Study of Ecophysiological Responses of the Antarctic Fruticose Lichen Cladonia borealis Using the PAM Fluorescence System under Natural and Laboratory Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Site Description and Lichen Identification
2.2. C. borealis Photosynthetic Activity at Night and Dawn
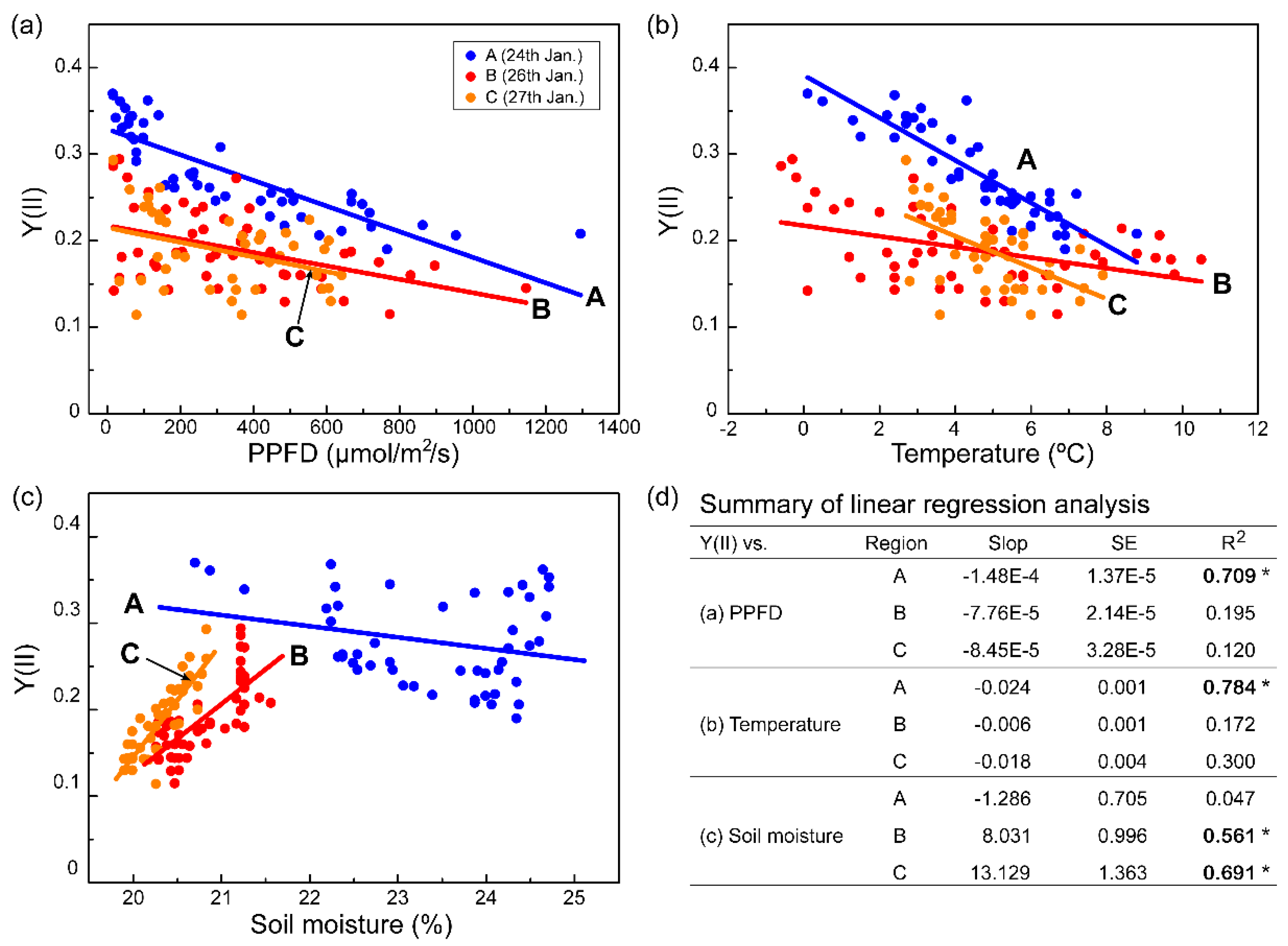
2.3. Water Deficiency Is a Limiting Factor for Photosynthesis of C. borealis
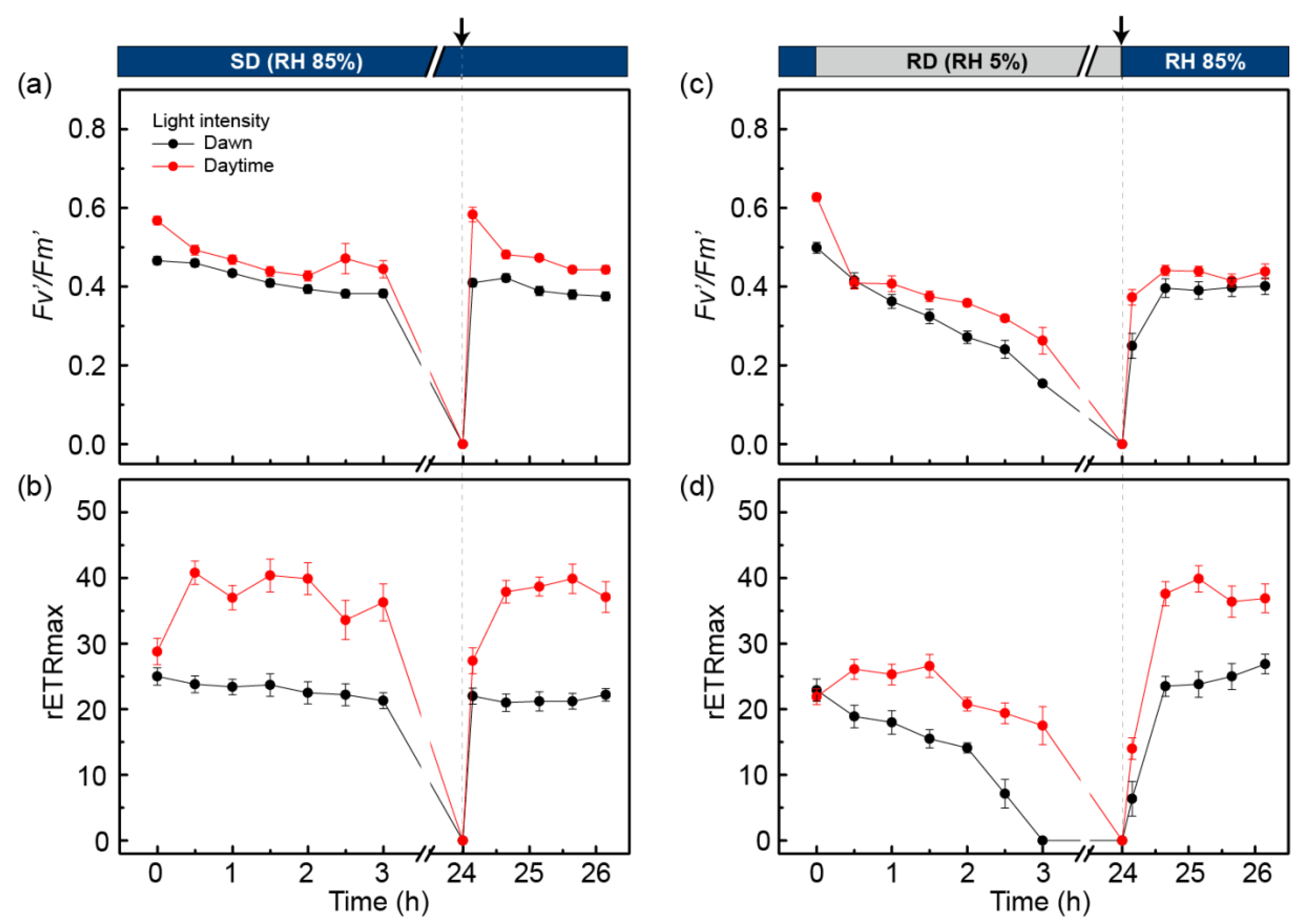
2.4. Photoinhibition Response of C. borealis under a Laboratory Mimic of Microclimate Conditions
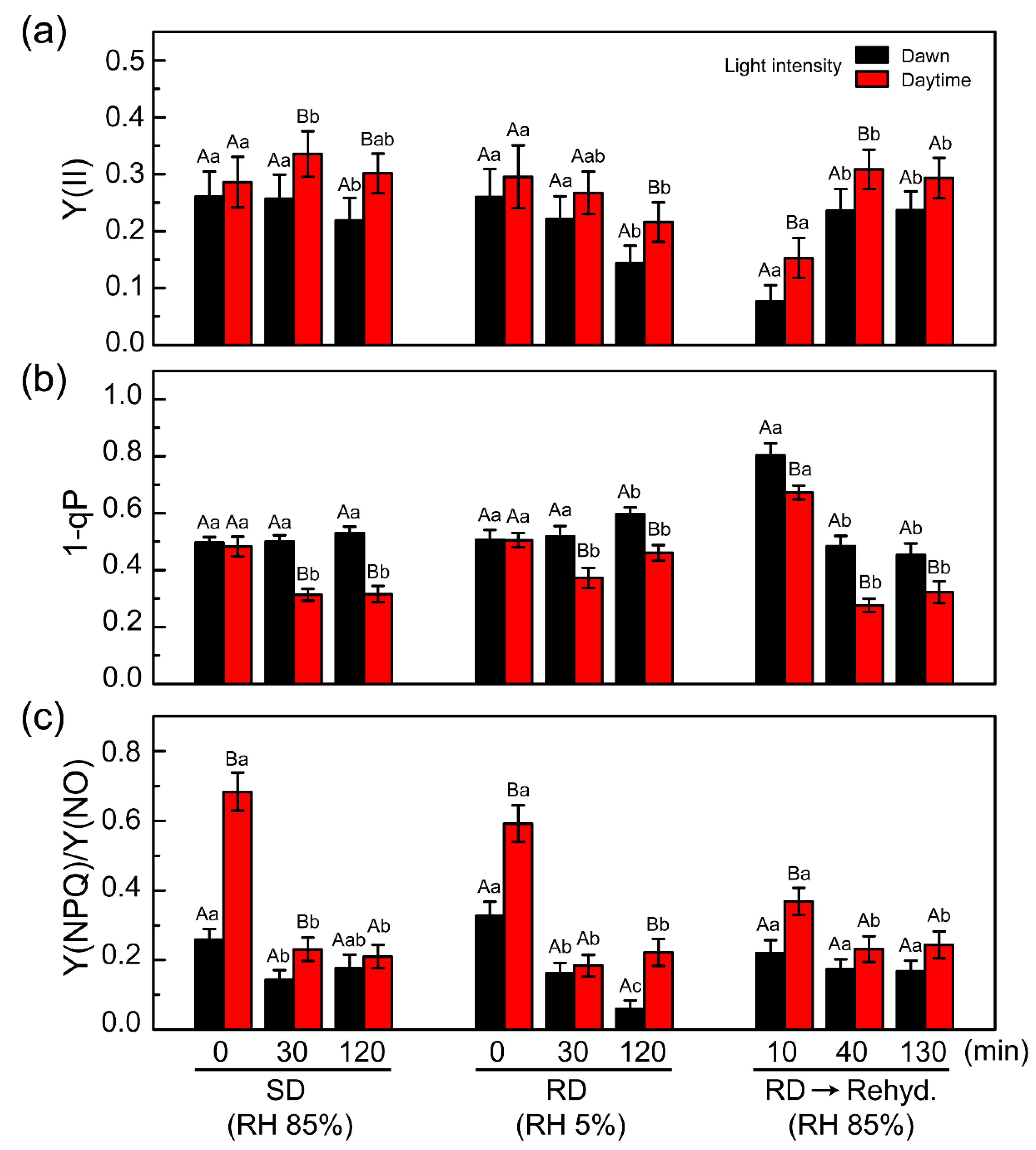
2.5. PSII Photochemistry Changes in C. borealis during the Dehydration Response
2.6. Major Photobiont of C. borealis Is Asterochloris Irregularis
3. Materials and Methods
3.1. Microclimate and Chlorophyll Fluorescence Measurement in the Field
3.2. Genomic DNA Extraction and Lichen Identification
3.3. Desiccation and Rehydration Treatment
3.4. Chlorophyll Fluorescence Measurement in the Laboratory
3.5. High-Throughput Amplicon Sequencing for Photobiont Diversity
3.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Y(II) | Quantum efficiency of photosystem II |
| Y(NO) | Quantum yield of nonregulated and non-photochemical energy dissipation at photosystem II |
| Y(NPQ) | Quantum yield of non-photochemical quenching at photosystem II |
| NPQ | non-photochemical quenching |
| ETR | Electron transfer rate |
| PSII | Photosystem II |
| PSI | Photosystem I |
| OEC | Oxygen evolving center |
| PQ | Plastoquinone |
| ROS | Reactive oxygen species |
| PPFD | Photosynthetic photon flux density |
| RH | Relative humidity |
| RWC | Relative water content |
| RD | Rapid desiccation |
| SD | Slow desiccation |
| RLC | Rapid light curve |
| AWS | Automatic weather station |
References
- Bewley, J.D. Physiological Aspects of Desiccation Tolerance. Annu. Rev. Plant Physiol. 1979, 30, 195–238. [Google Scholar] [CrossRef]
- Kranner, I. Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytol. 2002, 154, 451–460. [Google Scholar] [CrossRef]
- Rundel, P.W. Water relations. In Handbook of Lichenology; CRC Press, Inc.: Boca Raton, FL, USA, 1988; Volume 2, pp. 17–36. [Google Scholar]
- Green, T.A.; Schroeter, B.; Sancho, L.G. Plant life in Antarctica. In Functional Plant Ecology; CRC Press, Inc.: Boca Raton, FL, USA, 2007; pp. 408–453. [Google Scholar]
- Rudolph, E.D. Lichen ecology and microclimate studies at Cape Hallett, Antarctica. In Biometeorogy, Proceedings of the Third International Biometeorological Congress; Pergamon Press: Oxford, UK, 1966; Volume 2, pp. 900–910. [Google Scholar]
- Kappen, L. Ecophysiological relationships in different climatic regions. In Handbook of Lichenology; CRC Press, Inc.: Boca Raton, FL, USA, 1988; Volume 2, pp. 37–100. [Google Scholar]
- Lange, O.L. Ecophysiology of photosynthesis: Performance of poikilohydric lichens and homoiohydric Mediterranean sclerophylls. J. Ecol. 1988, 76, 915–937. [Google Scholar] [CrossRef]
- Nash, T.H., III; Moser, T.J.; Link, S.O. Nonrandom variation of gas exchange within arctic lichens. Can. J. Bot. 1980, 58, 1181–1186. [Google Scholar] [CrossRef]
- Oliver, M.J.; Derek Bewley, J. Desiccation-tolerance of plant tissues: A mechanistic overview. Hortic. Rev. 1996, 18, 171–213. [Google Scholar]
- Alpert, P.; Oliver, M.J. Drying without dying. In Desiccation and Survival in Plants: Drying without Dying; CABI Publishing: Wallingford, CT, USA, 2002; pp. 3–43. [Google Scholar]
- Proctor, M.C.; Pence, V.C. Vegetative tissues: Bryophytes, vascular resurrection plants and vegetative propagules. In Desiccation and Plant Survival; CABI Publishing: Wallingford, CT, USA, 2002; pp. 207–237. [Google Scholar]
- Proctor, M.C.; Smirnoff, N. Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: Chlorophyll fluorescence and inhibitor experiments. J. Exp. Bot. 2000, 51, 1695–1704. [Google Scholar] [CrossRef]
- Kranner, I.; Birtić, S. A modulating role for antioxidants in desiccation tolerance. Integr. Comp. Biol. 2005, 45, 734–740. [Google Scholar] [CrossRef]
- Palmqvist, K. Tansley review No. 117 carbon economy in lichens. New Phytol. 2000, 148, 11–36. [Google Scholar] [CrossRef]
- Heber, U. Photoprotection of green plants: A mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta 2008, 228, 641–650. [Google Scholar] [CrossRef]
- Komura, M.; Yamagishi, A.; Shibata, Y.; Iwasaki, I.; Itoh, S. Mechanism of strong quenching of photosystem II chlorophyll fluorescence under drought stress in a lichen, Physciella melanchla, studied by subpicosecond fluorescence spectroscopy. Biochim. Biophys. Acta 2010, 1797, 331–338. [Google Scholar] [CrossRef]
- Veerman, J.; Vasil’ev, S.; Paton, G.D.; Ramanauskas, J.; Bruce, D. Photoprotection in the lichen Parmelia sulcata: The origins of desiccation-induced fluorescence quenching. Plant Physiol. 2007, 145, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, T.D.; Król, M.; Huner, N.P.; Campbell, D.A. Seasonal changes in chlorophyll fluorescence quenching and the induction and capacity of the photoprotective xanthophyll cycle in Lobaria pulmonaria. Can. J. Bot. 2002, 80, 255–261. [Google Scholar] [CrossRef]
- MacKenzie, T.D.; MacDonald, T.M.; Dubois, L.A.; Campbell, D.A. Seasonal changes in temperature and light drive acclimation of photosynthetic physiology and macromolecular content in Lobaria pulmonaria. Planta 2001, 214, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, A.; Deltoro, V.I.; Barreno, E.; Valle-Tascon, S.D. Changes in in vivo chlorophyll fluorescence quenching in lichen thalli as a function of water content and suggestion of zeaxanthin-associated photoprotection. Physiol. Plant. 1997, 101, 93–102. [Google Scholar] [CrossRef]
- Kranner, I.; Cram, W.J.; Zorn, M.; Wornik, S.; Yoshimura, I.; Stabentheiner, E.; Pfeifhofer, H.W. Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc. Natl. Acad. Sci. USA 2005, 102, 3141–3146. [Google Scholar] [CrossRef]
- Zorn, M.; Pfeifhofer, H.W.; Grill, D.; Kranner, I. Responses of plastid pigments to desiccation and rehydration in the desert lichen Ramalina maciformis. Symbiosis Rehovot 2001, 31, 201–212. [Google Scholar]
- Sancho, L.G.; Pintado, A.; Navarro, F.; Ramos, M.; De Pablo, M.A.; Blanquer, J.M.; Raggio, J.; Valladares, F.; Green, T.G.A. Recent warming and cooling in the Antarctic Peninsula region has rapid and large effects on lichen vegetation. Sci. Rep. 2017, 7, 5689. [Google Scholar] [CrossRef]
- Sancho, L.G.; Pintado, A.; Green, T. Antarctic studies show lichens to be excellent biomonitors of climate change. Diversity 2019, 11, 42. [Google Scholar] [CrossRef]
- Laguna-Defior, C.; Pintado, A.; Green, T.A.; Blanquer, J.M.; Sancho, L.G. Distributional and ecophysiological study on the Antarctic lichens species pair Usnea antarctica/Usnea aurantiaco-atra. Polar Biol. 2016, 39, 1183–1195. [Google Scholar] [CrossRef]
- Barták, M.; Hájek, J.; Očenášová, P. Photoinhibition of photosynthesis in Antarctic lichen Usnea antarctica. I. Light intensity-and light duration-dependent changes in functioning of photosystem II. Czech Polar Rep. 2012, 2, 42–51. [Google Scholar] [CrossRef]
- Očenášová, P.; Barták, M.; Hájek, J. Photoinhibition of photosynthesis in Antarctic lichen Usnea antarctica. II. Analysis of non-photochemical quenching mechanisms activated by low to medium light doses. Czech Polar Rep. 2014, 4, 90–99. [Google Scholar] [CrossRef]
- Balarinová, K.; Barták, M.; Hazdrová, J.; Hájek, J.; Jílková, J. Changes in photosynthesis, pigment composition and glutathione contents in two Antarctic lichens during a light stress and recovery. Photosynthetica 2014, 52, 538–547. [Google Scholar] [CrossRef]
- Olech, M. Lichenological assessment of the Cape Lions Rump, King George Island, South Shetland Islands a baseline for monitoring biological changes. Pol. Polar Res. 1994, 15, 111–130. [Google Scholar]
- Olech, M. Annotated Checklist of Antarctic Lichens and Lichenicolous Fungi; Institute of Botany of the Jagiellonian University: Kraków, Poland, 2001; p. 145. [Google Scholar]
- Øvstedal, D.O.; Smith, R.L. Lichens of Antarctica and South Georgia: A Guide to Their Identification and Ecology; Cambridge University Press: Cambridge, UK, 2001; p. 411. [Google Scholar]
- Stenroos, S. Taxonomy and distribution of the lichen family Cladoniaceae in the Antarctic and peri-Antarctic regions. Cryptogam. Bot. 1993, 3, 310–344. [Google Scholar]
- Ahti, T. Cladoniaceae. Flora Neotropica Monograph 78; Organization for Flora Neotropica, New York Botanical Garden: New York, NY, USA, 2000; p. 362. [Google Scholar]
- Brodo, I.M.; Sharnoff, S.D.; Sharnoff, S. Lichens of North America; Yale University Press: New Haven, NJ, USA, 2001; p. 828. [Google Scholar]
- Kim, J.H.; Ahn, I.-Y.; Hong, S.G.; Andreev, M.; Lim, K.-M.; Oh, M.J.; Koh, Y.J.; Hur, J.-S. Lichen flora around the Korean Antarctic Scientific Station, King George Island, Antarctic. J. Microbiol. 2006, 44, 480–491. [Google Scholar] [PubMed]
- Osyczka, P.; Olech, M. The lichen genus Cladonia of King George Island, South Shetland Islands, Antarctica. Pol. Polar Res. 2005, 26, 107–123. [Google Scholar]
- Park, C.H.; Jeong, G.; Hong, S.G. Possible multiple introductions of Cladonia borealis to King George Island. Antarct. Sci. 2012, 24, 359–366. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.K.; Hur, J.-S.; Andreev, M.; Hong, S.G. Diversity of the lichenized fungi in King George Island, Antarctica, revealed by phylogenetic analysis of partial large subunit rDNA sequences. J. Microbiol. Biotechnol. 2008, 18, 1016–1023. [Google Scholar]
- Park, C.H.; Kim, K.M.; Elvebakk, A.; Kim, O.S.; Jeong, G.; Hong, S.G. Algal and fungal diversity in Antarctic lichens. J. Eukaryot. Microbiol. 2015, 62, 196–205. [Google Scholar] [CrossRef]
- Inoue, T.; Kudoh, S.; Uchida, M.; Tanabe, Y.; Inoue, M.; Kanda, H. Effects of substrate differences on water availability for Arctic lichens during the snow-free summers in the High Arctic glacier foreland. Polar Sci. 2014, 8, 397–412. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H. Desiccation-Tolerance in Lichens: A Review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. Photoinhibition in lichens depends on cortical characteristics and hydration. Lichenologist 2004, 36, 133–143. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Gauslaa, Y.; Nybakken, L.; Bilger, W. UV-induction of sun-screening pigments in lichens. New Phytol. 2003, 158, 91–100. [Google Scholar] [CrossRef]
- Barták, M.; Gloser, J.; Hájek, J. Visualized photosynthetic characteristics of the lichen Xanthoria elegans related to daily courses of light, temperature and hydration: A field study from Galindez Island, maritime Antarctica. Lichenologist 2005, 37, 433–443. [Google Scholar] [CrossRef]
- Lange, O.L.; Green, T.A. High thallus water content severely limits photosynthetic carbon gain of central European epilithic lichens under natural conditions. Oecologia 1996, 108, 13–20. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Máguas, C.; Adams, W.W.; Meyer, A.; Kilian, E.; Lange, O.L. Effect of high light on the efficiency of photochemical energy conversion in a variety of lichen species with green and blue-green phycobionts. Planta 1990, 180, 400–409. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of photosystem II. In International Review of Cell and Molecular Biology; Elsevier: Oxford, UK, 2013; Volume 300, pp. 243–303. [Google Scholar]
- Lange, O. Photosynthesis of soil-crust biota as dependent on environmental factors. In Biological Soil Crusts: Structure, Function, and Management; Springer: Heidelberg, Germany, 2001; Volume 150, pp. 217–240. [Google Scholar]
- Darrouzet-Nardi, A.; Reed, S.C.; Grote, E.E.; Belnap, J. Observations of net soil exchange of CO2 in a dryland show experimental warming increases carbon losses in biocrust soils. Biogeochemistry 2015, 126, 363–378. [Google Scholar] [CrossRef]
- Reiter, R.; Höftberger, M.; Green, T.A.; Türk, R. Photosynthesis of lichens from lichen-dominated communities in the alpine/nival belt of the Alps–II: Laboratory and field measurements of CO2 exchange and water relations. Flora-Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 34–46. [Google Scholar] [CrossRef]
- Harris, G.P. The ecology of corticolous lichens: II. The relationship between physiology and the environment. J. Ecol. 1971, 59, 441–452. [Google Scholar] [CrossRef]
- Hájek, J.; Barták, M.; Gloser, J. Effects of thallus temperature and hydration on photosynthetic parameters of Cetraria islandica from contrasting habitats. Photosynthetica 2001, 39, 427–435. [Google Scholar] [CrossRef]
- Büdel, B.; Scheidegger, C. Thallus morphology and anatomy. Lichen Biol. 1996, 2, 40–68. [Google Scholar]
- Lange, O.L. Photosynthetic productivity of the epilithic lichen Lecanora muralis: Long-term field monitoring of CO2 exchange and its physiological interpretation. I. Dependence of photosynthesis on water content, light, temperature, and CO2 concentration from laboratory measurements. Flora-Morphol. Distrib. Funct. Ecol. Plants 2002, 197, 233–249. [Google Scholar]
- Lange, O.L. Photosynthetic productivity of the epilithic lichen Lecanora muralis: Long-term field monitoring of CO2 exchange and its physiological interpretation: II. Diel and seasonal patterns of net photosynthesis and respiration. Flora-Morphol. Distrib. Funct. Ecol. Plants 2003, 198, 55–70. [Google Scholar] [CrossRef]
- Gasulla, F.; de Nova, P.G.; Esteban-Carrasco, A.; Zapata, J.M.; Barreno, E.; Guéra, A. Dehydration rate and time of desiccation affect recovery of the lichenic algae Trebouxia erici: Alternative and classical protective mechanisms. Planta 2009, 231, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Nabe, H.; Funabiki, R.; Kashino, Y.; Koike, H.; Satoh, K. Responses to desiccation stress in bryophytes and an important role of dithiothreitol-insensitive non-photochemical quenching against photoinhibition in dehydrated states. Plant Cell Physiol. 2007, 48, 1548–1557. [Google Scholar] [CrossRef][Green Version]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of Q A redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209. [Google Scholar] [CrossRef]
- Kooten, O.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Krause, G.; Vernotte, C.; Briantais, J.-M. Photoinduced quenching of chlorophyll fluorescence in intact chloroplasts and algae. Resolution into two components. Biochim. Biophys. Acta 1982, 679, 116–124. [Google Scholar] [CrossRef]
- Krause, G.H.; Jahns, P. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: Characterization and function. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 463–495. [Google Scholar]
- Allen, J.F.; Bennett, J.; Steinback, K.E.; Arntzen, C.J. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 1981, 291, 25. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Quick, W.P.; Stitt, M. An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochim. Biophys. Acta 1989, 977, 287–296. [Google Scholar] [CrossRef]
- Walters, R.G.; Horton, P. Resolution of components of non-photochemical chlorophyll fluorescence quenching in barley leaves. Photosynth. Res. 1991, 27, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Štepigová, J.; Gauslaa, Y.; Cempirkova-Vrablikova, H.; Solhaug, K. Irradiance prior to and during desiccation improves the tolerance to excess irradiance in the desiccated state of the old forest lichen Lobaria pulmonaria. Photosynthetica 2008, 46, 286. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Becerril, J.; García-Plazaola, J. Unravelling the roles of desiccation-induced xanthophyll cycle activity in darkness: A case study in Lobaria pulmonaria. Planta 2010, 231, 1335–1342. [Google Scholar] [CrossRef]
- Heber, U.; Lange, O.L.; Shuvalov, V.A. Conservation and dissipation of light energy as complementary processes: Homoiohydric and poikilohydric autotrophs. J. Exp. Bot. 2006, 57, 1211–1223. [Google Scholar] [CrossRef]
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef]
- Bačkor, M.; Peksa, O.; Škaloud, P.; Bačkorová, M. Photobiont diversity in lichens from metal-rich substrata based on ITS rDNA sequences. Ecotoxicol. Environ. Saf. 2010, 73, 603–612. [Google Scholar] [CrossRef]
- Beiggi, S.; Piercey-Normore, M.D. Evolution of ITS ribosomal RNA secondary structures in fungal and algal symbionts of selected species of Cladonia sect. Cladonia (Cladoniaceae, Ascomycotina). J. Mol. Evol. 2007, 64, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.M.; Reis, R.A.; Cruz, L.M.; Stocker-Wörgötter, E.; Grube, M.; Iacomini, M. Molecular studies of photobionts of selected lichens from the coastal vegetation of Brazil. FEMS Microbiol. Ecol. 2005, 54, 381–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelsen, M.; Gargas, A. Actin type intron sequences increase phylogenetic resolution: An example from Asterochloris (Chlorophyta: Trebouxiphceae). Lichenologist 2006, 38, 35–440. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Gargas, A. Dissociation and horizontal transmission of codispersing lichen symbionts in the genus Lepraria (Lecanorales: Stereocaulaceae). New Phytol. 2008, 177, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Peksa, O.; Škaloud, P. Do photobionts influence the ecology of lichens? A case study of environmental preferences in symbiotic green alga Asterochloris (Trebouxiophyceae). Mol. Ecol. 2011, 20, 3936–3948. [Google Scholar] [CrossRef]
- Piercey-Normore, M.D.; De Priest, P.T. Algal switching among lichen symbioses. Am. J. Bot. 2001, 88, 1490–1498. [Google Scholar] [CrossRef]
- Skaloud, P.; Peksa, O. Evolutionary inferences based on ITS rDNA and actin sequences reveal extensive diversity of the common lichen alga Asterochloris (Trebouxiophyceae, Chlorophyta). Mol. Phylogenetics Evol. 2010, 54, 36–46. [Google Scholar] [CrossRef]
- Škaloud, P.; Steinová, J.; Řídká, T.; Vančurová, L.; Peksa, O. Assembling the challenging puzzle of algal biodiversity: Species delimitation within the genus Asterochloris (Trebouxiophyceae, Chlorophyta). J. Phycol. 2015, 51, 507–527. [Google Scholar] [CrossRef]
- Yahr, R.; Vilgalys, R.; Depriest, P.T. Strong fungal specificity and selectivity for algal symbionts in Florida scrub Cladonia lichens. Mol. Ecol. 2004, 13, 3367–3378. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Pfündel, E.; Korhonen, J.F.; Juurola, E. A new monitoring PAM fluorometer (MONI-PAM) to study the short-and long-term acclimation of photosystem II in field conditions. Photosynth. Res. 2008, 96, 173–179. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Sun, W.Q. Methods for the study of water relations under desiccation stress. In Desiccation and Survival in Plants: Drying without Dying; CABI Publishing: Willingford, UK, 2002; pp. 47–91. [Google Scholar]

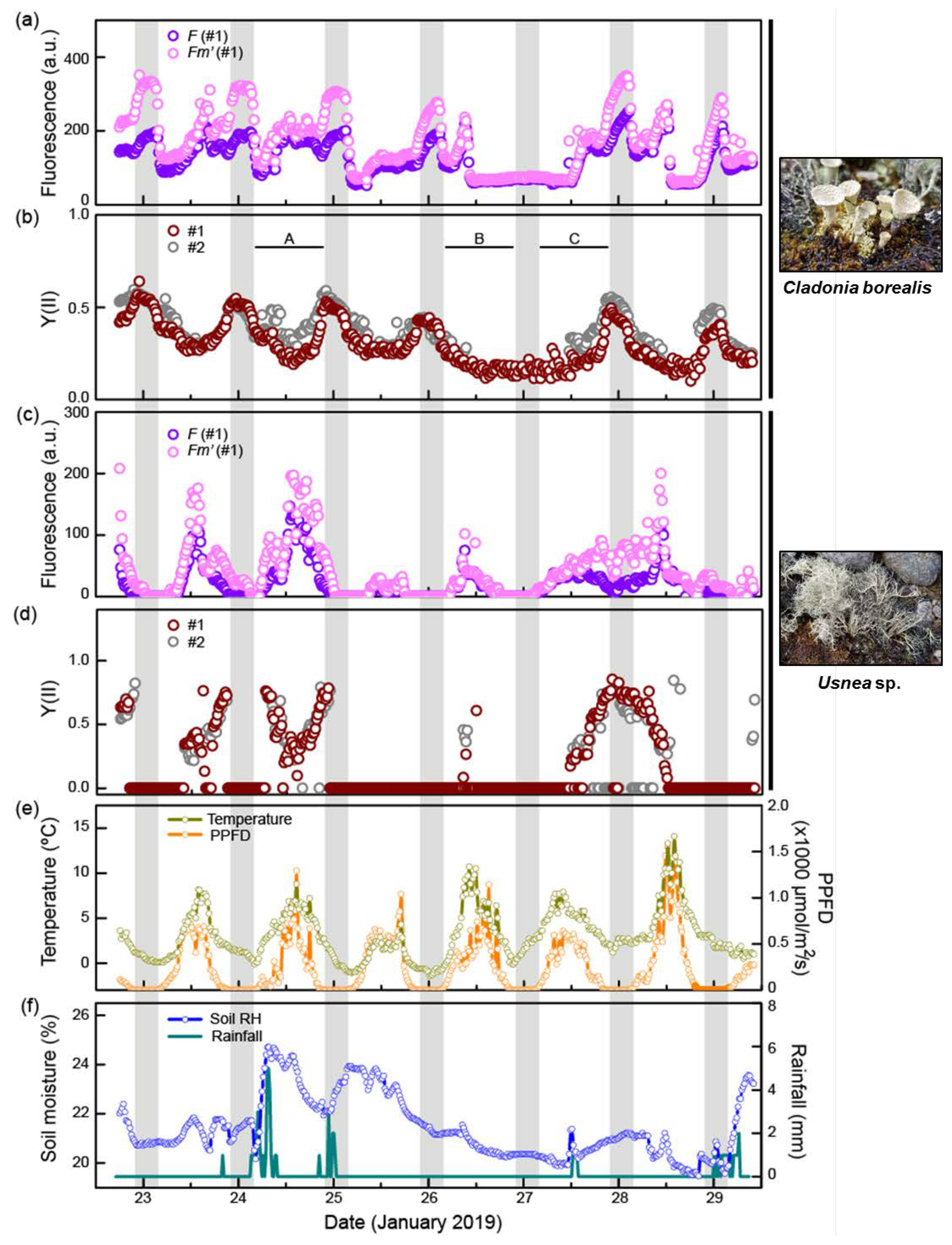
| Night | Dawn | Daytime | |
|---|---|---|---|
| Time range | 22:00–4:00 | 4:00–6:00 | 6:00–22:00 |
| Total data points | 133 | 42 | 316 |
| PPFD (μmol/m2/s) | 4 (± 2) | 47 (± 37) | 357 (± 289) |
| Temperature (°C) | 1.5 (± 1.3) | 1.5 (± 1.5) | 4.7 (± 2.9) |
| C. borealis | |||
| Y(II) | 0.41 (± 0.16) | 0.31 (± 0.12) | 0.26 (± 0.15) |
| Inactivation (%) * | 7.1% | 7.1 % | 14.1% |
| Usnea sp. | |||
| Y(II) | 0.09 (± 0.23) | 0.06 (± 0.19) | 0.19 (± 0.25) |
| Inactivation (%) | 87.6% | 91.7% | 58.9% |
| Id | NCBI Accession | Species | ANT#1 | ANT#2 | ||
|---|---|---|---|---|---|---|
| Copy No. | % | Copy No. | % | |||
| OTU01 | AM906000 | Asterochloris irregularis | 389,307 | 98.994 | 358,306 | 99.051 |
| OTU02 | MH415413 | Asterochloris irregularis voucher VancurovaO5 | 1902 | 0.484 | 56 | 0.015 |
| OTU03 | MF465900 | Coccomyxa antarctica isolate FACHB-2140 | 475 | 0.121 | 183 | 0.051 |
| OTU04 | FJ626732 | Trebouxia irregularis strain SAG 33.85 | 367 | 0.093 | 367 | 0.101 |
| OTU05 | HQ404871 | Coenochloris signiensis strain CCCryo 135-01 | 108 | 0.027 | 14 | 0.004 |
| OTU06 | KX147269 | Trebouxia jamesii isolate O2131C0001ASBM100076 | 104 | 0.026 | 424 | 0.117 |
| OTU07 | KY559180 | Trebouxia sp. ‘vagua’ isolate IB97 | 103 | 0.026 | 53 | 0.015 |
| OTU08 | KX181276 | Trebouxia impressa isolate L2239p | 95 | 0.024 | 10 | 0.003 |
| OTU09 | JX435372 | Uncultured Chlorophyta clone ALCE9 | 94 | 0.024 | 59 | 0.016 |
| OTU10 | JX435341 | Uncultured Chlorophyta clone ALBA5 | 84 | 0.021 | 176 | 0.049 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.M.; Lee, H.; Hong, S.G.; Lee, J. Study of Ecophysiological Responses of the Antarctic Fruticose Lichen Cladonia borealis Using the PAM Fluorescence System under Natural and Laboratory Conditions. Plants 2020, 9, 85. https://doi.org/10.3390/plants9010085
Cho SM, Lee H, Hong SG, Lee J. Study of Ecophysiological Responses of the Antarctic Fruticose Lichen Cladonia borealis Using the PAM Fluorescence System under Natural and Laboratory Conditions. Plants. 2020; 9(1):85. https://doi.org/10.3390/plants9010085
Chicago/Turabian StyleCho, Sung Mi, Hyoungseok Lee, Soon Gyu Hong, and Jungeun Lee. 2020. "Study of Ecophysiological Responses of the Antarctic Fruticose Lichen Cladonia borealis Using the PAM Fluorescence System under Natural and Laboratory Conditions" Plants 9, no. 1: 85. https://doi.org/10.3390/plants9010085
APA StyleCho, S. M., Lee, H., Hong, S. G., & Lee, J. (2020). Study of Ecophysiological Responses of the Antarctic Fruticose Lichen Cladonia borealis Using the PAM Fluorescence System under Natural and Laboratory Conditions. Plants, 9(1), 85. https://doi.org/10.3390/plants9010085






