1. Introduction
Miscanthus sacchariflorus (Maxim.) Hack, a perennial herbaceous plant belonging to the Poaceae family, has been recognized as a potential bioenergy crop [
1,
2,
3].
M. sacchariflorus grows primarily in the temperate regions of Asia and eastern Russia [
4,
5] at various altitudes, though not above 2000 m [
3,
6,
7,
8]. Recently,
M. sacchariflorus has been indicated as a possible bioenergy crop due to its potential to outperform other bioenergy crops with regard to annual biomass production and its ability to grow in a wide range of environments with minimal inputs [
2,
3,
7,
8,
9].
M. sacchariflorus competes poorly with weeds during its initial years of establishment, with significant negative effects to its growth and biomass due to deprivation of nutrients, space, light, and moisture [
10]. Comparatively, most weeds have excellent adaptive features and can germinate in diverse climatic conditions [
11]. Most of these weeds have a shorter dormancy period and prolific seed production, producing intense competition for resources with crops in cultivated fields. For example,
Bidens frondosa and
Digitaria sp. produce a large quantity of seeds, which are easily dispersed over large distances and possess the potential to germinate in diverse climatic conditions [
12,
13,
14].
Chenopodium album, a fast-growing broad-leaved weed, has been reported to be relatively insensitive or resistant to herbicides such as triazine [
15,
16]. Barnyard grass, another vigorously growing weed, can produce over one million viable seeds per plant [
17,
18] and is resistant to multiple herbicides including chloroacetamide (butachlor) and acetanilide (propanil); propagation of this grass causes significant losses in crop yields every year [
19,
20]. Moreover, a number of previous studies have reported that the allelochemicals released by these weeds to the surrounding environment can directly or indirectly inhibit the biomass production and yield of cultivated crops [
18,
21]. For example,
Erigeron canndensis presents strong competition to food crops in terms of growth and also produces phytotoxic chemicals that can suppress the growth of cultivated crops [
22,
23,
24,
25]. Earlier studies have indicated that the allelopathic properties of weeds play an important role in the invasive and adaptative capacities of perennial broad-leaved and grass weeds in cultivated fields [
26]. Miscanthus is considered as an invasive bio energy crops [
27]. Invasive plants produce phytochemical with strong allelopathic properties to suppress co-occurring plant species [
28,
29]. However, the effects of
M. sacchariflorus on weed germination have not been documented yet.
Indiscriminate use of herbicides for the control of weeds in order to increase cultivated plant growth and food production has yielded negative effects to non-targeted organisms, including human beings [
30]. Consumption of potentially toxic chemical residues can trigger a wide range of effects on human health, including cancers, genetic disorders, endocrine disruption, and neurological problems [
31]. Moreover, some studies have indicated that the improper and excessive use of herbicides is causing contamination of soil, air, water, and food resources. Herbicides used in the field can contaminate water resources due to soil runoff or leaching and cause genetic damage and physiological effects [
32,
33,
34,
35,
36]. Mechanical weed control, on the other hand, is not only damaging to the root systems of cultivated plants, but also labor-intensive and time-consuming. Novel methods of controlling weeds are therefore, urgently needed to meet the demands of modern agriculture. As compared to conventional herbicides, those derived from natural products or their analogs have the potential to be safer and more environmentally friendly.
Many plant species produce phytochemicals that enable them to inhibit or suppress the germination or growth of other plants [
37,
38]. These biochemicals are also called allelochemicals and generally constitute phenolic compounds, saponins, terpenes, steroids, alkaloids, and quinones [
39]. These compounds accumulate in the soil through plant residual decomposition, root exudation, and shoot leaching processes [
40,
41,
42]. Various studies have reported the isolation of single allelopathic compounds and their application for the development of herbicides [
43,
44,
45,
46]. Moreover, a number of allelochemicals from potentially allelopathic plants species have already been isolated and have proven to successfully inhibit weed germination and growth [
47], making them environmentally friendly compared to chemical herbicides [
48]. Most of these allelochemicals are beneficial to the soil, as they improve the nutrient concentration and enhance the microbial activity of the soil [
49,
50]. Moreover, most of these allelo-compounds are partially or completely soluble in water, which simplifies their application even in the absence of surfactants [
51]. Dayan et al. [
52] argued that the absence of halogenated substitutes, the lower concentrations of allelopathins necessary for activity, and the higher specific properties due to diversity in the allelopathins make these compounds more environmentally friendly than chemical herbicides [
53]. Accordingly, scientific interest in controlling weeds through allelochemicals has recently increased. Studies have reported that the allelochemical components of some plant species effectively inhibit the germination and growth of weeds, which are effects primarily attributed to the phenolic compounds present in the sample extracts [
47,
54]. However, no study has yet been conducted to evaluate allelopathic weed management systems for
M. sacchariflorus under field conditions.
Therefore, the primary objective of the present study was to investigate the possible allelopathic effects of M. sacchariflorus extracts on the seed germination and growth of selected weed species. Further, we documented the major allelochemicals present in M. sacchariflorus leaf extracts using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS).
4. Materials and Methods
4.1. Chemicals
Analytical standards used in this study were of analytical grade. All analytical standards were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The methanol used for the extraction process was purchased from J.T. Baker (Phillipsburg, NJ, USA).
4.2. Plant Material
M. sacchariflorus used in this study was cultivated in the experimental fields of Kangwon National University, at Chuncheon, Kangwon-Do, South Korea (37°56′09.96″ N; 127°46′55.21″ E) at an average altitude of 100 m. All of the experimental plots were arranged in a completely randomized block design. The experimental plots consisted of ten longitudinal rows separated by 1.5 m. Each plot consisted of 1.5 m2, with one M. sacchariflorus plant placed in each plot. The temperature of the cultivated field ranged from −27.9 °C (January) to 39.5 °C (August). The soil of the experimental field had a sandy loam texture. The M. sacchariflorus experimental field was irrigated during the initial year of establishment on a regular basis. The research field was located in a temperate monsoon climate, with a wet and humid summer. M. sacchariflorus plants were harvested at maturity from the cultivated field and chopped into small pieces (about 4 cm) with an electric cutter and dried at room temperature for 48 h. The dried, chopped pieces of M. sacchariflorus were applied to each plot after one week of cultivation. Absence of M. sacchariflorus mulch was used as a control.
4.3. Effect of M. sacchariflorus Mulch on the Biomass of Weeds
The fresh weight and dry weight of the weeds were measured at 70 days after preparation of the experimental field. Weeds grown in the experimental field in both the treated and control plots were collected from 1-m2 quadrats from each plot. The fresh weight of the weeds was recorded immediately after uprooting the weeds, with a digital balance. Dry weight of the weeds was recorded after drying in an oven at 70 °C for 48 h.
4.4. Preparation of Plant Extracts
To prepare the aqueous extracts, the leaves of M. sacchariflorus were collected from field at maturity. The sample was dried at room temperature for 48 h. The dried sample was ground in a blender. The powder sample (500 g) was mixed with one liter distilled water and the homogenate was kept in room temperature (25 °C) for 24 h. The mixture was filtered using Buchner funnel and filter paper to separate the debris. The obtained aqueous extracts were used for assessing allelopathic effects on weeds.
Allelochemical compounds present in the M. sacchariflorus extracts was quantified using an LC-MS/MS system. The fresh leaf samples of M. sacchariflorus were collected from the growing field and dried at 25 °C for 24 h, then ground into powder. Dried plant samples were weighed (1 g) and dissolved in 20 mL of 80% methanol for two days at room temperature (25 °C). The extract solutions were filtered through No. 1 Whatman filters and the filtrate was gathered, following which the methanol was evaporated under reduced pressure using a rotary evaporator at 41 °C (Eyela, SB-1300, Shanghai Eyela Co. Ltd. Shanghai, China). The obtained crude methanolic extracts were stored at −20 °C for further use. On the experiment day, the plant extracts were re-dissolved in 80% HPLC graded methanol (0.01 mL).
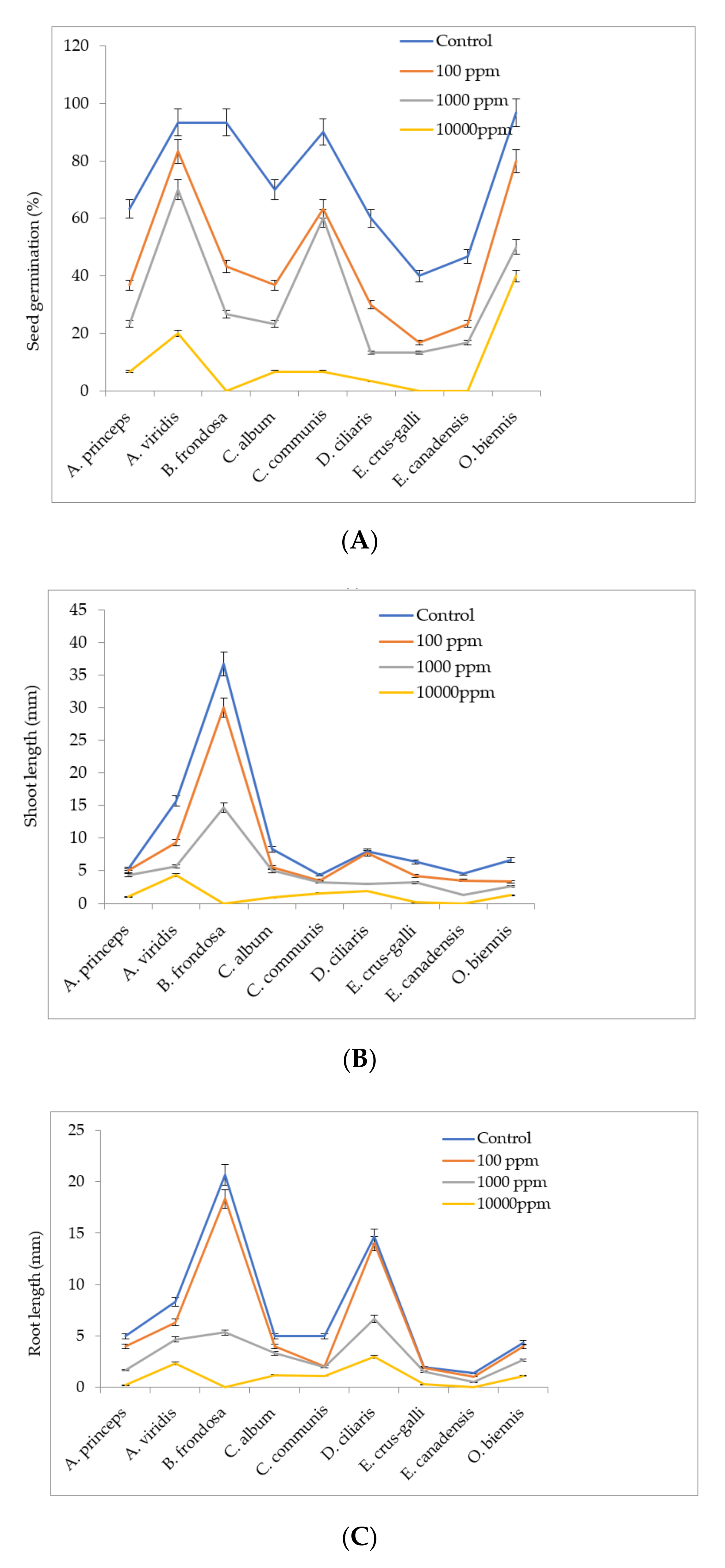
4.5. Effect of M. sacchariflorus Extracts and Allelopathic Compounds on Seed Germination
Seeds of the nine most prominent weeds found in the M. sacchariflorus field were collected in November 2018 and 2019 from the experimental fields. The harvested seeds were cleaned and separated from their pods, air-dried at room temperature for a week, and then stored in darkness at 4 °C until further use. Healthy seeds of uniform size were selected and soaked for 24 h in distilled water to remove dirt and germination-inhibiting compounds from the surface of the seeds. Initially, 5 mL of M. sacchariflorus methanolic extracts (100 ppm to 1000 ppm) was placed in a sterilized 9 cm diameter petri dishes lined with a double layer of filter papers (No. 1 Whatman) and evaporated to dryness for 12 h at 26 °C. After evaporation, 3 mL of distilled water were added onto the filter paper. Twenty seeds from each weed species were placed on filter papers. Sterile deionized water was used as a control. Three replicates of each treatment were performed and all treatments were maintained at 25 ± 1 °C for 40 days in a growth chamber with an 8:16 light and dark cycle. The effects of the methanolic extracts of M. sacchariflorus on weed seed germination and seedling growth (shoot length, root length) were measured after one week of incubation.
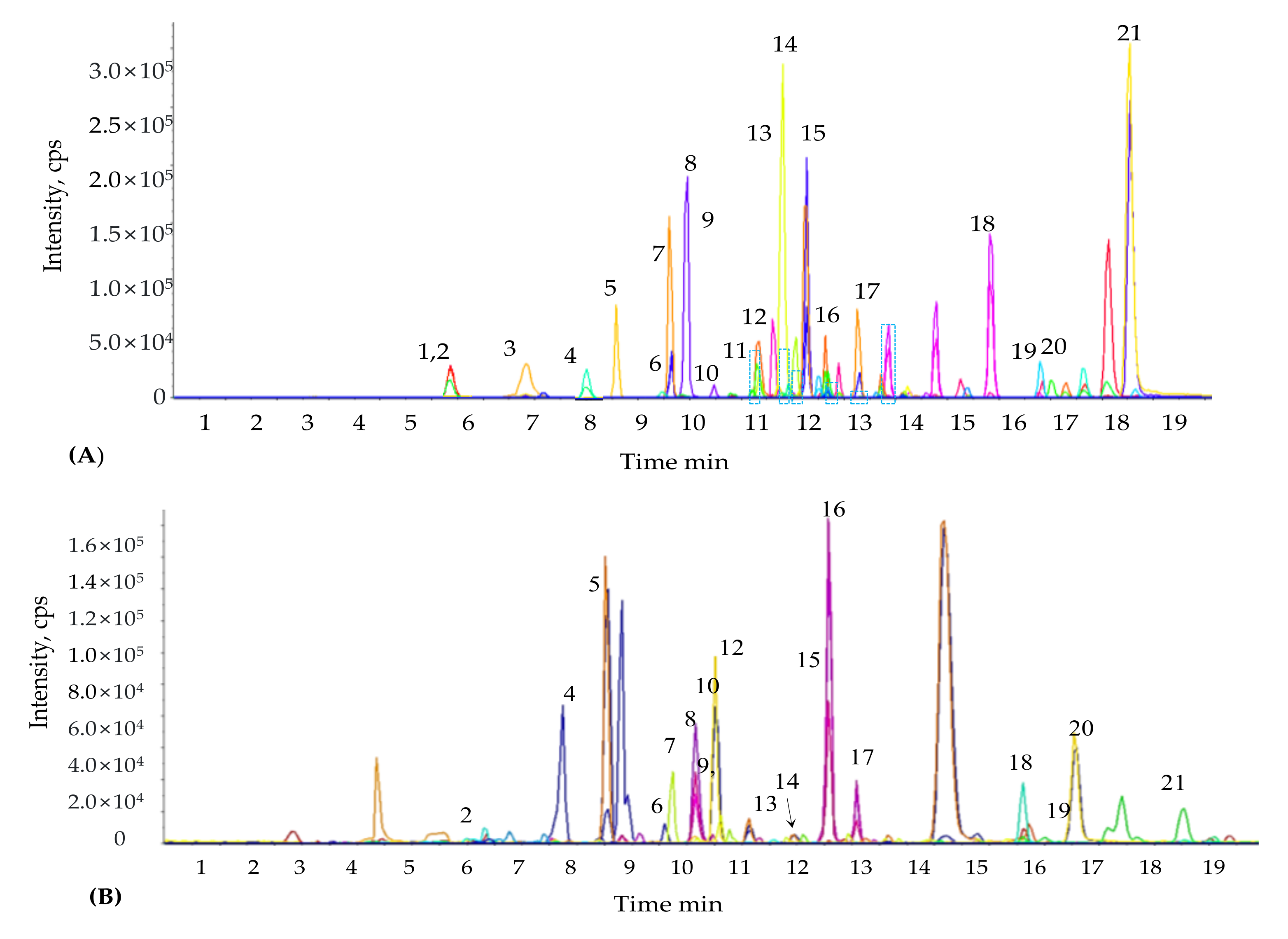
4.6. Estimation of Phenolic Compounds by Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (LC-MS/MS)
The concentration and composition of phenolic compounds were identified in
M. sacchariflorus using a liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) system, as described previously [
111]. Pumps (Agilent 1200, Agilent Technologies, Palo Alto, CA, USA) and an autosampler (Agilent 1100 series, Agilent Technologies, Palo Alto, CA, USA) were coupled to an API 2000 series mass spectrometer (Applied Biosystems, ON, Canada) integrated to the LC system. The chromatographic separation of phenolic compounds was performed using a C18 column (4.6 × 250 mm, 5 μm). The mass spectrometer was used in negative ion mode. The following parameters were used to determine the phenolic compounds present in the samples: nebulizer gas pressure, drying gas pressure, collision gas pressure, and curtain gas pressure set at 40, 70, 2, and 20 psi, respectively; drying gas temperature and capillary voltage were set at 350 °C and 4.5 kV, respectively. The mobile phase comprised 0.1% formic acid (HCOOH) (
v/v) in water (solution A) and acetonitrile (C
2H
3N) in water (solution B). The following mobile phase gradient elution programs were performed to efficiently separate the compounds: 10–40% B for 0–10 min; 40–50% B for 10–20 min; 50–100% B for 20–25 min; 100–10% B for 25–26 min; and 10% B for 26–30 min. During the experiment, the temperature of the column was set at 25 °C. The sample injection volume was 10 µL. The mobile phase was eluted at a constant flow rate of 0.7 mL min
−1. Mass-spectrometry data were recorded in multiple reactions monitoring (MRM) mode. Different mass spectrometric parameters such as entrance potential (EP), collision energy (CE), declustering potential (DP), cell entrance potential (CEP), and collision cell exit potential (CXP) were determined for each MRM transition that was monitored. Analysis of allelochemicals for each sample was performed in triplicate.
4.7. Effect of M. sacchariflorus Extracts on Photosynthetic Pigment Contents
To extract chlorophyll a, chlorophyll b, and carotenoids, 0.5 g fresh leaf samples of weeds from the treated and control plots were taken and homogenized with 10 mL of 80% acetone. Homogenized samples were centrifuged for 4000 rpm for 10 min at 4 °C. The supernatants were separated from the mixture and collected in a cuvette for further use. To measure the chlorophyll a, chlorophyll b, and carotenoid contents, the spectrophotometric method proposed by Lichtentaler and Wellburn [
112] was employed using a Shimadju UV-VS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The concentrations of photosynthetic pigments present in the extracts were estimated using the following equations:
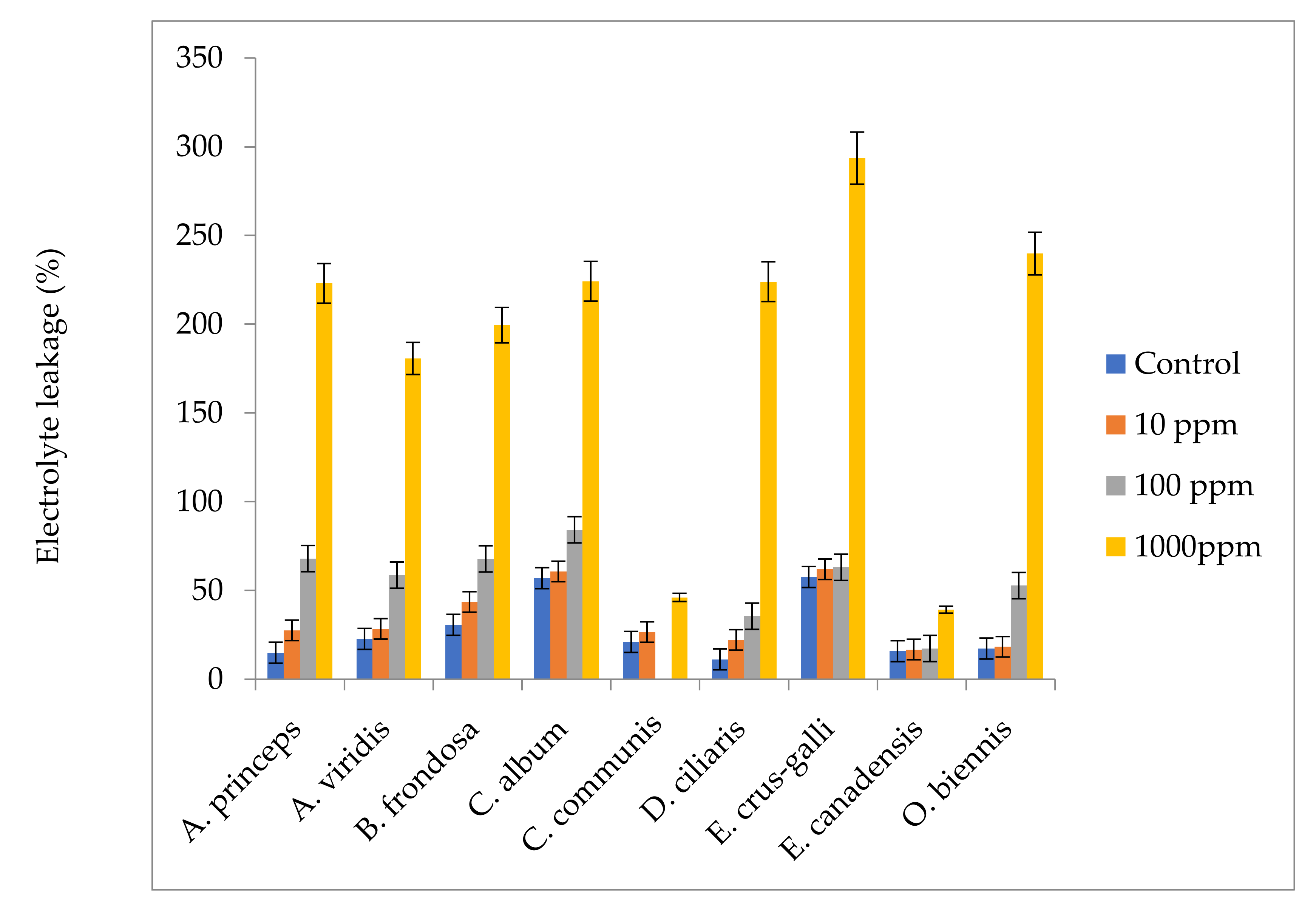
4.8. Effect of M. sacchariflorus Extracts on Electrolyte Leakage
Leaf discs (2 g) from fresh young leaves were cut into 2 cm segments and rinsed in deionized water, then placed in petri dishes containing 20 mL of distilled water for 24 h at 25 °C. The samples were then washed in an orbital shaker (Orbit Shaker, Lab-Line, Dubuque, USA) at room temperature. The initial electrical conductivity (EC0) of the samples was measured at 15 min intervals using a digital conductometer (Digimed DM-3, Digicron, Sao Paulo, Brazil) [
113]. The petri dishes containing cut samples were placed in a boiling water bath for 30 min and then cooled at 25 °C. The electrical conductivity of the cooled samples was then measured (ECf). Electrolyte leakage (EL) was determined according to the following equation: EL = (ECf−EC0), where ECf is the electrical conductivity at the final time and EC0 is the initial electrical conductivity.
4.9. Antioxidant Enzyme Assay
For superoxide dismutase (SOD) activity, fresh seedling leaves (1 g) of treated and non-treated (control) weeds were homogenized in a mortar and pestle using liquid nitrogen in 5 mL of extraction buffer (20 mM Tris-HCl in 1% polyvinylpyrrolidone, pH 7.4) following the method described by Beauchamp and Fridovich [
114]. The extracted solution was filtered to remove the debris, and the homogenate was centrifuged at 10,000
g for 30 min at 4 °C. The supernatant was separated from the solution and then used for antioxidant assays. An initial reaction solution was prepared by mixing 50 mM phosphate buffer (pH 7.8), 75 μM nitroblue tetrazolium, 13 mM methionine, 100 nM EDTA, 2 μM riboflavin, and dd H
2O. After two minutes, 50 μL of the enzyme extract was added. The absorbance value of the reaction mixture was then recorded at 560 nm. One unit of SOD activity (U) was defined as the quantity of enzyme required for 50% inhibition of the initial reaction rate.
Ascorbate peroxidase (APX) activity was estimated as described by Nakano and Asada [
115]. Initially, 50 mM potassium phosphate (pH 7.0), 0.5 mM ascorbic acid (C
6H
8O
6), 2% hydrogen peroxide (H
2O
2), 0.2 mM Ethylenediaminetetraacetic acid (EDTA), and 0.1 mL enzyme extract were mixed to a final volume of 3 mL. The absorbance value of the mixture was measured via Shimadju UV-VS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) at 290 nm. The quantity of ascorbate oxidized was calculated using the extinction coefficient (2.8 mM
−1 cm
−1). APX was defined as 1 mmol mL
−1 per min at 25 °C cm
−1). One unit of ascorbate oxidized as 1 mmol mL
−1 ascorbate oxidized per min at 25 °C.
Catalase (CAT) assay indicates the variation in the quantity of H
2O
2 due to the presence of CAT in the test samples, indicating the potency of the activator/inhibitors under assessment. The catalase (CAT) assay was conducted following the methods described by Aebi [
116]. Initially, various extracts with different concentrations were added to the 4 uL of plant extracts in 67 mM sodium potassium phosphate at pH 7.4. The mixture was further incubated for 10 min at 37 °C. Then, 1.2 mL of H
2O
2 (0.6%) was added to the mixture and the change in absorbance was assessed at 240 nm for 2 min. Sodium azide was used as a positive control.
Polyphenol oxidase activity (PPO) was determined following the method described by Saeidian [
117]. The reaction mixture consisted of 100 mM phosphate buffer (pH 7.0), 1 mL (0.1 M) catechol, and 0.5 mL of enzyme extracts mixed to a final volume of 3 mL. The mixture was incubated at room temperature for five minutes. The PPO activity assay was carried out using a Shimadju UV-VS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) at 420 nm.
4.10. Statistical Analysis
The data shown represent the mean ± SD. The data were statistically evaluated using analysis of variance (ANOVA) and significant differences between the means were assessed using Duncan’s multiple range tests at significance levels of p < 0.05 and p < 0.01. Interrelationships among seed germination and growth, pigment contents, phenolic compounds, electrolyte ion leakage, and antioxidant enzyme activities were determined by Pearson’s correlation coefficient using SPSS version 20 (SPSS, 2011).