Integrative Transcriptome and Proteome Analysis Identifies Major Molecular Regulation Pathways Involved in Ramie (Boehmeria nivea (L.) Gaudich) under Nitrogen and Water Co-Limitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Sampling
2.2. RNA Sample Preparation and Transcriptome Analyses
2.3. Protein Sample Preparation and Proteomic Analysis
2.4. Association Analysis of Transcriptome and Proteome Profiles
2.5. Statistical Analysis
3. Results
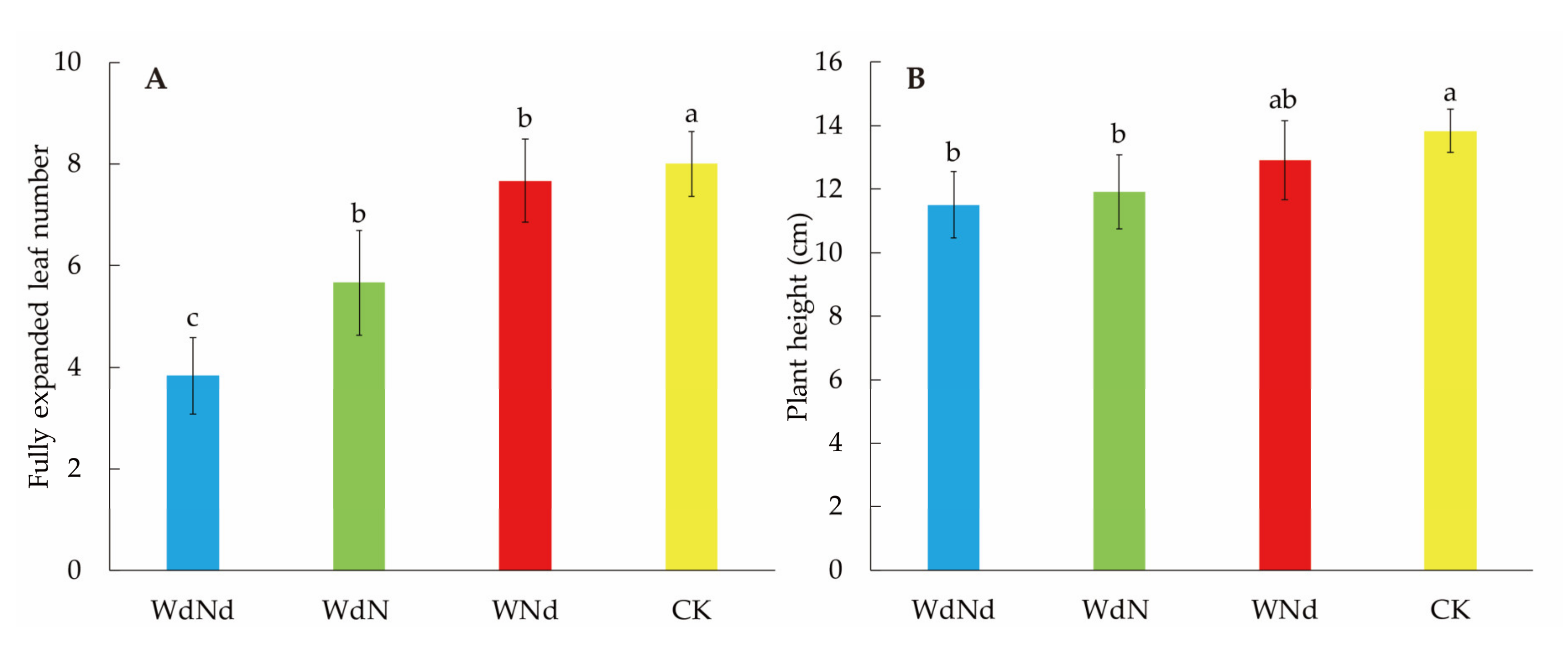
3.1. Seedling Morphological Differences among Different Nitrogen–Water Conditions
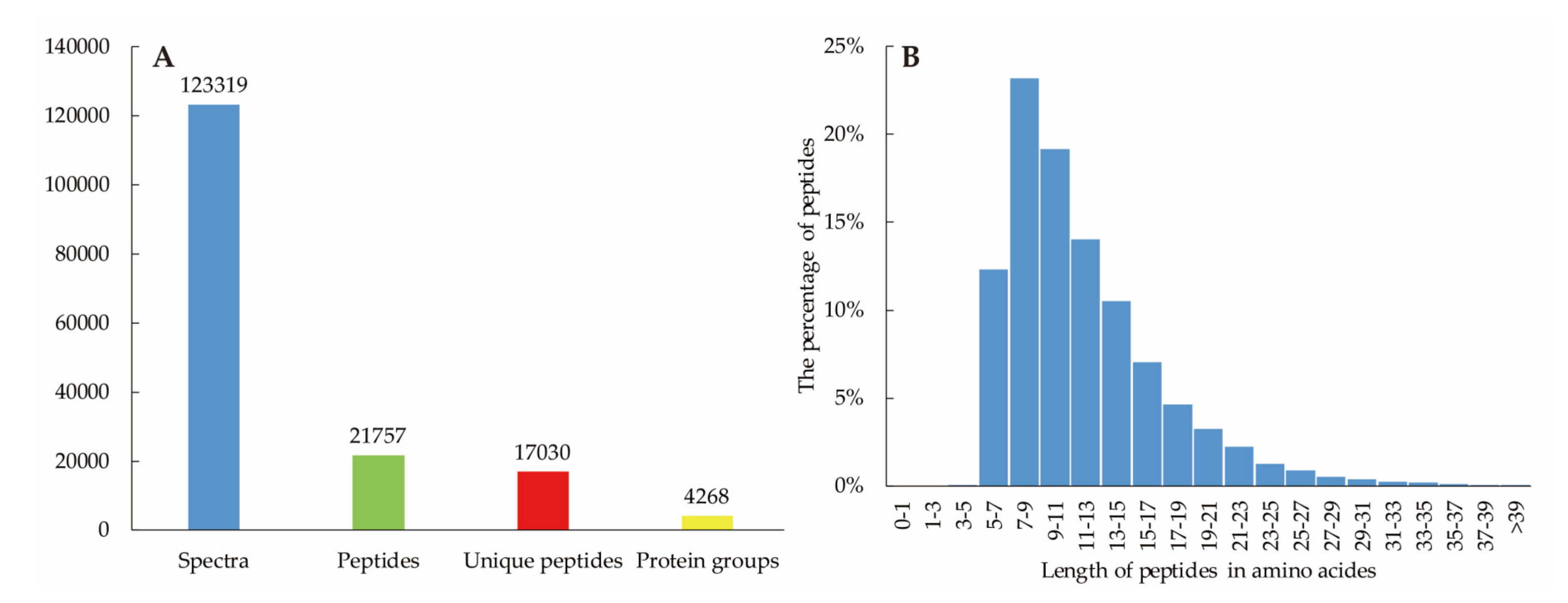
3.2. Overview of Ramie Transcriptome and Proteome
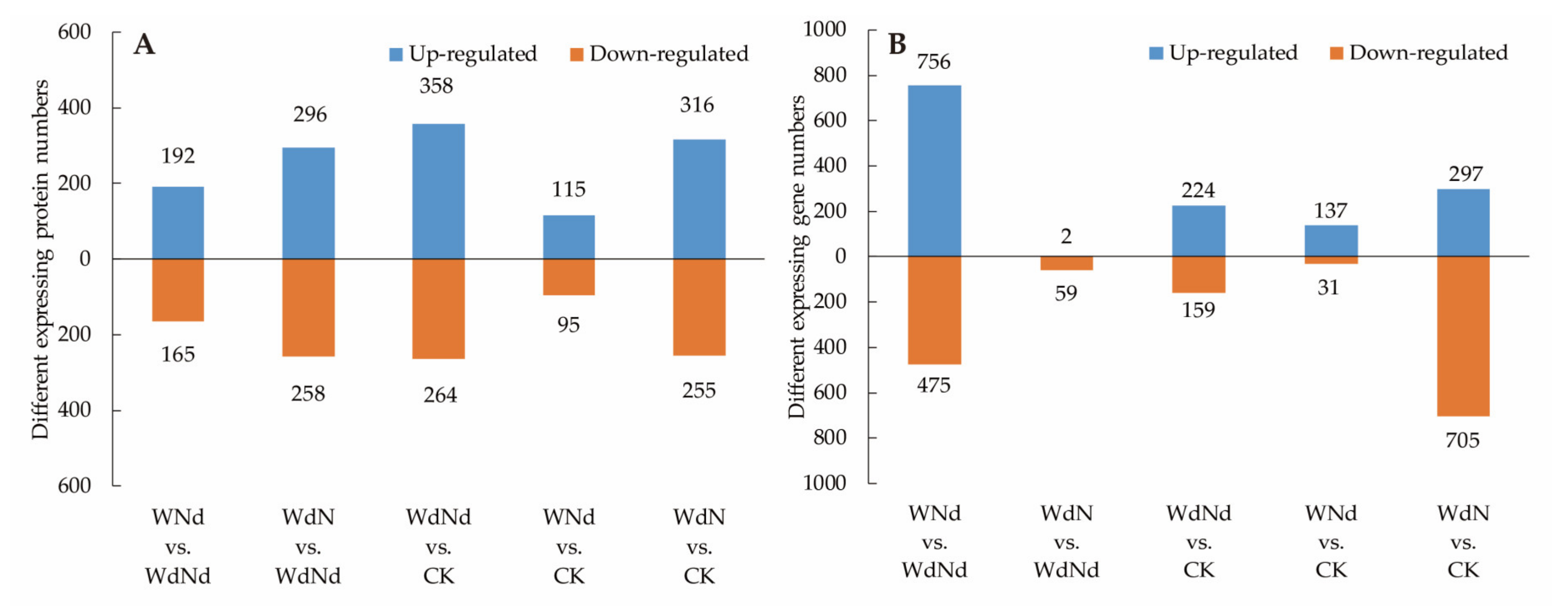
3.3. Identified DEGs and DEPs Involved in Ramie Nitrogen and Water Stress Responses
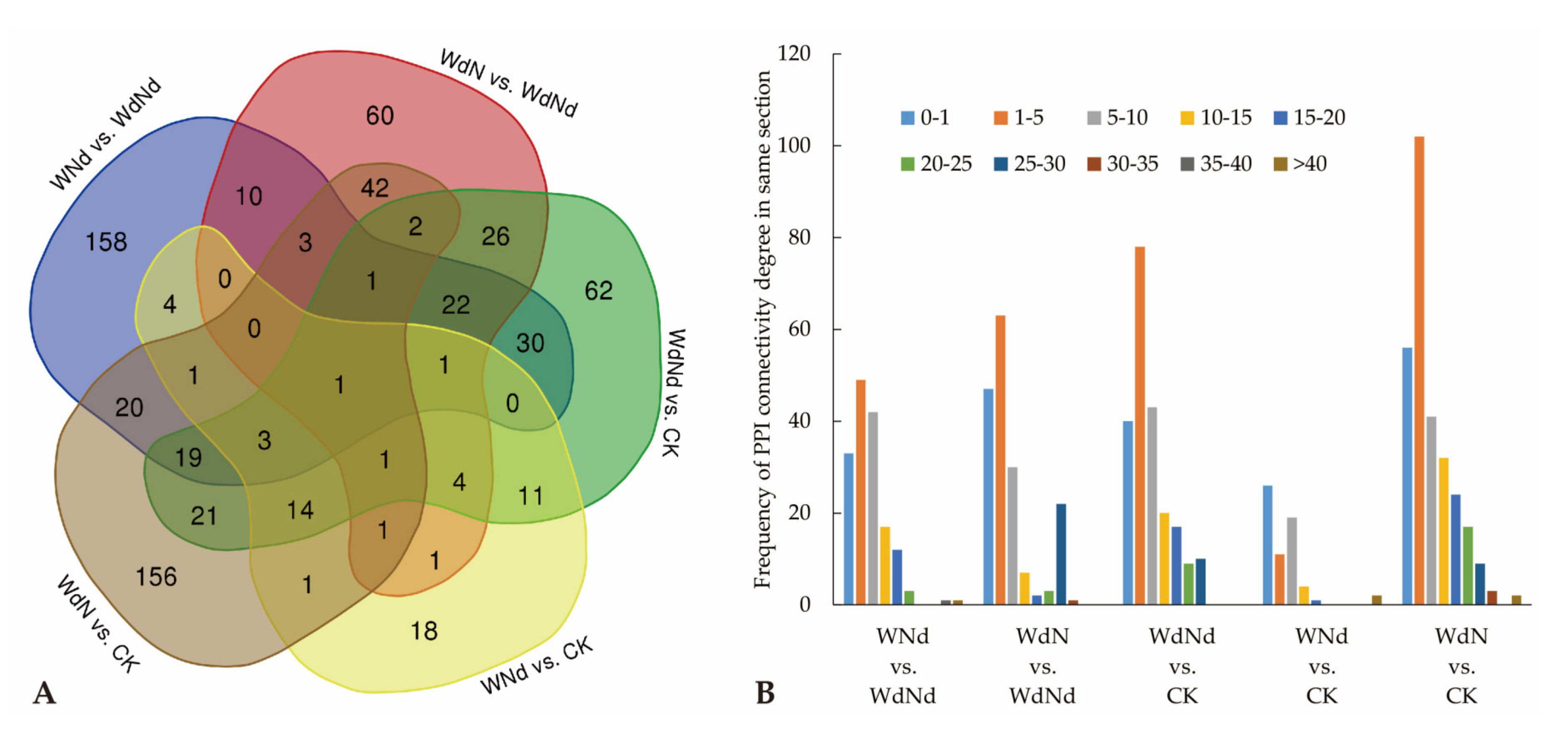
3.4. Correlation of Transcript and Protein Profiles in Ramie under Water and Nitrogen Stress
3.5. GO Enrichment Analysis Based on DEPs Correlated to DEGs
3.6. KEGG Pathway Enrichment Analysis Based on Transcriptome and Proteome
4. Discussion
4.1. Ramie Growth under Different Water and N Conditions
4.2. Photosynthesis and Ethylene Responding in Ramie under Water and N Stresses
4.3. Glycolysis in Ramie under Water and N Stresses
4.4. Nitrogen Metabolism in Ramie under Water and N Stresses
4.5. Global View on Water and N Interactions in Ramie at Molecular Level
4.6. Advice for Improving Water and N Synergistic Effects in Ramie
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Buchmann, N.; Sprent, J.; Buckley, T.N.; Turnbull, T.L. Crops, nitrogen, water: Are legumes friend, foe, or misunderstood ally? Trends Plant Sci. 2018, 23, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Quemada, M.; Gabriel, J.L. Approaches for increasing nitrogen and water use efficiency simultaneously. Glob. Food Secur. 2016, 9, 29–35. [Google Scholar] [CrossRef]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, J.; Wang, Q.; Zhou, K.; Mao, K.; Ma, F. Overexpression of MdEPF2 improves water use efficiency and reduces oxidative stress in tomato. Environ. Exp. Bot. 2019, 162, 321–332. [Google Scholar] [CrossRef]
- DeMalach, N.; Zaady, E.; Kadmon, R. Contrasting effects of water and nutrient additions on grassland communities: A global meta-analysis. Global Ecol. Biogeogr. 2017, 269, 983–992. [Google Scholar] [CrossRef]
- Lü, X.; Dijkstra, F.A.; Kong, D.; Wang, Z.; Han, X. Plant nitrogen uptake drives responses of productivity to nitrogen and water addition in a grassland. Sci. Rep. 2015, 4, 4817. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Tasisa, B.Y.; Baskin, J.M.; Baskin, C.C.; Lü, X.; Zhou, D. Divergent responses to water and nitrogen addition of three perennial bunchgrass species from variously degraded typical steppe in Inner Mongolia. Sci. Total Environ. 2019, 647, 1344–1350. [Google Scholar] [CrossRef]
- Dziedek, C.; von Oheimb, G.; Calvo, L.; Fichtner, A.; Kriebitzsch, W.; Marcos, E.; Pitz, W.T.; Härdtle, W. Does excess nitrogen supply increase the drought sensitivity of European beech (Fagus sylvatica L.) seedlings? Plant Ecol. 2016, 217, 393–405. [Google Scholar] [CrossRef]
- Luan, M.B.; Jian, J.B.; Chen, P.; Chen, J.H.; Chen, J.H.; Gao, Q.; Gao, G.; Zhou, J.H.; Chen, K.M.; Guang, X.M.; et al. Draft genome sequence of ramie, Boehmeria nivea (L.) Gaudich. Mol. Ecol. Resour. 2018, 18, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.M. Fiber Crops; The University Presses of Florida: Gainesville, FL, USA, 1975. [Google Scholar]
- Tan, Z.; Wang, C.; Yi, Y.; Wang, H.; Li, M.; Zhou, W.; Tan, S.; Li, F. Extraction and purification of chlorogenic acid from ramie (Boehmeria nivea L. Gaud) leaf using an ethanol/salt aqueous two-phase system. Sep. Purif. Technol. 2014, 132, 396–400. [Google Scholar] [CrossRef]
- Kipriotis, E.; Heping, X.; Vafeiadakis, T.; Kiprioti, M.; Alexopoulou, E. Ramie and kenaf as feed crops. Ind. Crop. Prod. 2015, 68, 126–130. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W. Stabilized nanoscale zerovalent iron mediated cadmium accumulation and oxidative damage of Boehmeria nivea (l.) Gaudich cultivated in cadmium contaminated sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Gong, W.; Yan, L.; Zhu, Z.; Hu, Z.; Peng, Y. Biodegradation of ramie stalk by Flammulina velutipes: Mushroom production and substrate utilization. AMB Express 2017, 7, 171. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, S.; Tang, Q.; Yu, Y.; Tang, S. Identification of drought stress-responsive transcription factors in ramie (Boehmeria nivea L. Gaud). BMC Plant Biol. 2013, 13, 130. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, S.; Fu, L.; Yu, Y.; Tang, Q.; Tang, S. Morphological and physiological changes of ramie (Boehmeria nivea L. Gaud) in response to drought stress and GA3 treatment. Russ. J. Plant Physl. 2013, 60, 749–755. [Google Scholar] [CrossRef]
- Ullah, S.; Anwar, S.; Rehman, M.; Khan, S.; Zafar, S.; Liu, L.; Peng, D. Interactive effect of gibberellic acid and NPK fertilizer combinations on ramie yield and bast fibre quality. Sci. Rep. 2017, 7, 10647. [Google Scholar] [CrossRef]
- Ullah, S.; Liu, L.; Anwar, S.; Tuo, X.; Khan, S.; Wang, B.; Peng, D. Effects of fertilization on ramie (Boehmeria nivea L.) growth, yield and fiber quality. Sustainability 2016, 8, 887. [Google Scholar] [CrossRef]
- Hofer, D.; Suter, M.; Buchmann, N.; Luscher, A. Nitrogen status of functionally different forage species explains resistance to severe drought and post-drought overcompensation. Agr. Ecosyst. Environ. 2017, 236, 312–322. [Google Scholar] [CrossRef]
- Sperandio, M.V.L.; Santos, L.A.; Bucher, C.A.; Fernandes, M.S.; de Souza, S.R. Isoforms of plasma membrane H+-ATPase in rice root and shoot are differentially induced by starvation and resupply of NO3− or NH4+. Plant Sci. 2011, 180, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, X.; Chen, Q.; Wang, L.; Qi, K.; Yin, H.; Qiao, X.; Wang, P.; Zhang, S.; Wu, J.; et al. Dynamic transcriptome analysis of root nitrate starvation and re-supply provides insights into nitrogen metabolism in pear (Pyrus bretschneideri). Plant Sci. 2018, 277, 322–333. [Google Scholar] [CrossRef]
- Dossa, K.; Li, D.; Wang, L.; Zheng, X.; Yu, J.; Wei, X.; Fonceka, D.; Diouf, D.; Liao, B.; Cisse, N.; et al. Dynamic transcriptome landscape of sesame (Sesamum indicum L.) under progressive drought and after rewatering. Genom. Data 2017, 11, 122–124. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- McAlister, G.C.; Huttlin, E.L.; Haas, W.; Ting, L.; Jedrychowski, M.P.; Rogers, J.C.; Kuhn, K.; Pike, I.; Grothe, R.A.; Blethrow, J.D.; et al. Increasing the multiplexing capacity of tmts using reporter ion isotopologues with isobaric masses. Anal. Chem. 2012, 84, 7469–7478. [Google Scholar] [CrossRef]
- Lan, P.; Li, W.; Schmidt, W. Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Mol. Cell. Proteom. 2012, 11, 1156–1166. [Google Scholar] [CrossRef]
- Kuss, C.; Gan, C.S.; Gunalan, K.; Bozdech, Z.; Sze, S.K.; Preiser, P.R. Quantitative proteomics reveals new insights into erythrocyte invasion by plasmodium falciparum. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Casas-Vila, N.; Bluhm, A.; Sayols, S.; Dinges, N.; Dejung, M.; Altenhein, T.; Kappei, D.; Altenhein, B.; Roignant, J.; Butter, F. The developmental proteome of Drosophila melanogaster. Genome Res. 2017, 27, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Futcher, B.; Latter, G.I.; Monardo, P.; McLaughlin, C.S.; Garrels, J.I. A sampling of the yeast proteome. Mol. Cell. Biol. 1999, 19, 7357–7368. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Frendo, P.; Ladrera, R.; Zabalza, A.; Puppo, A.; Arrese-Igor, C.; González, E.M. Nitrogen fixation control under drought stress. Localized or sustemic? Plant Pysiol. 2007, 143, 1968–1974. [Google Scholar] [CrossRef]
- Zhong, C.; Bai, Z.; Zhu, L.; Zhang, J.; Zhu, C.; Huang, J.; Jin, Q.; Cao, X. Nitrogen-mediated alleviation of photosynthetic inhibition under moderate water deficit stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2019, 157, 269–282. [Google Scholar] [CrossRef]
- Badhan, S.; Kole, P.; Ball, A.; Mantri, N. RNA sequencing of leaf tissues from two contrasting chickpea genotypes reveals mechanisms for drought tolerance. Plant Physiol. Biochem. 2018, 129, 295–304. [Google Scholar] [CrossRef]
- An, X.; Zhang, J.; Liao, Y.; Liu, L.; Peng, D.; Wang, B. Senescence is delayed when ramie (Boehmeria nivea L.) is transformed with the isopentyl transferase (ipt) gene under control of the SAG12 promoter. FEBS Open Bio. 2017, 7, 636–644. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Montaldi, E.; Puntarulo, S. Oxidative stress, antioxidant capacity and ethylene production during ageing of cut carnation (Dianthus caryophyllus) petals. J. Exp. Bot. 1996, 47, 595–601. [Google Scholar] [CrossRef][Green Version]
- Gergoff, G.; Chaves, A.; Bartoli, C.G. Ethylene regulates ascorbic acid content during dark-induced leaf senescence. Plant Sci. 2010, 178, 207–212. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, J.; Zhang, Z.; Li, H.; Yang, B.; Chen, W.; Dai, X.; Li, X.; Yang, S.; Liu, L.; et al. Integrative transcriptome and proteome analysis identifies major metabolic pathways involved in pepper fruit development. J. Proteome Res. 2019, 18, 982–994. [Google Scholar] [CrossRef]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, X.Y.; Gong, Q.Q.; Xie, C.; Fan, W.; Yang, J.L.; Lin, Q.S.; Zheng, S.J. Root proteome of rice studied by iTRAQ provides integrated insight into aluminum stress tolerance mechanisms in plants. J. Proteom. 2014, 98, 189–205. [Google Scholar] [CrossRef]
- Deng, G.; Liu, L.J.; Zhong, X.Y.; Lao, C.Y.; Wang, H.Y.; Wang, B.; Zhu, C.; Shah, F.; Peng, D.X. Comparative proteome analysis of the response of ramie under N, P and K deficiency. Planta 2014, 239, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Gao, G.; Yu, C.; Zhu, A.; Chen, P.; Chen, K.; Chen, J.; Xiong, H. Transcriptome analysis of high-NUE (T29) and low-NUE (T13) genotypes identified different responsive patterns involved in nitrogen stress in ramie (Boehmeria nivea (L.) Gaudich). Plants 2020, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Zhu, S.; Wang, Z.; Qin, L.; Alam, M.A.; Xie, J.; Yuan, Z. Proteome response of Dunaliella parva induced by nitrogen limitation. Algal Res. 2017, 23, 196–202. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, J.; Jie, Y.; Xing, H.; Zhong, Y.; She, W.; Wei, G.; Yu, W.; Ma, Y. A ramie (Boehmeria nivea) bZIP transcription factor BnbZIP3 positively regulates drought, salinity and heavy metal tolerance. Mol. Breed. 2016, 36, 120. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Wang, Y.; Tang, S.; Dai, Q.; Zhu, S.; Liu, T. Identification of Dof transcription factors in ramie (Boehmeria nivea L. Gaud) and their expression in response to different nitrogen treatments. 3 Biotech 2018, 8, 496. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, S.; Tang, S.; Liu, T. Identification of drought, cadmium and root-lesion nematode infection stress-responsive transcription factors in ramie. Open Life Sci. 2016, 11, 191–199. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; He, Y.; Liu, L.; Wang, B.; Peng, D. Isolation and functional characterization of SUCROSE SYNTHASE 1 and SUCROSE TRANSPORTER 2 promoters from ramie (Boehmeria nivea L. Gaudich). Gene 2019, 685, 114–124. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; Peng, D.; Liu, L.; Dai, L.; Chen, C.; Wang, B. Identification and expression characterization of the Phloem Protein 2 (PP2) genes in ramie (Boehmeria nivea L. Gaudich). Sci. Rep. 2018, 8, 10734. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, C.; Chen, P.; Wang, Y.; Tan, L.; Chen, J.; Xiong, H. Characterization of a glutamine synthetase gene BnGS1-2 from ramie (Boehmeria nivea L. Gaud) and biochemical assays of BnGS1-2-over-expressing transgenic tobacco. Acta Physiol. Plant 2015, 37, 1742. [Google Scholar] [CrossRef]
- DiMario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant carbonic anhydrases: Structures, locations, evolution, and physiological roles. Mol. Plant 2017, 10, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M. The multifunctional face of plant carbonic anhydrase. Plant Physiol. Bioch. 2017, 112, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, T.R.; Lemaire, G.; Sadras, V.O.; Gastal, F. Water use efficiency in perennial forage species: Interactions between nitrogen nutrition and water deficit. Field Crop. Res. 2018, 222, 1–11. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, K.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wang, L. Comparative transcriptome analysis of genes involved in the response of resistant and susceptible peach cultivars to water stress. Sci. Hortic. 2019, 245, 29–38. [Google Scholar] [CrossRef]
- Huang, C.; Wei, G.; Luo, Z.; Xu, J.; Zhao, S.; Wang, L.; Jie, Y. Effects of nitrogen on ramie (Boehmeria nivea) hybrid and its parents grown under field conditions. J. Agric. Sci. 2014, 6, 230–243. [Google Scholar] [CrossRef]
- Postma, J.A.; Schurr, U.; Fiorani, F. Dynamic root growth and architecture responses to limiting nutrient availability: Linking physiological models and experimentation. Biotechnol. Adv. 2014, 32, 53–65. [Google Scholar] [CrossRef]
- Simons, M.; Saha, R.; Amiour, N.; Kumar, A.; Guillard, L.; Clement, G.; Miquel, M.; Li, Z.; Mouille, G.; Lea, P.J.; et al. Assessing the metabolic impact of nitrogen availability using a compartmentalized maize leaf genome-scale model. Plant Physiol. 2014, 166, 1659–1674. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef]
- De Silva, M.; Siriwardena, D.P.; Sandaruwan, C.; Priyadarshana, G.; Karunaratne, V.; Kottegoda, N. Urea-silica nanohybrids with potential applications for slow and precise release of nitrogen. Mater. Lett. 2020, 272, 127839. [Google Scholar] [CrossRef]
- Dong, W.; Guo, J.; Xu, L.; Song, Z.; Zhang, J.; Tang, A.; Zhang, X.; Leng, C.; Liu, Y.; Wang, L.; et al. Water regime-nitrogen fertilizer incorporation interaction: Field study on methane and nitrous oxide emissions from a rice agroecosystem in Harbin, China. J. Environ. Sci. 2018, 64, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.R.; Rufty, T.W. Nitrogen and water resources commonly limit crop yield increases, not necessarily plant genetics. Glob. Food Secur. 2012, 1, 94–98. [Google Scholar] [CrossRef]
- Sadras, V.O.; Richards, R.A. Improvement of crop yield in dry environments: Benchmarks, levels of organisation and the role of nitrogen. J. Exp. Bot. 2014, 65, 1981–1995. [Google Scholar] [CrossRef] [PubMed]

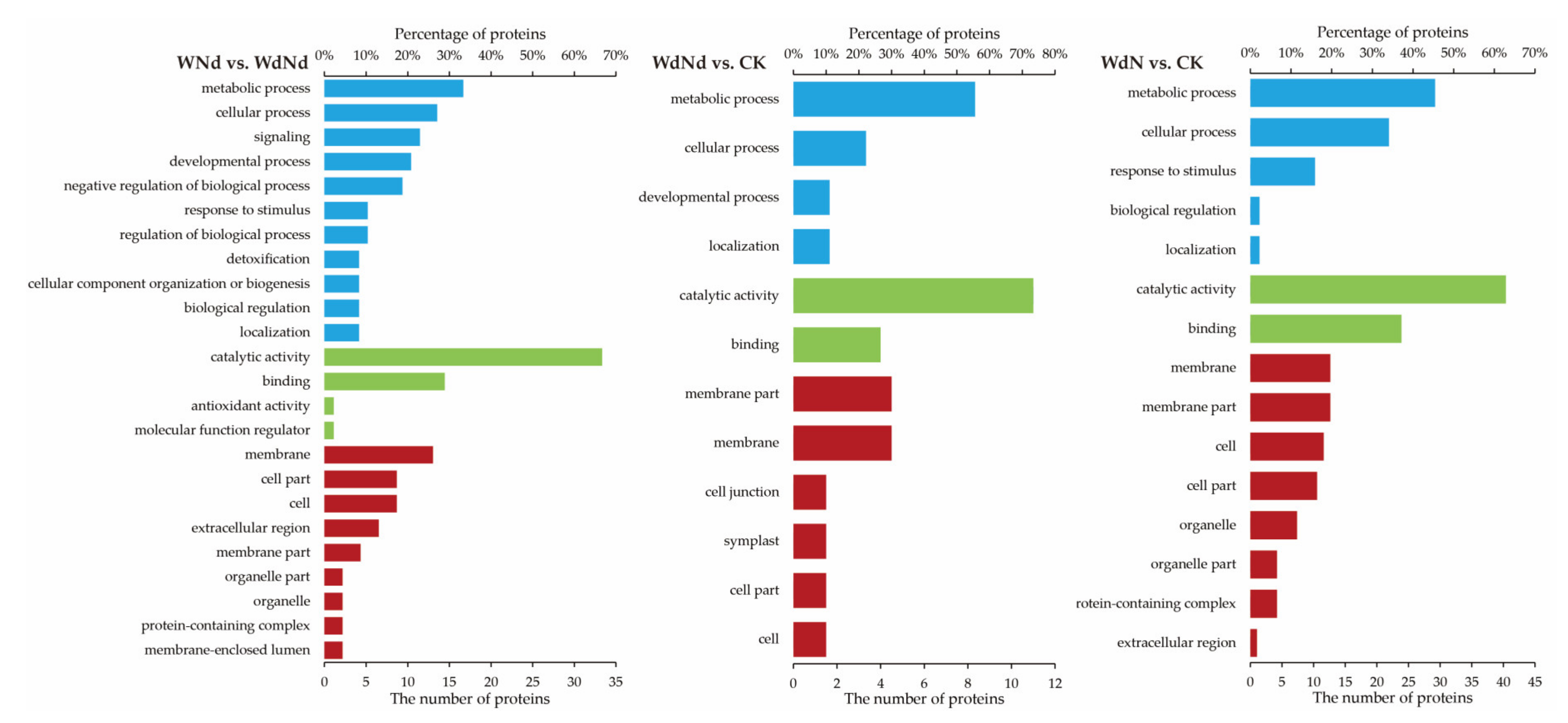
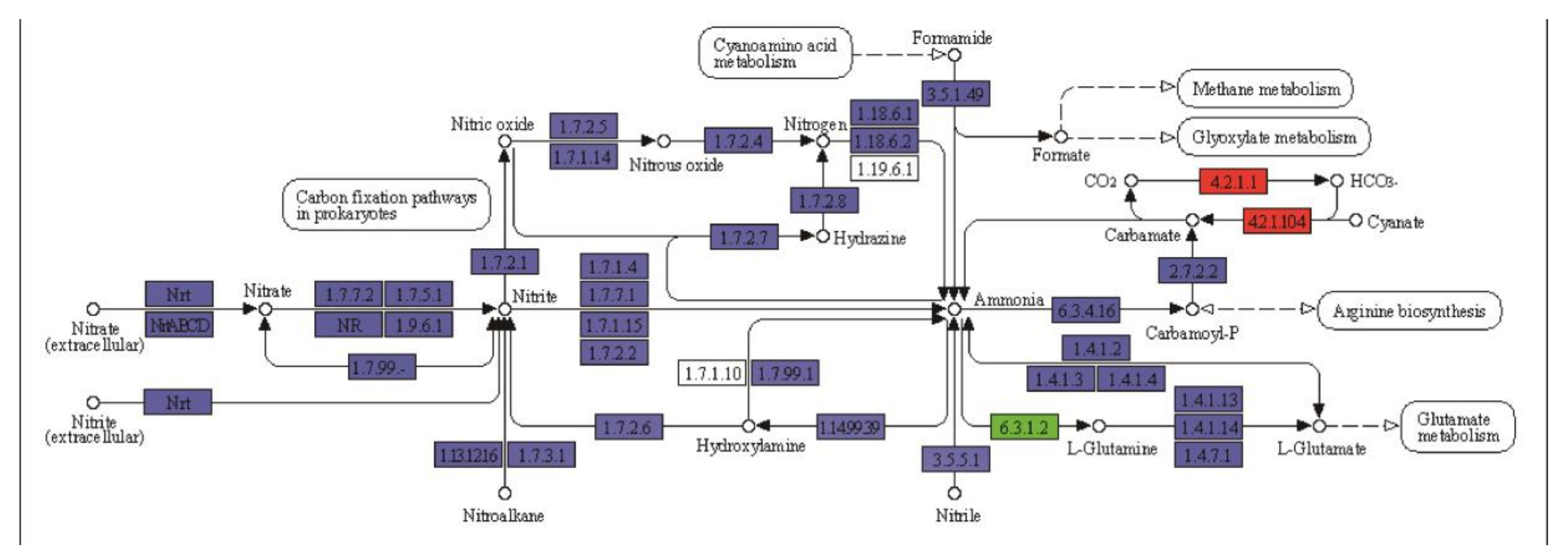
| Treatments | WdNd | WNd | WdN | CK |
|---|---|---|---|---|
| Ca(NO3)2·4(H2O) | - | - | 1653.1 | 826.5 |
| KNO3 | - | - | 404.4 | 202.2 |
| KCl | - | - | 447.3 | 223.7 |
| K2SO4 | 871.3 | 435.7 | - | - |
| KH2PO4 | 272.2 | 136.1 | 272.2 | 136.1 |
| MgSO4·7H2O | 985.9 | 493.0 | 985.9 | 493.0 |
| CaCl2 | 1109.8 | 554.9 | 333.0 | 166.5 |
| Database | Number of Unigenes | Percentage (%) |
|---|---|---|
| NCBI non-redundant protein sequences (Nr) | 56,802 | 36.72 |
| Swiss-Prot | 35,660 | 23.05 |
| Protein family (Pfam) | 39,358 | 25.44 |
| Gene Ontology (GO) | 26,711 | 17.27 |
| KEGG Orthology (KO) | 11,912 | 7.7 |
| Annotated in all databases | 6,414 | 4.15 |
| Annotated in at least one database | 64,848 | 41.92 |
| Total Unigenes | 154,691 | 100 |
| Metabolic Processes | Regulation Trend | WNd vs. WdNd | WdN vs. WdNd | WdNd vs. CK | WNd vs. CK | WdN vs. CK |
|---|---|---|---|---|---|---|
| Photosynthesis-related | Up | 3/16 | 0/14 | 0/12 | 0/5 | 1/9 |
| Down | 1/4 | 4/15 | 2/24 | 0/11 | 21/20 | |
| Ethylene responding | Up | 13/1 | 0/0 | 0/0 | 0/0 | 5/1 |
| Down | 2/0 | 0/0 | 8/1 | 0/0 | 13/2 | |
| Glycolysis-related | Up | 54/7 | 0/19 | 11/11 | 9/1 | 24/15 |
| Down | 21/9 | 1/6 | 4/16 | 1/7 | 46/15 | |
| Nitrogen-related | Up | 63/2 | 0/4 | 9/1 | 6/1 | 23/5 |
| Down | 30/4 | 8/2 | 9/2 | 3/1 | 74/2 |
| Comparisons | WNd vs. WdNd | WdN vs. WdNd | WdNd vs. CK | WNd vs. CK | WdN vs. CK |
|---|---|---|---|---|---|
| DEPs/DEGs-SameTrend | 0.5046 | NA | −0.6018 | NA | 0.5542 |
| DEPs/DEGs-Opposite | NA | NA | NA | NA | −0.2571 |
| DEPs/NDEGs | 0.4773 | 0.0186 | 0.4028 | 0.1212 | 0.2402 |
| NDEPs/DEGs | 0.3743 | 1 | 0.2913 | 0.1133 | 0.2989 |
| NDEPs/NDEGs | 0.2255 | −0.0666 | 0.2371 | −0.0231 | 0.1198 |
| Comparisons | KEGG Pathways | Number of Proteins | Number of Genes |
|---|---|---|---|
| WNd vs. WdNd | Amino sugar and nucleotide sugar metabolism | 8 | 16 |
| Pentose and glucuronate interconversions | 5 | 8 | |
| Metabolism of xenobiotics by cytochrome P450 | 3 | 7 | |
| Drug metabolism—cytochrome P450 | 3 | 7 | |
| WdN vs. CK | Phenylpropanoid biosynthesis | 8 | 10 |
| Photosynthesis—antenna proteins | 5 | 10 | |
| WdNd vs. CK | Pentose and glucuronate interconversions | 9 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gao, G.; Chen, P.; Chen, K.; Wang, X.; Bai, L.; Yu, C.; Zhu, A. Integrative Transcriptome and Proteome Analysis Identifies Major Molecular Regulation Pathways Involved in Ramie (Boehmeria nivea (L.) Gaudich) under Nitrogen and Water Co-Limitation. Plants 2020, 9, 1267. https://doi.org/10.3390/plants9101267
Chen J, Gao G, Chen P, Chen K, Wang X, Bai L, Yu C, Zhu A. Integrative Transcriptome and Proteome Analysis Identifies Major Molecular Regulation Pathways Involved in Ramie (Boehmeria nivea (L.) Gaudich) under Nitrogen and Water Co-Limitation. Plants. 2020; 9(10):1267. https://doi.org/10.3390/plants9101267
Chicago/Turabian StyleChen, Jikang, Gang Gao, Ping Chen, Kunmei Chen, Xiaofei Wang, Lianyang Bai, Chunming Yu, and Aiguo Zhu. 2020. "Integrative Transcriptome and Proteome Analysis Identifies Major Molecular Regulation Pathways Involved in Ramie (Boehmeria nivea (L.) Gaudich) under Nitrogen and Water Co-Limitation" Plants 9, no. 10: 1267. https://doi.org/10.3390/plants9101267
APA StyleChen, J., Gao, G., Chen, P., Chen, K., Wang, X., Bai, L., Yu, C., & Zhu, A. (2020). Integrative Transcriptome and Proteome Analysis Identifies Major Molecular Regulation Pathways Involved in Ramie (Boehmeria nivea (L.) Gaudich) under Nitrogen and Water Co-Limitation. Plants, 9(10), 1267. https://doi.org/10.3390/plants9101267







