Surface Characterization, Antimicrobial Activity, and Biocompatibility of Autopolymerizing Acrylic Resins Coated with Reynoutria elliptica Extract
Abstract
1. Introduction
2. Results
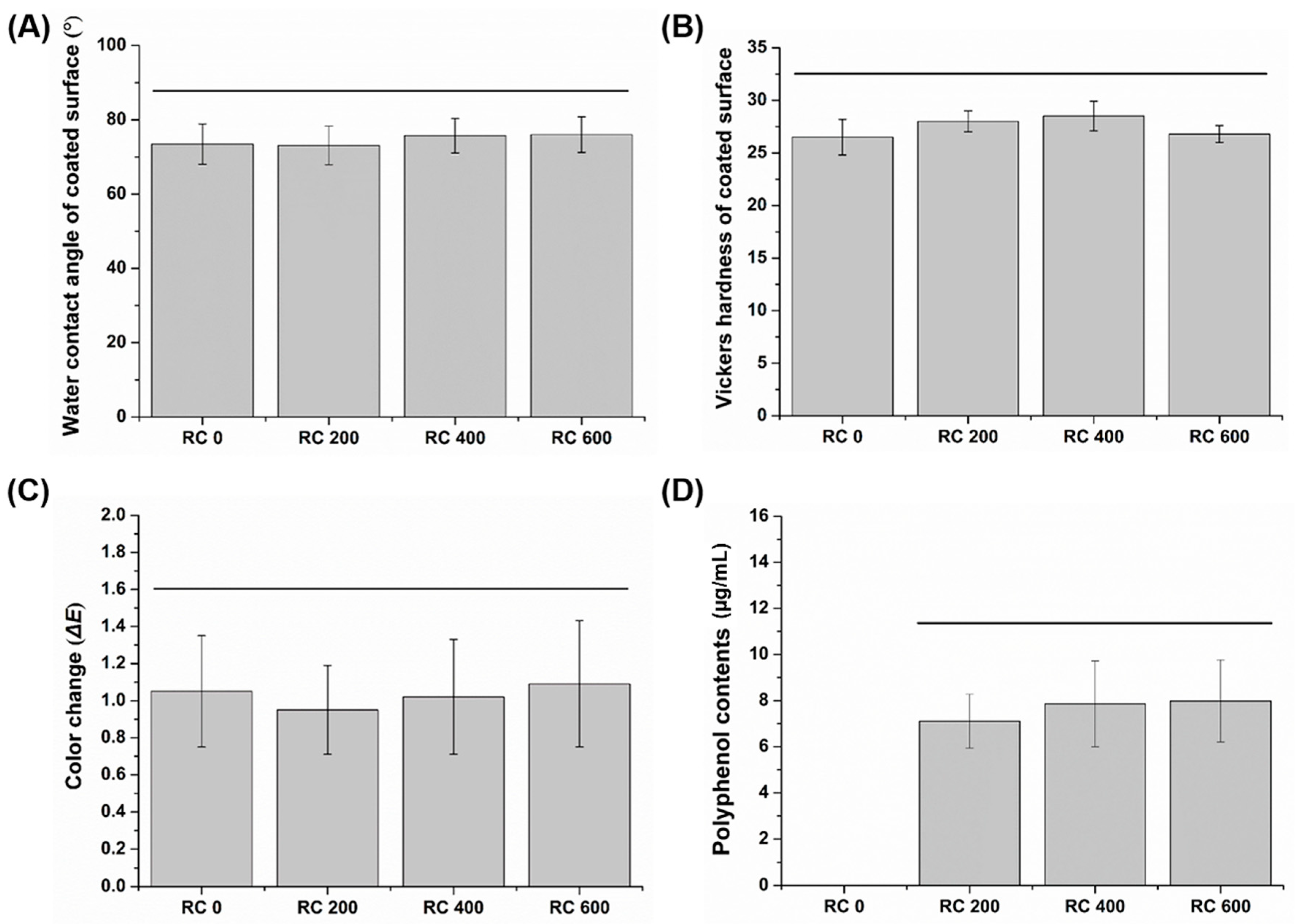
2.1. Water Contact Angle of the Coated Surface
2.2. Microhardness of the Coated Surface
2.3. Color Characterization
2.4. Analysis of Polyphenol Content
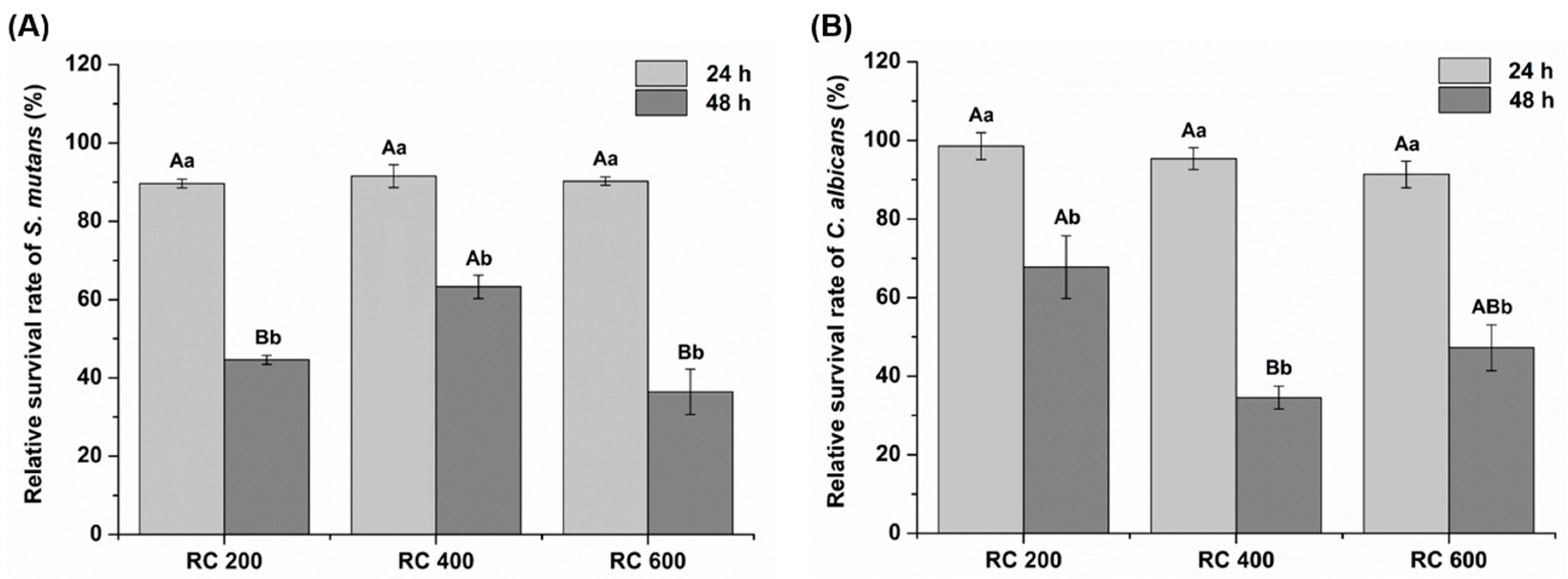
2.5. Antimicrobial Activity
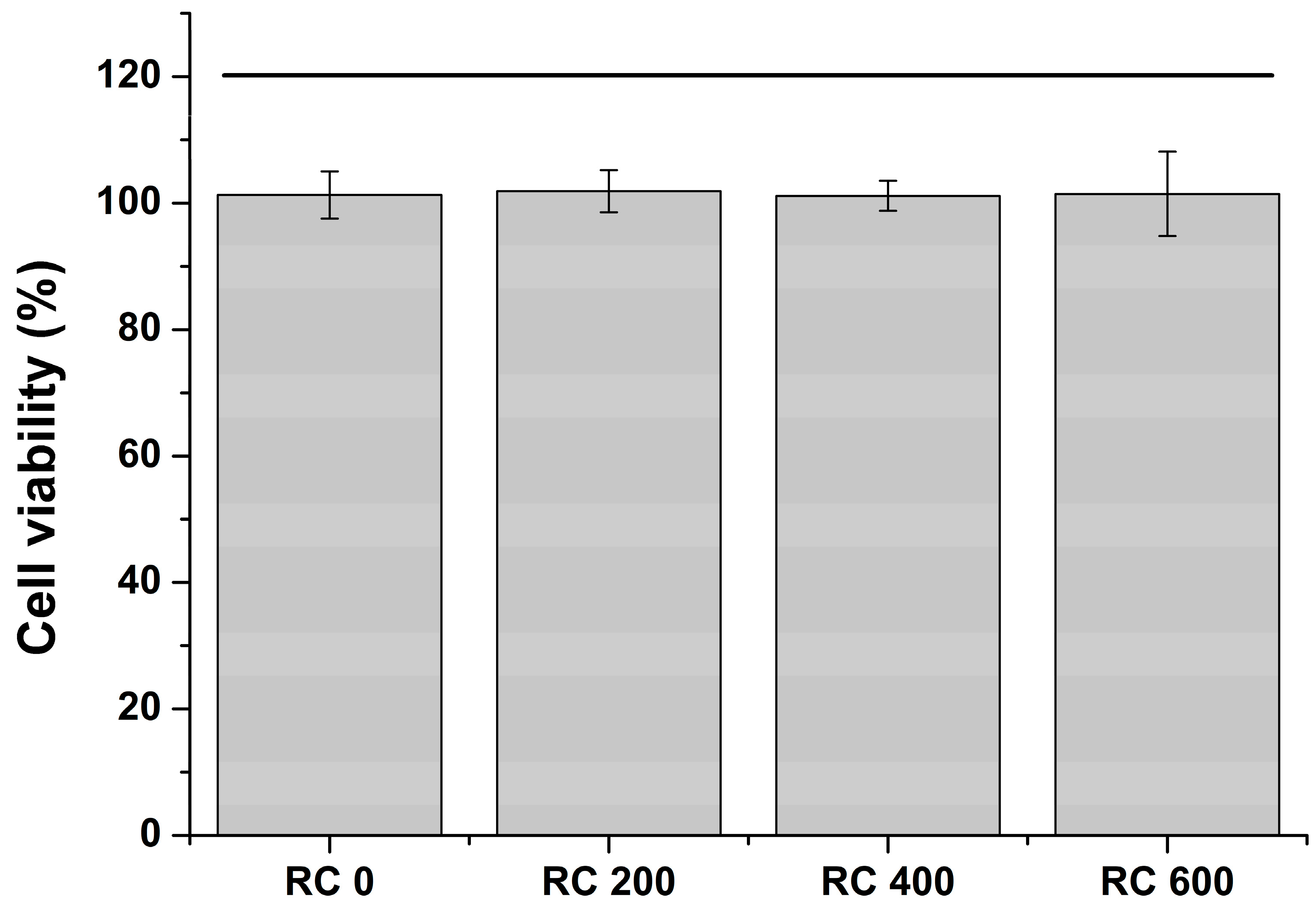
2.6. Cell Viability
3. Discussion
4. Materials and Methods
4.1. Coating Resin Containing R. Elliptica Extract
4.2. Coating Treatment on Acrylic Resin Disks
4.3. Surface Characterization
4.4. Analysis of the Coated Sample Extracts
4.5. Antimicrobial Test
4.6. Cell Viability Test
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Albanna, R.H.; Farawanah, H.M.; Aldrees, A.M. Microbial evaluation of the effectiveness of different methods for cleansing clear orthodontic retainers: A randomized clinical trial. Angle Orthod. 2017, 87, 460–465. [Google Scholar] [CrossRef]
- Apratim, A.; Shah, S.S.; Sinha, M.; Agrawal, M.; Chhaparia, N.; Abubakkar, A. Denture hygiene habits among elderly patients wearing complete dentures. J. Contemp. Dent. Pract. 2013, 14, 1161–1164. [Google Scholar] [CrossRef]
- Zuo, W.; Feng, D.; Song, A.; Gong, H.; Zhu, S. Effects of organic-inorganic hybrid coating on the color stability of denture base resins. J. Prosthet. Dent. 2016, 115, 103–108. [Google Scholar] [CrossRef]
- Littlewood, S.J.; Millett, D.T.; Doubleday, B.; Bearn, D.R.; Worthington, H.V. Retention procedures for stabilising tooth position after treatment with orthodontic braces. Cochrane Database Syst. Rev. 2016, CD002283. [Google Scholar] [CrossRef] [PubMed]
- Dikbas, I.; Gurbuz, O.; Unalan, F.; Koksal, T. Impact strength of denture polymethyl methacrylate reinforced with different forms of E-glass fibers. Acta Odontol. Scand. 2013, 71, 727–732. [Google Scholar] [CrossRef]
- Khasawneh, S.; Al-Wahadni, A. Control of denture plaque and mucosal inflammation in denture wearers. J. Ir. Dent. Assoc. 2002, 48, 132–138. [Google Scholar]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, L.; Guida, A.; Petruzzi, M.; Serpico, R. Candida-associated denture stomatitis. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e139-43. [Google Scholar] [CrossRef]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef]
- Batoni, G.; Pardini, M.; Giannotti, A.; Ota, F.; Giuca, M.R.; Gabriele, M.; Campa, M.; Senesi, S. Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur. J. Oral Sci. 2001, 109, 388–392. [Google Scholar] [CrossRef]
- Hibino, K.; Wong, R.W.; Hägg, U.; Samaranayake, L.P. The effects of orthodontic appliances on Candida in the human mouth. Int. J. Paediatr. Dent. 2009, 19, 301–308. [Google Scholar] [CrossRef]
- Baena-Monroy, T.; Moreno-Maldonado, V.; Franco-Martínez, F.; Aldape-Barrios, B.; Quindós, G.; Sánchez-Vargas, L.O. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral Cir. Bucal 2005, 10 (Suppl. S1), E27–E39. [Google Scholar]
- Loesche, W.J.; Rowan, J.; Straffon, L.H.; Loos, P.J. Association of Streptococcus mutants with human dental decay. Infect. Immun. 1975, 11, 1252–1260. [Google Scholar] [CrossRef]
- Iosif, L.; Preoteasa, C.T.; Murariu-Măgureanu, C.; Preoteasa, E. Clinical study on thermography, as modern investigation method for Candida-associated denture stomatitis. Rom. J. Morphol. Embryol. 2016, 57, 191–195. [Google Scholar]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust. Dent. J. 1998, 43, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.C.; da Silva-Neto, J.P.; Dantas, T.S.; Naves, L.Z.; das Neves, F.D.; da Mota, A.S. Bacterial adhesion and surface roughness for different clinical techniques for acrylic polymethyl methacrylate. Int. J. Dent. 2016, 8685796. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Kuroki, K.; Hayashi, T.; Sato, K.; Asai, T.; Okano, M.; Kominami, Y.; Takahashi, Y.; Kawai, T. Effect of self-cured acrylic resin added with an inorganic antibacterial agent on Streptococcus mutans. Dent. Mater. J. 2010, 29, 277–285. [Google Scholar] [CrossRef]
- Hannah, V.E.; O’Donnell, L.; Robertson, D.; Ramage, G. Denture Stomatitis: Causes, cures and prevention. Prim. Dent. J. 2017, 6, 46–51. [Google Scholar] [CrossRef]
- Liu, S.Y.; Tonggu, L.; Niu, L.N.; Gong, S.Q.; Fan, B.; Wang, L.; Zhao, J.H.; Huang, C.; Pashley, D.H.; Tay, F.R. Antimicrobial activity of a quaternary ammonium methacryloxy silicate-containing acrylic resin: A randomised clinical trial. Sci. Rep. 2016, 6, 21882. [Google Scholar] [CrossRef]
- Addy, M.; Shaw, W.C.; Hansford, P.; Hopkins, M. The effect of orthodontic appliances on the distribution of Candida and plaque in adolescents. Br. J. Orthod. 1982, 9, 158–163. [Google Scholar] [CrossRef]
- Shay, K. Denture hygiene: A review and update. J. Contemp. Dent. Pract. 2000, 1, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Jagger, R.; Vowles, R.; Moran, J. Comparative stain removal properties of four commercially available denture cleaning products: An in vitro study. Int. J. Dent. Hyg. 2011, 9, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Kubo, K.; Saito, T.; Obata, T.; Wada, T.; Yanagisawa, K.; Sakurai, K. Surface morphology of silicone soft relining material after mechanical and chemical cleaning. J. Prosthodont. Res. 2018, 62, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Sato, Y.; Owada, G.; Minakuchi, S. Effectiveness of a combination denture-cleaning method versus a mechanical method: Comparison of denture cleanliness, patient satisfaction, and oral health-related quality of life. J. Prosthodont. Res. 2018, 62, 353–358. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Evans, J.L.; Hamlet, S.; Love, R.M. Incorporation of antimicrobial agents in denture base resin: A systematic review. J. Prosthet. Dent. 2020. [Google Scholar] [CrossRef]
- Pusateri, C.R.; Monaco, E.A.; Edgerton, M. Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch. Oral Biol. 2009, 54, 588–594. [Google Scholar] [CrossRef]
- Feng, D.; Gong, H.; Zhang, J.; Guo, X.; Yan, M.; Zhu, S. Effects of antibacterial coating on monomer exudation and the mechanical properties of denture base resins. J. Prosthet. Dent. 2017, 117, 171–177. [Google Scholar] [CrossRef]
- Fitjer, L.C.; Jonas, I.E.; Kappert, H.F. Corrosion susceptibility of lingual wire extensions in removable appliances. An in vitro study. J. Orofac. Orthop. 2002, 63, 212–226. [Google Scholar] [CrossRef]
- Budtz-Jørgensen, E. Materials and methods for cleaning dentures. J. Prosthet. Dent. 1979, 42, 619–623. [Google Scholar] [CrossRef]
- Ayaz, E.A.; Altintas, S.H.; Turgut, S. Effects of cigarette smoke and denture cleaners on the surface roughness and color stability of different denture teeth. J. Prosthet. Dent. 2014, 112, 241–248. [Google Scholar] [CrossRef]
- Hong, G.; Murata, H.; Li, Y.; Sadamori, S.; Hamada, T. Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J. Prosthet. Dent. 2009, 101, 205–213. [Google Scholar] [CrossRef]
- Tan, C.M.; Tsoi, J.K.; Seneviratne, C.J.; Matinlinna, J.P. Evaluation of the Candida albicans removal and mechanical properties of denture acrylics cleaned by a low-cost powered toothbrush. J. Prosthodont. Res. 2014, 58, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Peracini, A.; Andrade, I.M.; Paranhos Hde, F.; Silva, C.H.; de Souza, R.F. Behaviors and hygiene habits of complete denture wearers. Braz. Dent. J. 2010, 21, 247–252. [Google Scholar] [CrossRef]
- Shinonaga, Y.; Arita, K. Antibacterial effect of acrylic dental devices after surface modification by fluorine and silver dual-ion implantation. Acta Biomater. 2012, 8, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Rantala, L.I.; Lastumäki, T.M.; Peltomäki, T.; Vallittu, P.K. Fatigue resistance of removable orthodontic appliance reinforced with glass fibre weave. J. Oral Rehabil. 2003, 30, 501–506. [Google Scholar] [CrossRef]
- Kuroiwa, A.; Nomura, Y.; Ochiai, T.; Sudo, T.; Nomoto, R.; Hayakawa, T.; Kanzaki, H.; Nakamura, Y.; Hanada, N. Antibacterial, hydrophilic effect and mechanical properties of orthodontic resin coated with UV-responsive photocatalyst. Materials 2018, 11, 889. [Google Scholar] [CrossRef]
- Zeitoun, R.; Najjar, F.; Wehbi, B.; Khalil, A.; Fayyad-Kazan, M.; Dagher-Hamalian, C.; Faour, W.H.; El-Makhour, Y. Chemical composition, antioxidant and anti-inflammatory activity evaluation of the lebanese propolis Extract. Curr. Pharm. Biotechnol. 2019, 20, 84–96. [Google Scholar] [CrossRef]
- Jaradat, N.; AlMasri, M.; Zaid, A.N.; Othman, D.G. Pharmacological and phytochemical screening of Palestinian traditional medicinal plants Erodium laciniatum and Lactuca orientalis. J. Complement. Integr. Med. 2017, 15. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choi, Y.R.; Lee, M.J.; Kang, M.K. Antimicrobial effects against oral pathogens and cytotoxicity of Glycyrrhiza uralensis extract. Plants 2020, 9, 838. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, S.I.; Lee, K.B.; Yoo, Y.C.; Ryu, S.Y.; Song, K.S. Neuraminidase inhibitors from Reynoutria elliptica. Arch. Pharm. Res. 2003, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Choi, T.W.; Kim, C.; Nam, D.; Lee, S.G.; Jang, H.J.; Lee, J.H.; Um, J.Y.; Jung, S.H.; Shim, B.S.; et al. Anti-inflammatory activities of Reynoutria elliptica through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Immunopharm. Immunotoxicol. 2012, 34, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of oral-derived stem cells in dental and maxillofacial applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Im, H.-G.; Lee, S.-O. Growth Inhibition of Helicobacter pylorio by Reynoutria elliptica Migo. Korean J. Food Sci. Technol. 2003, 35, 1182–1187. [Google Scholar]
- Yang, S.Y.; Kang, M.K. Biocompatibility and antimicrobial activity of Reynoutria elliptica extract for dental application. Plants 2020, 9, 670. [Google Scholar] [CrossRef]
- Lyutakov, O.; Goncharova, I.; Rimpelova, S.; Kolarova, K.; Svanda, J.; Svorcik, V. Silver release and antimicrobial properties of PMMA films doped with silver ions, nano-particles and complexes. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 534–540. [Google Scholar] [CrossRef]
- Kamikawa, Y.; Hirabayashi, D.; Nagayama, T.; Fujisaki, J.; Hamada, T.; Sakamoto, R.; Kamikawa, Y.; Sugihara, K. In vitro antifungal activity against oral Candida species using a denture base coated with silver nanoparticles. J. Nanomater. 2014, 780410. [Google Scholar] [CrossRef]
- Lazarin, A.A.; Machado, A.L.; Zamperini, C.A.; Wady, A.F.; Spolidorio, D.M.; Vergani, C.E. Effect of experimental photopolymerized coatings on the hydrophobicity of a denture base acrylic resin and on Candida albicans adhesion. Arch. Oral Biol. 2013, 58, 1–9. [Google Scholar] [CrossRef]
- Arai, T.; Ueda, T.; Sugiyama, T.; Sakurai, K. Inhibiting microbial adhesion to denture base acrylic resin by titanium dioxide coating. J. Oral Rehabil. 2009, 36, 902–908. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef]
- Procópio, A.L.F.; da Silva, R.A.; Maciel, J.G.; Sugio, C.Y.C.; Soares, S.; Urban, V.M.; Neppelenbroek, K.H. Antimicrobial and cytotoxic effects of denture base acrylic resin impregnated with cleaning agents after long-term immersion. Toxicol. Vitr. 2018, 52, 8–13. [Google Scholar] [CrossRef]
- Ali, A.A.; Alharbi, F.A.; Suresh, C.S. Effectiveness of coating acrylic resin dentures on preventing Candida adhesion. J. Prosthodont. 2013, 22, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Addison, O.; Marquis, P.M.; Fleming, G.J. Quantifying the strength of a resin-coated dental ceramic. J. Dent. Res. 2008, 87, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Rendell, J.; Grasso, J.E.; Gay, T. Retention and stability of the maxillary denture during function. J. Prosthet. Dent. 1995, 73, 344–347. [Google Scholar] [CrossRef]
- Monsénégo, P.; Proust, J. Complete denture retention. Part I: Physical analysis of the mechanism. Hysteresis of the solid-liquid contact angle. J. Prosthet. Dent. 1989, 62, 189–196. [Google Scholar] [CrossRef]
- Sipahi, C.; Anil, N.; Bayramli, E. The effect of acquired salivary pellicle on the surface free energy and wettability of different denture base materials. J. Dent. 2001, 29, 197–204. [Google Scholar] [CrossRef]
- Grundke, K.; Michel, S.; Knispel, G.; Grundler, A. Wettability of silicone and polyether impression materials: Characterization by surface tension and contact angle measurements. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 598–609. [Google Scholar] [CrossRef]
- Jooste, C.; Geerts, G.; Adams, L. Comparison of the clinical abrasion resistance of six commercially available denture teeth. J. Prosthet. Dent. 1997, 77, 23–27. [Google Scholar] [CrossRef]
- Reis, K.R.; Bonfante, G.; Pegoraro, L.F.; Conti, P.C.; Oliveira, P.C.; Kaizer, O.B. In vitro wear resistance of three types of polymethyl methacrylate denture teeth. J. Appl. Oral. Sci. 2008, 16, 176–180. [Google Scholar] [CrossRef]
- Haselden, C.A.; Hobkirk, J.A.; Pearson, G.J.; Davies, E.H. A comparison between the wear resistance of three types of denture resin to three different dentifrices. J. Oral. Rehabil. 1998, 25, 335–339. [Google Scholar] [CrossRef]
- Chadwick, R.G.; McCabe, J.F.; Walls, A.W.; Storer, R. The effect of storage media upon the surface microhardness and abrasion resistance of three composites. Dent. Mater. 1990, 6, 123–128. [Google Scholar] [CrossRef]
- Konyashin, I.; Ries, B.; Hlawatschek, D.; Zhuk, Y.; Mazilkin, A.; Straumal, B.; Dorn, F.; Park, D. Wear-resistance and hardness: Are they directly related for nanostructured hard materials? Int. J. Refract. Met. Hard Mater. 2015, 49, 203–211. [Google Scholar] [CrossRef]
- Silva, M.J.; de Oliveira, D.G.; Marcillo, O.O.; Neppelenbroek, K.H.; Lara, V.S.; Porto, V.C. Effect of denture-coating composite on Candida albicans biofilm and surface degradation after disinfection protocol. Int. Dent. J. 2016, 66, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, R.; Yli-Urpo, A.; Seppä, L. Wear of dental restorative and prosthetic materials in vitro. Dent. Mater. 1989, 5, 35–37. [Google Scholar] [CrossRef]
- Koksal, T.; Dikbas, I. Color stability of different denture teeth materials against various staining agents. Dent. Mater. J. 2008, 27, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Smullen, J.; Koutsou, G.A.; Foster, H.A.; Zumbé, A.; Storey, D.M. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007, 41, 342–349. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO 10993–5: 2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Clifford, C.; Downes, S. A comparative study of the use of colorimetric assays in the assessment of biocompatibility. J. Mater. Sci. Mater. Med. 1996, 7, 637–643. [Google Scholar] [CrossRef]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-point bending tests of zirconia core/veneer ceramics for dental restorations. Int. J. Dent. 2013, 2013, 831976. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; Mori, G.; Dibello, V.; Marrelli, M.; Mirgaldi, R.; De Vito, D.; Tatullo, M. Anti-plaque and antimicrobial efficiency of different oral rinses in a 3-day plaque accumulation model. J. Biol. Regul. Homeost. Agents 2016, 30, 1173–1178. [Google Scholar]
- ISO. ISO 10993-12: 2012. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
| Experimental Groups | Streptococcus Mutans | Candida Albicans | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| RC 0 | 0.053 ± 0.003 a | 0.612 ± 0.008 a | 0.042 ± 0.002 a | 0.829 ± 0.049 a |
| RC 200 | 0.047 ± 0.001 b | 0.273 ± 0.007 c | 0.041 ± 0.001 a | 0.599 ± 0.071 b |
| RC 400 | 0.048 ± 0.002 b | 0.387 ± 0.018 b | 0.040 ± 0.001 a | 0.305 ± 0.025 c |
| RC 600 | 0.048 ± 0.001 b | 0.223 ± 0.035 c | 0.038 ± 0.001 a | 0.418 ± 0.052 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-Y.; Kang, M.-K. Surface Characterization, Antimicrobial Activity, and Biocompatibility of Autopolymerizing Acrylic Resins Coated with Reynoutria elliptica Extract. Plants 2020, 9, 1292. https://doi.org/10.3390/plants9101292
Yang S-Y, Kang M-K. Surface Characterization, Antimicrobial Activity, and Biocompatibility of Autopolymerizing Acrylic Resins Coated with Reynoutria elliptica Extract. Plants. 2020; 9(10):1292. https://doi.org/10.3390/plants9101292
Chicago/Turabian StyleYang, Song-Yi, and Min-Kyung Kang. 2020. "Surface Characterization, Antimicrobial Activity, and Biocompatibility of Autopolymerizing Acrylic Resins Coated with Reynoutria elliptica Extract" Plants 9, no. 10: 1292. https://doi.org/10.3390/plants9101292
APA StyleYang, S.-Y., & Kang, M.-K. (2020). Surface Characterization, Antimicrobial Activity, and Biocompatibility of Autopolymerizing Acrylic Resins Coated with Reynoutria elliptica Extract. Plants, 9(10), 1292. https://doi.org/10.3390/plants9101292






