chl a, Chl b and Chl (a+b) were then analyzed Enzyme using a microplate reader at 663 and 645 nm [42]. Chl a = 12.21A663 nm − 2.81A645 nm, Chl b = 20.13A645 nm − 5.03A663 nm and Chl (a+b) = 20.20A645 nm − 8.05A663 nm.
NO3−-N and NH4+-N were extracted with distilled water (10 mL) from oven-dried samples (0.2 g) at 100 °C for 1 h to analyze NO3−-N and NH4+-N. For NO3−-N measurement, a 0.1 mL aliquot was taken and added to 5% salicylic acid–sulfuric acid (w/v), the absorption values were measured using a microplate reader at 410 nm [43]. For NH4+-N measurement, a 0.2 mL aliquot was taken and added to 1 mL reaction solution (2.625 g sodium hydroxide and 1.5 mL hypochlorous acid to a constant volume to 50 mL) and 1 mL chromogenic solution (12.5 mg sodium nitrite and 3 g phenol to constant volume to 50 mL), and then was placed in a water bath at 37 °C for 30 min [44]. The absorption values were measured using a microplate reader at 624 nm. In addition, AAs content was measured using acid ninhydrin [45,46].
4.4. Enzyme Extraction and Analysis
NR activity from root and leaf samples was determined according to previous studies [47]. Fresh tissue of leaves and roots (0.15 g) was ground with phosphate buffer (100 mM, pH 7.5, 2 mL). The activity of NR measured the light absorption of residual NO2− at 540 nm. The soluble protein (SP) content was analyzed by the G-250 regent using BSA as a standard [48].
GS, GDH and GOGAT were extracted in leaves and roots, as described previously according to previous research [47]. The activity of GS was analyzed using hydroxylamine as a substrate and the production of γ-glutamylhydroxamate was calculated with acidified FeCl3 [49]. The activities of GDH and GOGAT were measured by producing NADH oxidation using a microplate reader at 340 nm [47].
4.5. Transcriptional Level Analysis of Genes
Total RNA from control and treated roots and leaves were extracted with the DP441-50T RNAprep pure plant kit from Tiangen Biotech (Beijing, China) according to the manufacturer’s instructions. Total RNA (2 mg) was reverse-transcribed using a Prime Script RT reagent cDNA kit (Takara). Afterwards, the synthetic cDNA was used as a template for real-time PCR amplification. The primers of NRT1.1, NR, GS and GOGAT were synthesized by Sangon Biotechnology (Shanghai, China). The relative transcriptional levels were calculated using 2−ΔΔCT based on the transcriptional levels of a target gene versus GAPDH (a reference gene in sweet potato) [50,51]. Quantitative RT-PCR primers and internal standard primers were shown in Table S1.
4.6. Statistical Analysis
Data were shown as average value (±SE), and were analyzed using Origin 2018 and SPSS 23.0 with at least three replications. ANOVA was used to compare the drought response with different letters and/or * via the least significant difference (LSD) test (p < 0.05).
5. Conclusions
Based on these results, a scheme summarizing the effects of drought on primary metabolism in leaves and roots of the sweet potato was proposed (Figure 14). In summary, leaf RWC of sweet potato seedlings showed a decreasing trend after drought stress. The drought-treated plants revealed severe growth inhibition, compared with the 0% PEG-treated plants. The unaffected NH4+-N assimilation in sweet potato leaves was probably due to the improved NO3− uptake and reduced NO3−-N pathway in drought-treated leaves, mainly determined by NR activity. Both NH4+-N content, GS and GOGAT activity in X32 roots was reduced under drought stress, whereas NR activity remained increased by drought stress, consistent with NO3−-N content in roots. The low NH4+ to NO3− ratio in X32 roots was mainly attributed to NH4+ uptake reductions instead of NO3− uptake. However, prolonged drought time did not decline NH4+ content and GS activity in N1 roots, but drought stress obviously reduced the transcriptional levels of genes GS2 and GOGAT in N1 roots. Indeed, drought stress enhanced root NH4+ to NO3− ratio in N1. Therefore, GS and GOGAT determined the response to drought in roots.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/10/1341/s1, Table S1: Specific primers for gene amplification. Table S2: Changes of Pn and gs in sweet potato leaves at one day after PEG-drought stress.
Author Contributions
Conception and design of the research, J.L. and Z.L.; performed experiments, H.X., T.X., J.Z., and K.S.; statistical analysis, H.X., T.X. and J.Z.; drafting the manuscript, H.X. and J.Z.; revision of manuscript for important intellectual content, H.X., T.X., J.L. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key R&D Project (2018YFD1000704, 2018YFD1000700), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJB210012), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX20-2317), China Agriculture Research System (CARS-10-B3), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Natural Science Foundation by Jiangsu Normal University (16XLR047).
Acknowledgments
Xushu 32 seedlings were obtained from the Sweet Potato Research Institute, Xuzhou Academy of Agricultural Sciences, China, and Ningzishu 1 seedlings were obtained from the Institute of Food Crops/Provincial Key Laboratory of Agrobiology, Jiangsu Academy of Agricultural Sciences. The authors would like to thanks Zhonghou Tang and Yizhi Xie for all of their assistance in sweet potato seedlings.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, D.; Li, Q.; Cao, Q.; Niu, F.; Xie, Y.; Tang, J.; Li, H. Development and prospect of sweetpotato industry and its technologies in China. Jiangsu J. Agric. Sci. 2012, 28, 969–973. (In Chinese) [Google Scholar]
- Ruan, L.; Chen, L.; Chen, Y.; He, J.; Zhang, W.; Gao, Z.; Zhang, Y. Expression of Arabidopsis HOMEODOMAIN GLABROUS 11 enhances tolerance to drought stress in transgenic sweet potato plants. J. Plant Biol. 2012, 55, 151–158. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, F.; Si, Z.; Huo, J.; Xing, L.; An, Y.; He, S.; Liu, Q. A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 2016, 14, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Q.; Du, Z.H.; Xie, B.T.; Zhang, H.Y.; Zhang, L.M.; Zhang, W.L. Effects of film mulching on water content in soil and root formation of summer sweet potato seedlings. Shangdong Agric. Sci. 2014, 46, 41–45+51. (In Chinese) [Google Scholar]
- Jiang, Z.; Lv, Y. High yield cultivation technology of sweet potato. Rain Fed Crops 2010, 30, 225–226. (In Chinese) [Google Scholar]
- Chowdhury, S.R.; Singh, R.; Kundu, D.K.; Antony, E.; Thakur, A.K.; Verma, H.N. Growth, dry-matter partitioning and yield of sweet potato (Ipomoea batatas L.) as influenced by soil mechanical impedance and mineral nutrition under different irrigation regimes. Adv. Hortic. Sci. 2002, 16, 25–29. [Google Scholar]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Boschiero, B.N.; Mariano, E.; Azevedo, R.A.; Ocheuze Trivelin, P.C. Influence of nitrate–ammonium ratio on the growth, nutrition, and metabolism of sugarcane. Plant Physiol. Biochem. 2019, 139, 246–255. [Google Scholar] [CrossRef]
- Nacry, P.; Bouguyon, E.; Gojon, A. Nitrogen acquisition by roots: Physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 2013, 370, 1–29. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, C.; Su, L.; Li, Y.; Zhao, Z. Nitrogen uptake and metabolism of Populus simonii in response to PEG-induced drought stress. Environ. Exp. Bot. 2016, 123, 78–87. [Google Scholar] [CrossRef]
- Duan, J.F.; Tian, H.; Gao, Y.J. Expression of nitrogen transporter genes in roots of winter wheat (Triticum aestivum L.) in response to soil drought with contrasting nitrogen supplies. Crop Pasture Sci. 2016, 67, 128–136. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Huber, S.C. Posttranslational regulation of nitrate reductase in higher plants. Plant Physiol. 1994, 106, 817. [Google Scholar] [CrossRef] [PubMed]
- Amâncio, S.; Stulen, I. Nitrogen Acquisition and Assimilation in Higher Plants; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 3. [Google Scholar]
- Cao, H.; Wang, X.; Zou, Y.; Shu, H. Effects of exogenous nitric oxide on the several enzymes of nitrofen metabolism in Maius hupehensis (Pamp.) rehd seedings under water stress. Acta Hortic. Sin. 2009, 36, 781–786, (In Chinese with English Abstract). [Google Scholar]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhong, C.; Zhu, C.; Zhu, L.; Zhang, J.; Wu, L.; Jin, Q. Ammonium uptake and metabolism alleviate PEG-induced water stress in rice seedlings. Plant Physiol. Biochem. 2018, 132, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yuan, F.; Song, J.; Wang, B. Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Funct. Plant Biol. 2016, 43, 244–253. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Jin, Y.; Li, W.; Zhang, L. Effects of phosphorus application on photosynthetic carbon and nitrogen metabolism, water use efficiency and growth of dwarf bamboo (Fargesia rufa) subjected to water deficit. Plant Physiol. Biochem. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, S.X. Effects of application of N and K fertilizers on nitrogen metabolism of two genetype varieties of maize under water-stressed condition. Plant Nutr. Fertil. Sci. 2007, 13, 554–560. (In Chinese) [Google Scholar]
- Wang, R.; Gao, M.; Ji, S.; Wang, S.; Meng, Y.; Zhou, Z. Carbon allocation, osmotic adjustment, antioxidant capacity and growth in cotton under long-term soil drought during flowering and boll-forming period. Plant Physiol. Biochem. 2016, 107, 137. [Google Scholar] [CrossRef]
- Chilundo, M.N.G.; Joel, A.; Wesstrom, I.; Rui, B.; Messing, I. Effects of reduced irrigation dose and slow release fertiliser on nitrogen use efficiency and crop yield in a semi-arid loamy sand. Agric. Water Manag. 2016, 168, 68–77. [Google Scholar] [CrossRef]
- Villordon, A.Q.; Clark, C.A. Variation in virus symptom development and root architecture attributes at the onset of storage root initiation in ‘Beauregard’ sweetpotato plants grown with or without nitrogen. PLoS ONE 2014, 9, e107384. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, S.C.; Ji, C.Y.; Lee, J.J.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Diverse antioxidant enzyme levels in different sweetpotato root types during storage root formation. Plant Growth Regul. 2015, 75, 155–164. [Google Scholar] [CrossRef]
- Wang, B.; Zhai, H.; He, S.; Zhang, H.; Ren, Z.; Zhang, D.; Liu, Q. A vacuolar Na+/H+ antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweetpotato. Sci. Hortic. 2016, 201, 153–166. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, Z.; Tang, D.; Luo, K.; Lu, H.; Liu, Y.; Dong, J.; Wang, X.; Lv, C.; Wang, J.; et al. Comparative transcriptome analysis reveals critical function of sucrose metabolism related-enzymes in starch accumulation in the storage root of sweet potato. Front. Plant Sci. 2017, 8, 914. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xie, B.T.; Wang, B.Q.; Dong, S.X.; Duan, W.X.; Zhang, L.M. Evaluation of drought tolerance and screening for drought-tolerant indicators in sweetpotato cultivars. Acta Agron. Sin. 2019, 49, 419–430. [Google Scholar] [CrossRef]
- Cho, K.-S.; Han, E.-H.; Kwak, S.-S.; Cho, J.-H.; Im, J.-S.; Hong, S.-Y.; Sohn, H.-B.; Kim, Y.-H.; Lee, S.-W. Expressing the sweet potato orange gene in transgenic potato improves drought tolerance and marketable tuber production. C. R. Biol 2016, 339, 207–213. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Liu, Q.; Xiang, D. Effects of drought stress on root development and physiological charateristics of sweet potato at seeding stage. Chin. J. Appl. Ecol. 2019, 30, 3155–3163. [Google Scholar]
- Yu, Y.; Xu, T.; Li, X.; Tang, J.; Ma, D.; Li, Z.; Sun, J. NaCl-induced changes of ion homeostasis and nitrogen metabolism in two sweet potato (Ipomoea batatas L.) cultivars exhibit different salt tolerance at adventitious root stage. Environ. Exp. Bot. 2016, 129, 23–36. [Google Scholar] [CrossRef]
- Ming, D.F.; Pei, Z.F.; Naeem, M.S.; Gong, H.J.; Zhou, W.J. Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J. Agron. Crop. Sci. 2012, 198, 14–26. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.Q.; Xia, H.Q.; Gao, Y.; Tang, Z.H.; Zhang, J.Y.; Li, Z.Y.; Liu, J.R. Effects of soil drought stress on photosynthesis and yield of sweet potato. J. Jiangsu Norm. Univ. 2019, 37, 21–25. (In Chinese) [Google Scholar]
- Zahoor, R.; Zhao, W.; Abid, M.; Dong, H.; Zhou, Z. Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Li, M.J.; Zhou, K.; Sun, T.T.; Hu, L.Y.; Li, C.Y.; Ma, F.W. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Obidiegwu, J.; Bryan, G.; Jones, H.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dugo, V.; Durand, J.-L.; Gastal, F. Water deficit and nitrogen nutrition of crops. A review. Agron. Sustain. Dev. 2010, 30, 529–544. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.d.M.; Ríos, J.J.; Blasco, B.; Rosales, M.Á.; Melgarejo, R.; Romero, L.; Ruiz, J.M. Ammonia production and assimilation: Its importance as a tolerance mechanism during moderate water deficit in tomato plants. J. Plant Physiol. 2011, 168, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Azedo-Silva, J.; Osorio, J.; Fonseca, F.; Correia, M.J. Effects of soil drying and subsequent re-watering on the activity of nitrate reductase in roots and leaves of Helianthus annuus. Funct. Plant Biol. 2004, 31, 611–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nie, L.L.; Feng, J.J.; Fan, P.X.; Chen, X.Y.; Guo, J.; Lv, S.L.; Bao, H.; Jia, W.T.; Tai, F.; Jiang, P.; et al. Comparative proteomics of root plasma membrane proteins reveals the involvement of calcium signalling in NaCl-facilitated nitrate uptake in Salicornia europaea. J. Exp. Bot. 2015, 66, 4497–4510. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Ding, L.; Shen, Q.; Guo, S. Ammonium enhances the tolerance of rice seedlings (Oryza sativa L.) to drought condition. Agric. Water Manag. 2009, 96, 1746–1750. [Google Scholar] [CrossRef]
- Sairam, R.K.; Shukla, D.S.; Saxena, D.C. Stress induced injury and antioxidant enzymes in relation to drought tolerance in wheat genotypes. Biol. Plant. 1997, 40, 357–364. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M.; Romero, L. Relationship between potassium fertilisation and nitrate assimilation in leaves and fruits of cucumber (Cucumis sativus) plants. Ann. Appl. Biol. 2002, 140, 241–245. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Rosen, H. A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 1957, 67, 10–15. [Google Scholar] [CrossRef]
- Liu, J.; Meng, Y.; Lv, F.; Chen, J.; Ma, Y.; Wang, Y.; Chen, B.; Zhang, L.; Zhou, Z. Photosynthetic characteristics of the subtending leaf of cotton boll at different fruiting branch nodes and their relationships with lint yield and fiber quality. Front. Plant Sci. 2015, 6, 747. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, W.; Yang, J.; Oosterhuis, D.M.; Loka, D.A.; Zhou, Z. Relationship between potassium fertilization and nitrogen metabolism in the leaf subtending the cotton (Gossypium hirsutum L.) boll during the boll development stage. Plant Physiol. Biochem. 2016, 101, 113–123. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Singh, N.K.; Cherry, J.H.; Locy, R.D. Nitrate uptake and utilization is modulated by exogenous gamma-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol. Biochem. 2010, 48, 443–450. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, Y.H.; Ji, C.Y.; Park, S.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Stable internal reference genes for the normalization of Real-time PCR in different sweetpotato cultivars subjected to abiotic stress conditions. PLoS ONE 2012, 7, 9. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).