Plant Triterpenoid Crosstalk: The Interaction of Brassinosteroids and Phytoecdysteroids in Lepidium sativum
Abstract
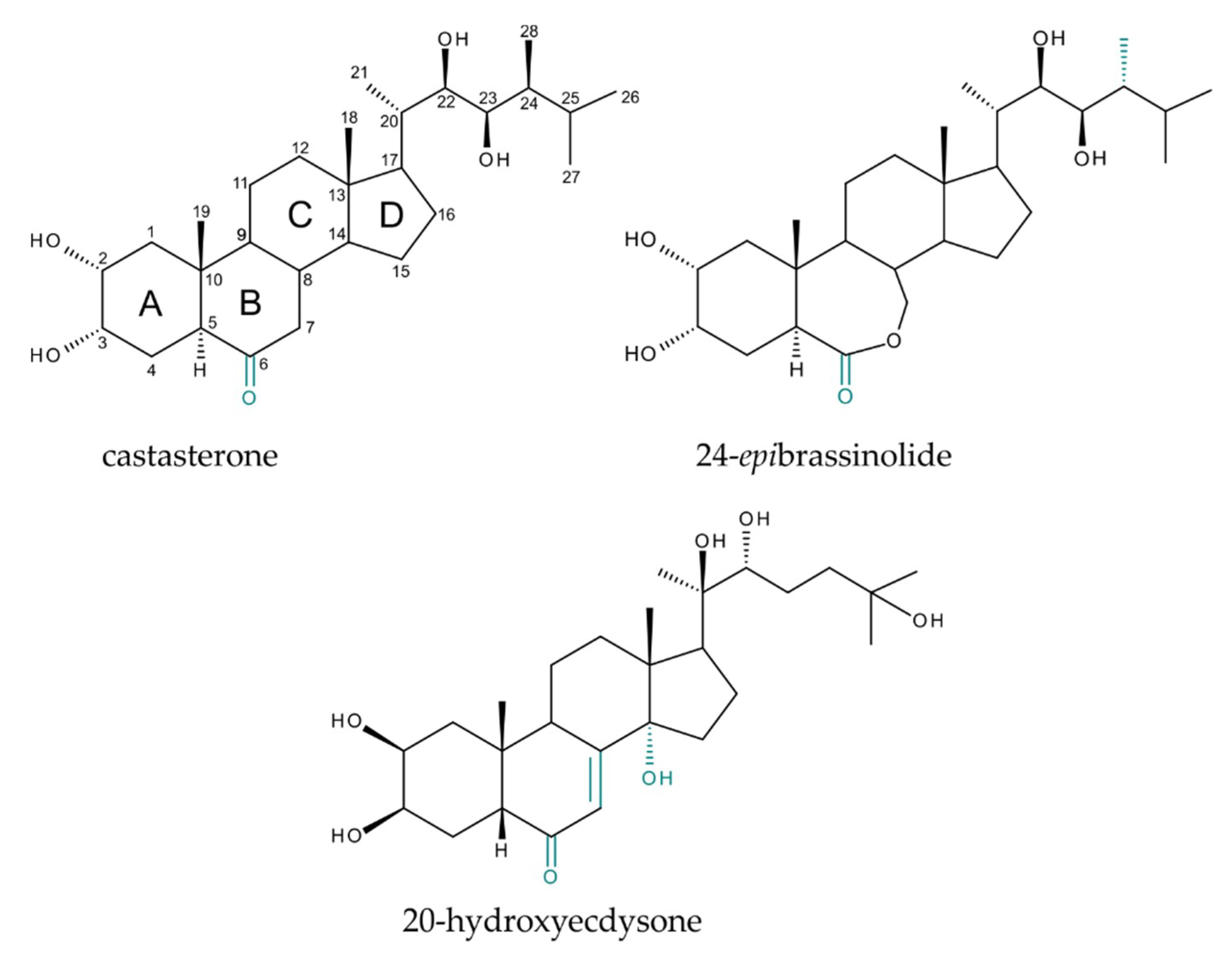
1. Introduction
2. Results and Discussion
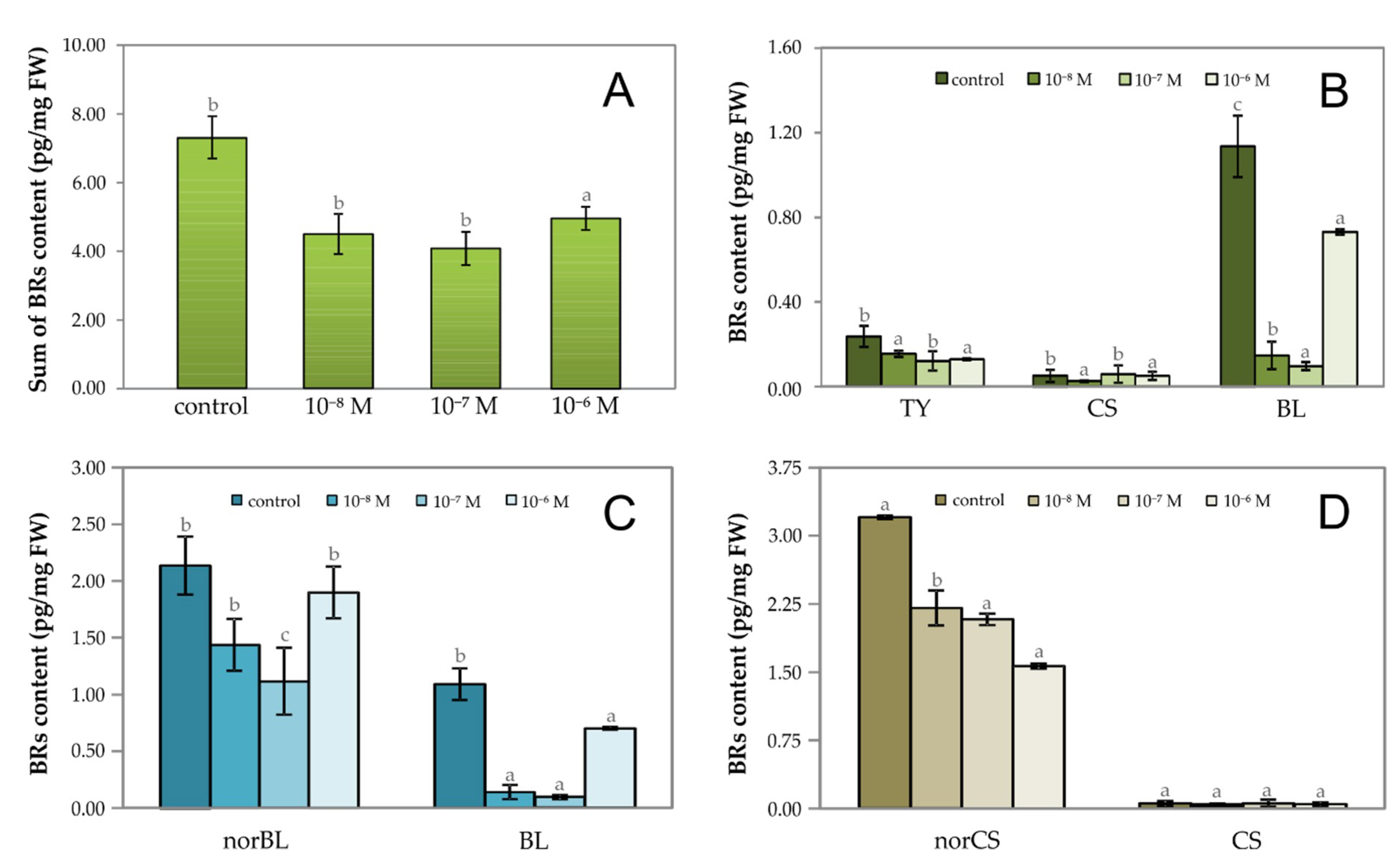
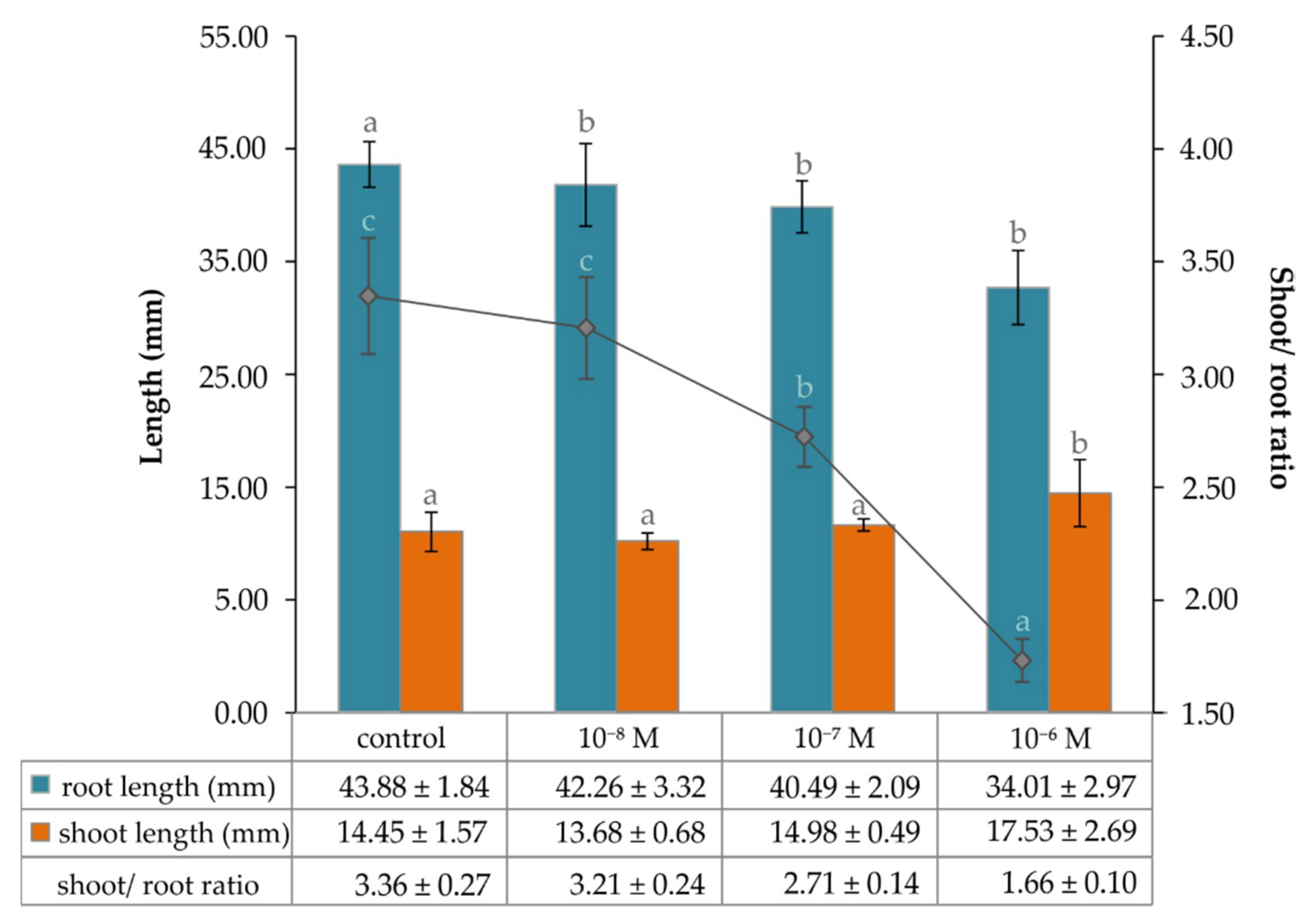
2.1. The Effect of Exogenous Phytoecdysteroid on Growth of Lepidium Seedlings and the Level of Endogenous Brassinosteroids
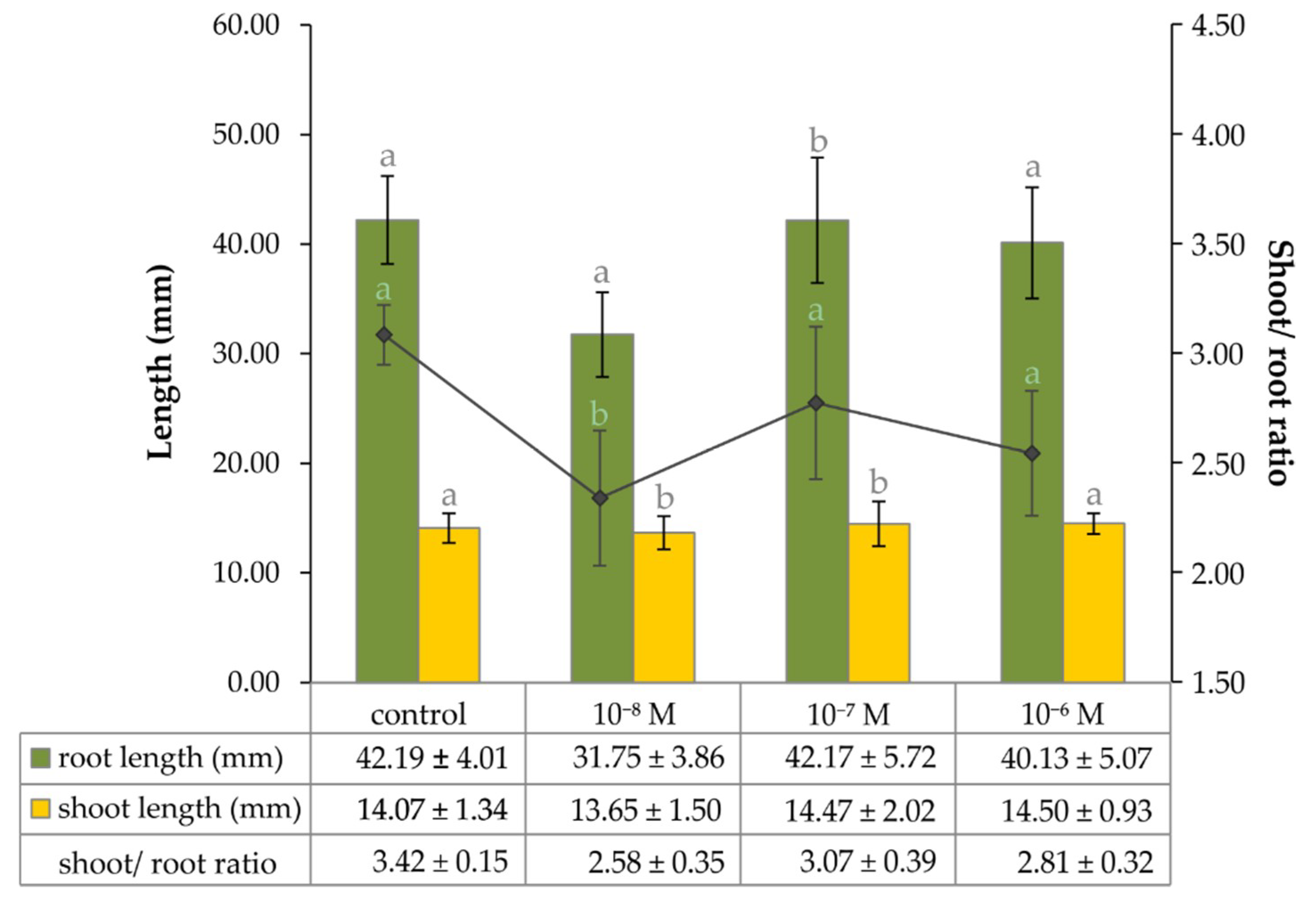
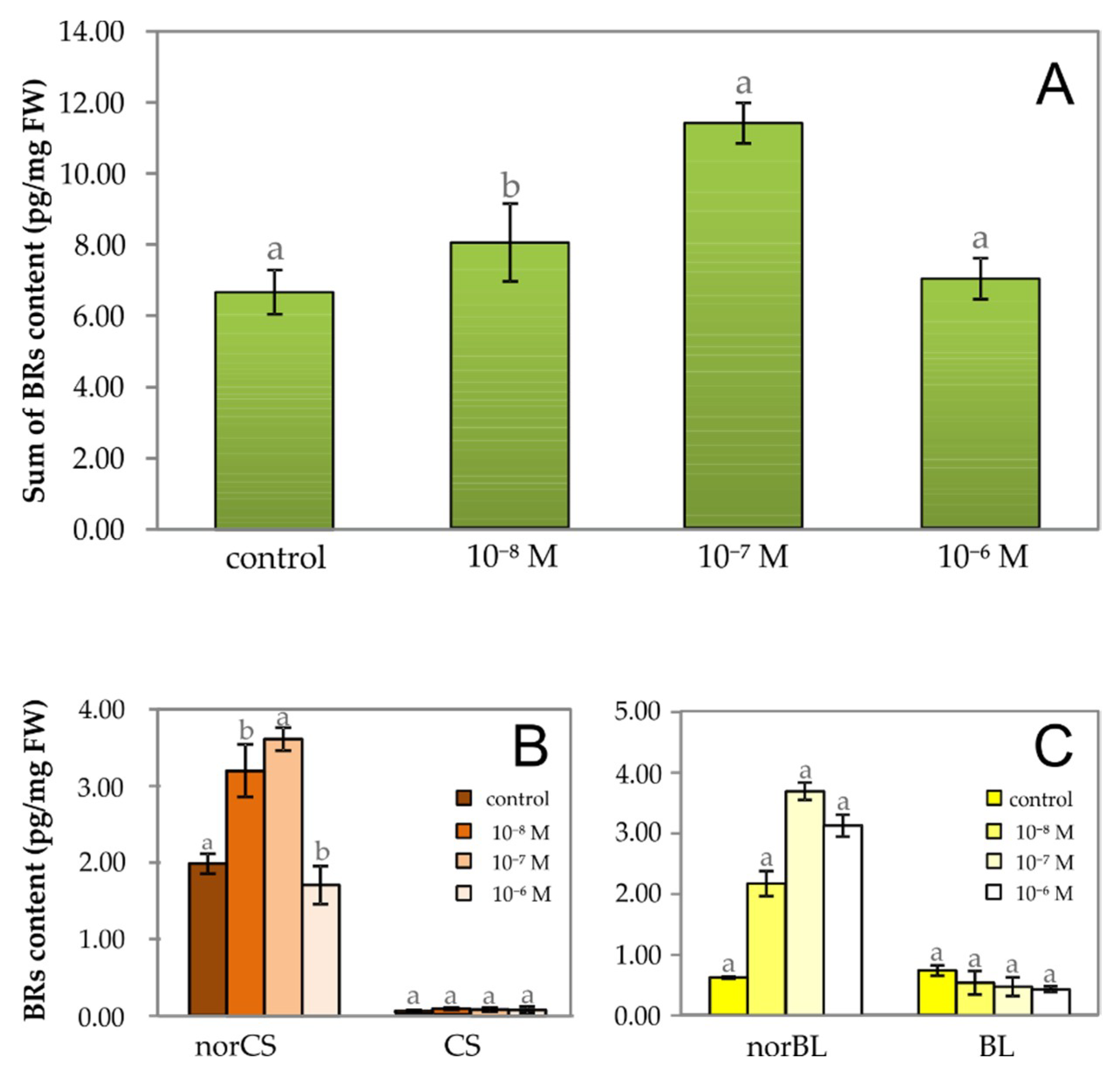
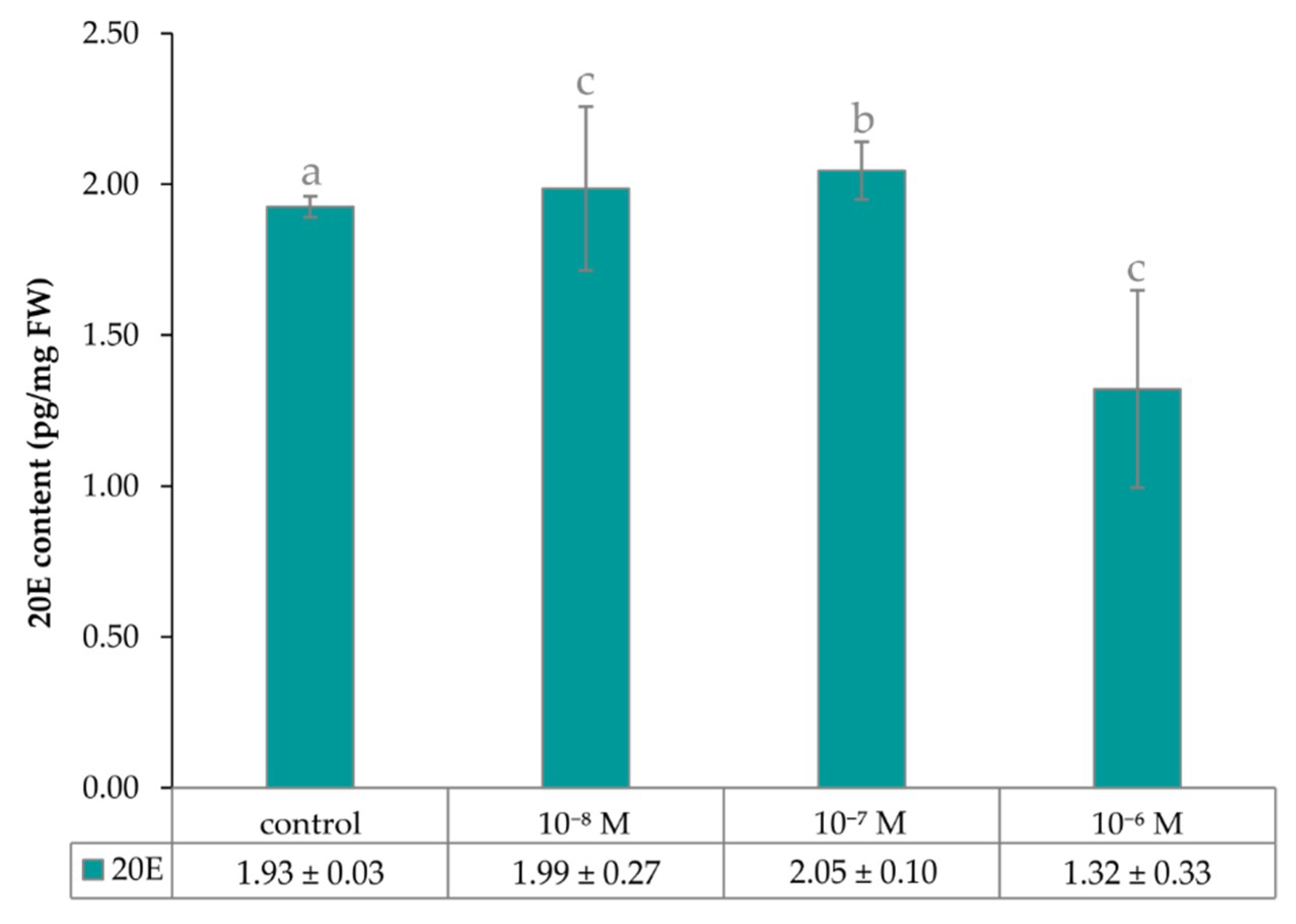
2.2. The Effect of Exogenous Brassinosteroid on Growth of Lepidium Seedlings and the Level of Endogenous Phytoecdysteroid
3. Material and Methods
3.1. Plant Material, Growing, and Cultivation Conditions
3.2. Sample Preparation and Analysis of Brassinosteroids by Ultra-High-Performance Liquid Chromatography–Electrospray Tandem Mass Spectrometry (UHPLC–ESI–MS/MS)
3.3. Sample Preparation and Analysis of Phytoecdysteroids by Ultra-High-Performance Liquid Chromatography–Electrospray Tandem Mass Spectrometry (UHPLC–ESI–MS/MS)
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies, P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. In Plant Hormones, Biosynthesis, Signal Transduction, Action! Davies, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1–15. [Google Scholar]
- Clouse, S.D. Brassinosteroids. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2011. [Google Scholar]
- Lafont, R.; Bouthier, A.; Wilson, I.D. Phytoecdysteroids: Structures, occurrence, biosynthseis and possible ecological significance. In Insect Chemical Ecology; Hrdy, I., Ed.; Academia: Prague, Czech Republic, 1991; pp. 197–214. [Google Scholar]
- Mandava, N.B. Plant growth-promoting brassinosteroids. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 23–52. [Google Scholar] [CrossRef]
- Watanabe, B. Structure-activity relationship studies of insect and plant steroid hormones. J. Pestic. Sci. 2015, 40, 146–151. [Google Scholar] [CrossRef]
- Caño-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-García, S.; Cheng, J.-C.; nam, K.H.; Li, J.M.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Sakurai, A. Brassinosteroids. Nat. Prod. Rep. 1997, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, R.; Horn, D.H.S. Distribution and Role of Insect Hormones in Plants. Endocrinology of Insects; A. R. Liss Inc.: New York, NY, USA, 1983; pp. 627–654. [Google Scholar]
- Kubo, I.; Hanke, F.J. Chemical methods for isolating and identifying phytochemicals biologically active in insects. In Insect Plant Interactions; Miller, J.R., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. 225–249. [Google Scholar]
- Dreier, S.I.; Towers, G.H.N. Activity of ecdysterone in selected plant growth bioassays. J. Plant Physiol. 1988, 132, 509–512. [Google Scholar] [CrossRef]
- Macháčková, I.; Vágner, M.; Sláma, K. Comparison between the effects of 20-hydroxyecdysone and phytohormones on growth and development in plants. Eur. J. Entomol. 1995, 92, 309–316. [Google Scholar]
- Rothová, O.; Holá, D.; Kočová, M.; Tůmová, L.; Hnilička, F.; Hniličková, H.; Kamlar, M.; Macek, T. 24-Epibrassinolide and 20-hydroxyecdysone affect photosynthesis differently in maize and spinach. Steroids 2014, 85, 44–57. [Google Scholar] [CrossRef]
- Kamlar, M.; Rothova, O.; Salajkova, S.; Tarkowska, D.; Drasar, P.; Kocova, M.; Harmatha, J.; Hola, D.; Kohout, L.; Macek, T. The effect of exogenous 24-epibrassinolide on the ecdysteroid content in the leaves of Spinacia oleracea L. Steroids 2015, 97, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tarkowská, D.; Novák, O.; Oklestkova, J.; Strnad, M. The determination of 22 natural brassinosteroids in a minute sample of plant tissue by UHPLC–ESI–MS/MS. Anal. Bioanal. Chem. 2016, 408, 6799–6812. [Google Scholar] [CrossRef]
- Lehmann, M.; Vorbrodt, H.-M.; Adam, G.; Koolman, J. Antiecdysteroid activity of brassinosteroids. Experientia 1988, 44, 355–356. [Google Scholar] [CrossRef]
- Voight, B.; Whiting, P.; Dinan, L. The ecdysteroid agonist/antagonist and brassinosteroid-like activities of synthetic brassinosteroid/ecdysteroid hybrid molecules. Cell. Mol. Life Sci. 2001, 58, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Rittenberg, D.; Foster, G.L. A new procedure for quantitative analysis by isotope dilution, with application to the determination of amino acids and fatty acids. J. Biol. Chem. 1940, 133, 737–744. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarkowská, D.; Krampolová, E.; Strnad, M. Plant Triterpenoid Crosstalk: The Interaction of Brassinosteroids and Phytoecdysteroids in Lepidium sativum. Plants 2020, 9, 1325. https://doi.org/10.3390/plants9101325
Tarkowská D, Krampolová E, Strnad M. Plant Triterpenoid Crosstalk: The Interaction of Brassinosteroids and Phytoecdysteroids in Lepidium sativum. Plants. 2020; 9(10):1325. https://doi.org/10.3390/plants9101325
Chicago/Turabian StyleTarkowská, Danuše, Eliška Krampolová, and Miroslav Strnad. 2020. "Plant Triterpenoid Crosstalk: The Interaction of Brassinosteroids and Phytoecdysteroids in Lepidium sativum" Plants 9, no. 10: 1325. https://doi.org/10.3390/plants9101325
APA StyleTarkowská, D., Krampolová, E., & Strnad, M. (2020). Plant Triterpenoid Crosstalk: The Interaction of Brassinosteroids and Phytoecdysteroids in Lepidium sativum. Plants, 9(10), 1325. https://doi.org/10.3390/plants9101325





