Mass Spectrometry Imaging of Specialized Metabolites for Predicting Lichen Fitness and Snail Foraging
Abstract
1. Introduction
2. Results
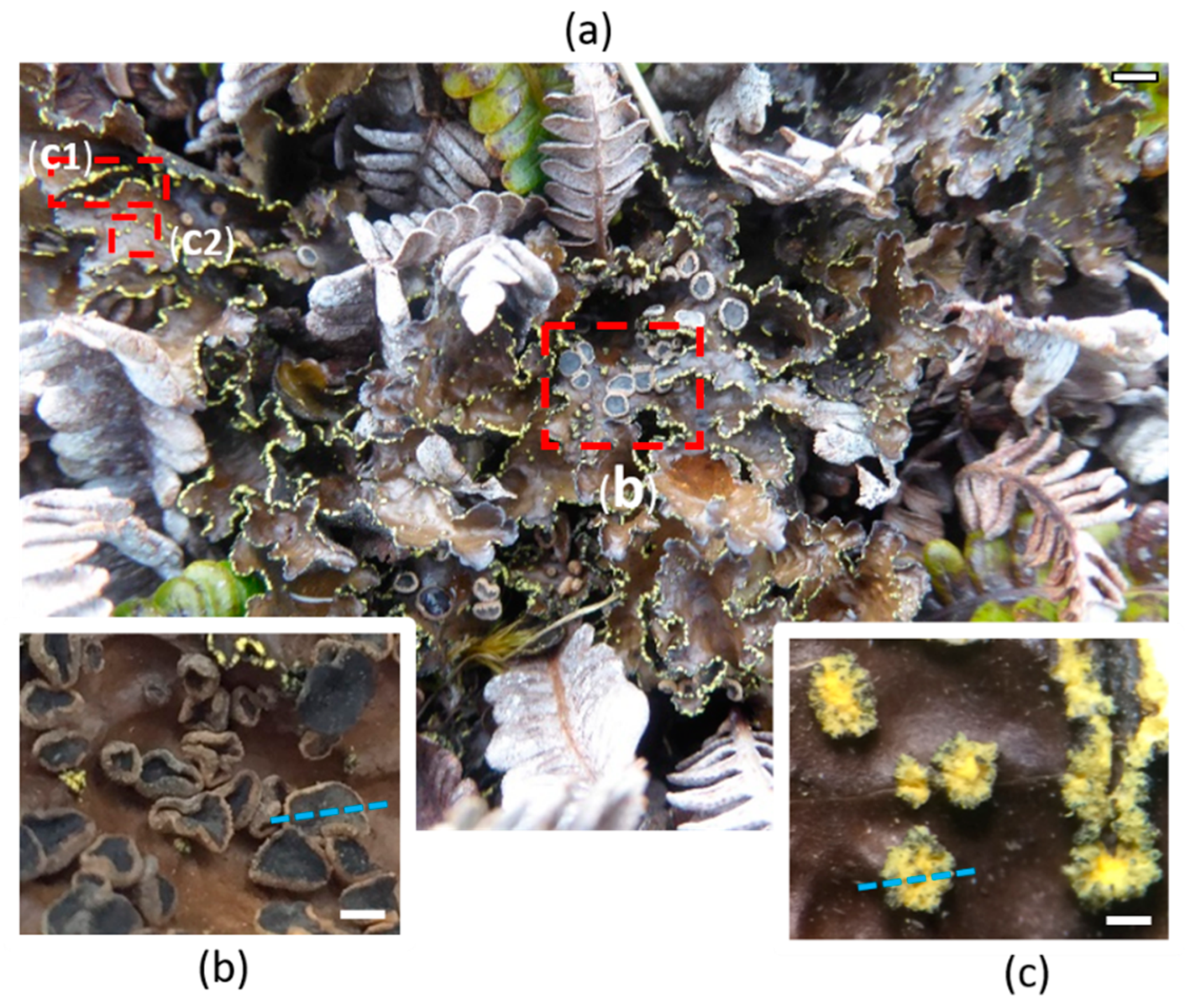
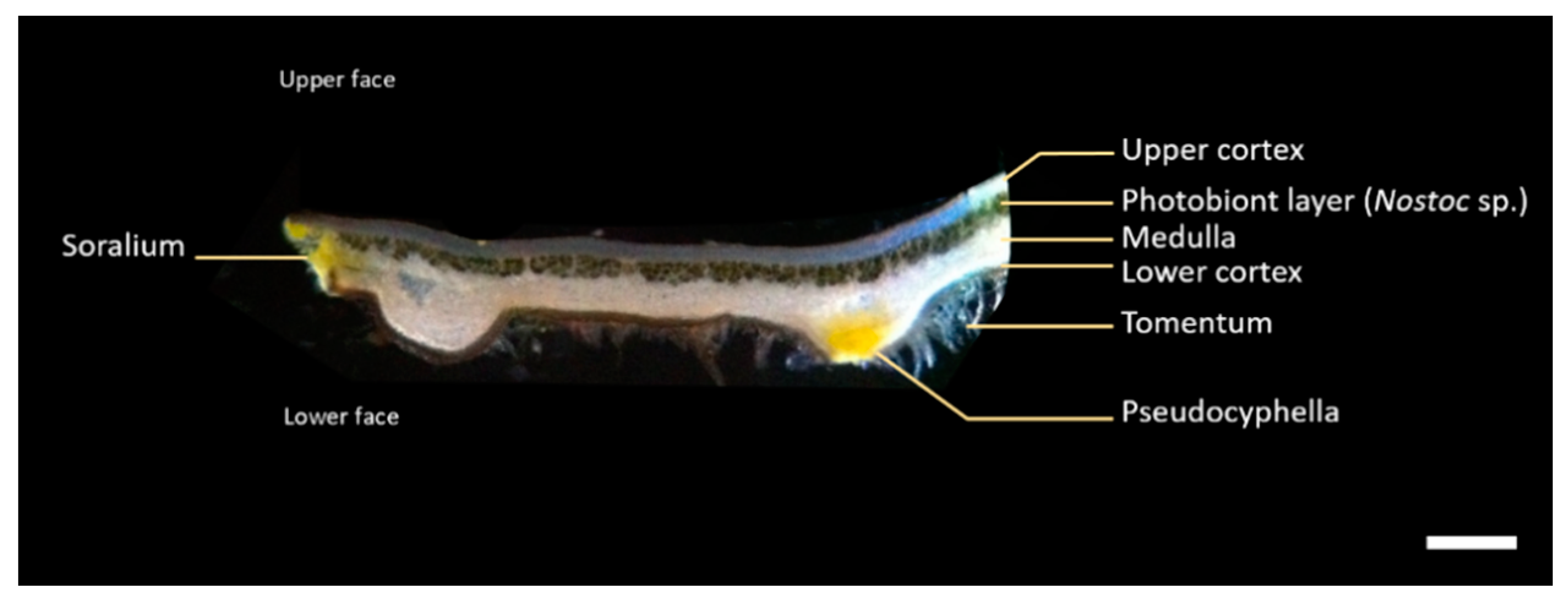
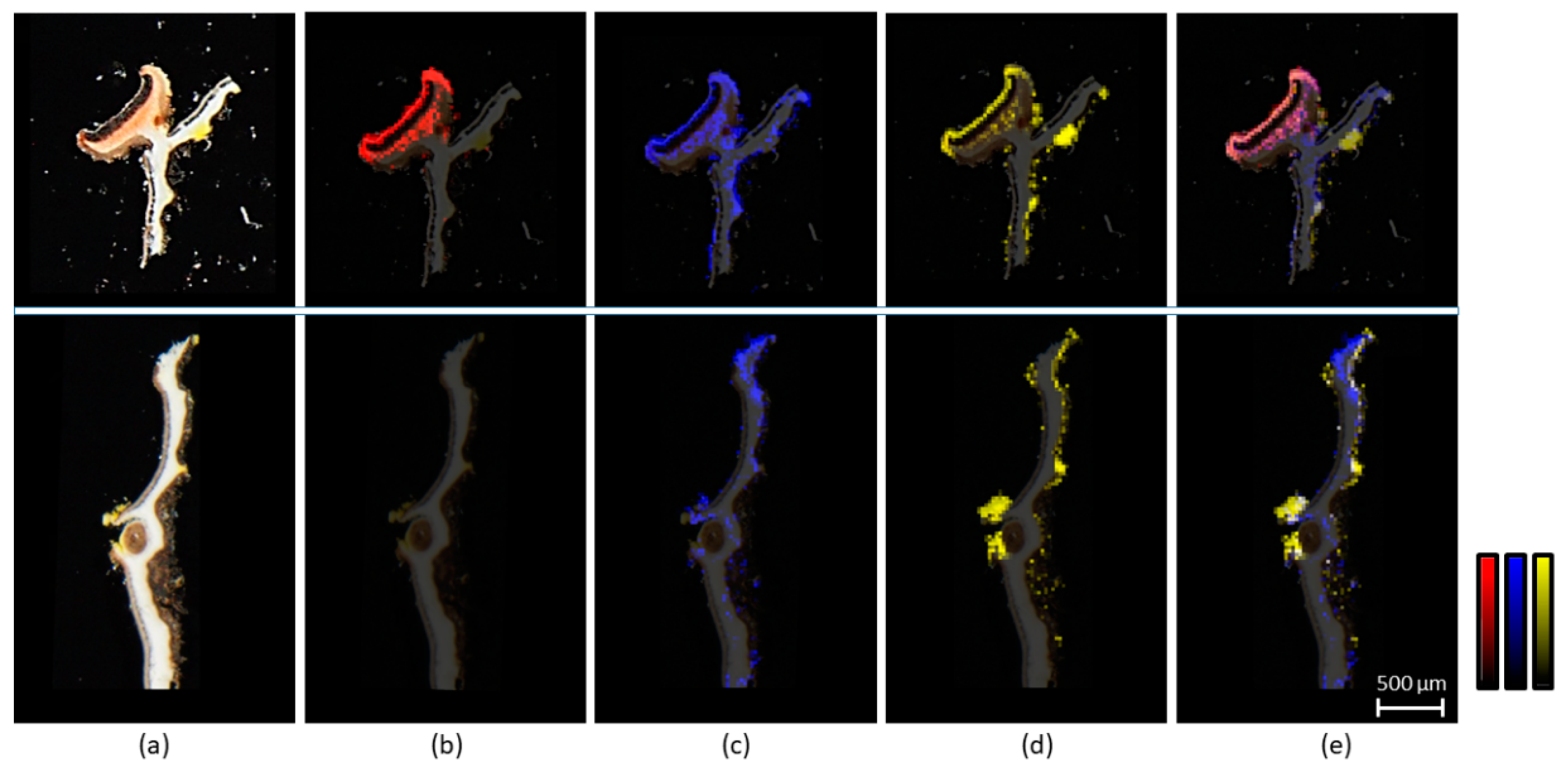
2.1. Morphological and Chemical Characterization of the Lichen Pseudocyphellaria crocata and Spatial Mapping of Metabolites by LDI-MSI
2.2. Quantification of Lichen Metabolites in P. crocata by HPLC-DAD-MS ESI(−)
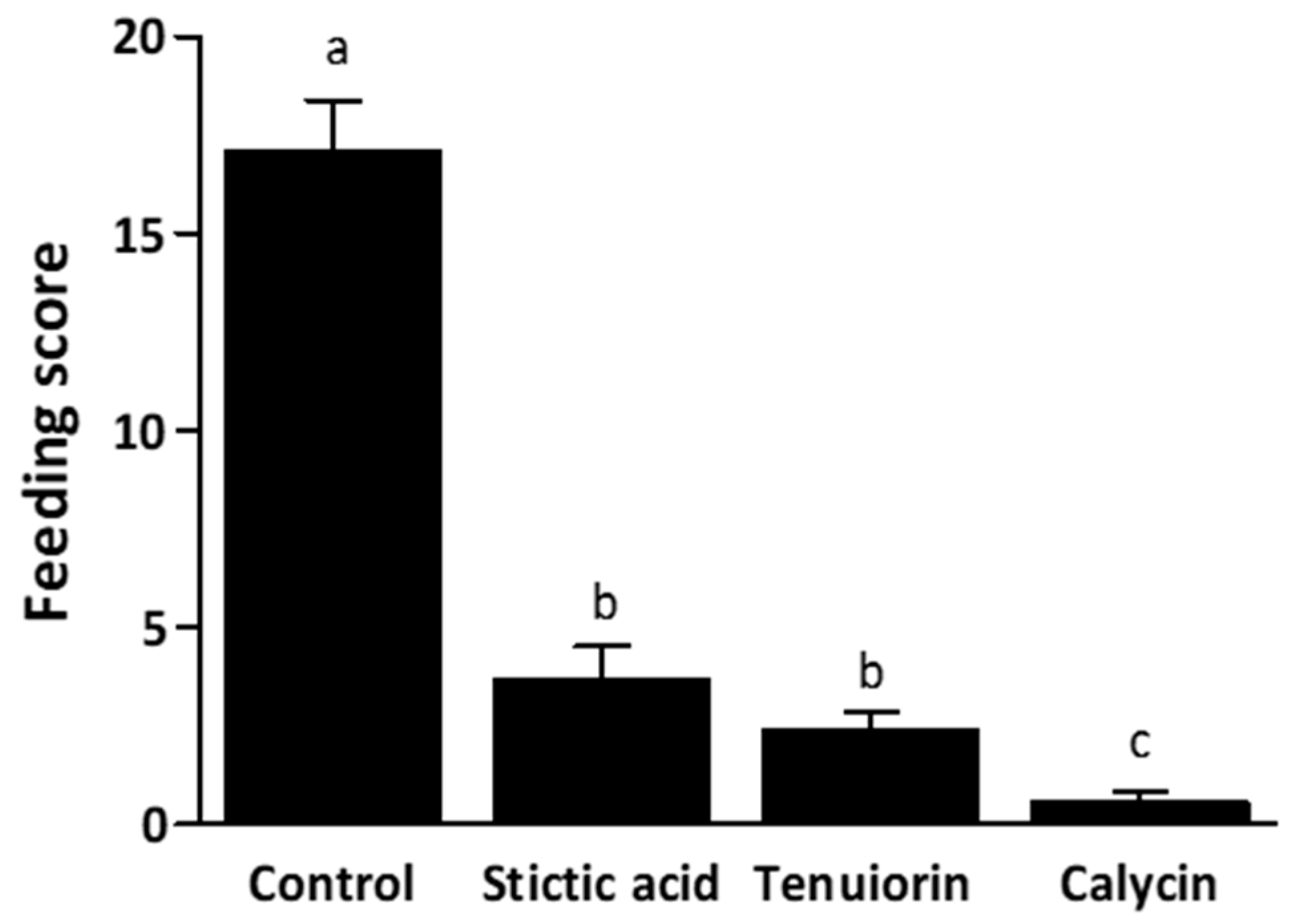
2.3. No-Choice Feeding Experiments with N. hookeri
3. Discussion
4. Materials and Methods
4.1. Biological Materials
4.2. Mass Spectrometry Imaging
4.3. HPLC-DAD-MS Analysis and Prediction of LogP and Water Solubility of Specialized Metabolites
4.4. Snails No Choice Experiments on Isolated Compounds
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Ethical Statement
Acknowledgments
Conflicts of Interest
References
- Lawrey, J.D. Chemical defense in lichen symbioses. In Defensive Mutualism in Microbial Symbiosis; White, J.F., Torres, M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 167–176. [Google Scholar]
- Schupp, P.; Eder, C.; Paul, V.; Proksch, P. Distribution of secondary metabolites in the sponge Oceanapia sp. and its ecological implications. Mar. Biol. 1999, 135, 573–580. [Google Scholar] [CrossRef]
- Kliebenstein, D. Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004, 27, 675–684. [Google Scholar] [CrossRef]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- McKey, D. Adaptive patterns in alkaloid physiology. Am. Nat. 1974, 108, 305–320. [Google Scholar] [CrossRef]
- Rhoades, D. Evolution of plant chemical defence against herbivores. In Herbivores: Their Interaction with Secondary Plant Metabolites’; Rosenthal, G., Janzen, D., Eds.; Academic Press: San Diego, CA, USA, 1979; pp. 3–54. [Google Scholar]
- Meldau, S.; Erb, M.; Baldwin, I.T. Defence on demand: Mechanisms behind optimal defence patterns. Ann. Bot. 2012, 110, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.A.; Mitchell-Olds, T. Testing the optimal defense hypothesis in nature: Variation for glucosinolate profiles within plants. PLoS ONE 2017, 12, e0180971. [Google Scholar]
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Naturforsch. C 2010, 65, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, M.; Koopmann, R.; Hormi, O.; Tuomi, J. Phenols in reproductive and somatic structures of lichens: A case of optimal defence? Oikos 2000, 91, 371–375. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Biochemical diversity and ecology of lichen-forming fungi: Lichen substances, chemosyndromic variation and origin of polyketide-type metabolites (biosynthetic pathways). In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; pp. 161–179. ISBN 978-81-322-2234-7. [Google Scholar]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances; Springer: Berlin, Germany; New York, NY, USA, 1996; ISBN 978-3-540-60811-0. [Google Scholar]
- Dworjanyn, S.A.; De Nys, R.; Steinberg, P.D. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar. Biol. 1999, 133, 727–736. [Google Scholar] [CrossRef]
- Conéjéro, G.; Noirot, M.; Talamond, P.; Verdeil, J.-L. Spectral analysis combined with advanced linear unmixing allows for histolocalization of phenolics in leaves of coffee trees. Front. Plant Sci. 2014, 5, 39. [Google Scholar]
- Talamond, P.; Verdeil, J.-L.; Conéjéro, G. Secondary metabolite localization by autofluorescence in living plant cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Perdian, D.C.; Song, Z.; Yeung, E.S.; Nikolau, B.J. Use of mass spectrometry for imaging metabolites in plants. Plant J. 2012, 70, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.; Schramm, K.; Jeschke, V.; Nemes, P.; Vertes, A.; Gershenzon, J.; Svatoš, A. Quantification of plant surface metabolites by matrix-assisted laser desorption–ionization mass spectrometry imaging: Glucosinolates on Arabidopsis thaliana leaves. Plant J. 2015, 81, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Burrell, M.M.; Earnshaw, C.J.; Clench, M.R. Imaging Matrix Assisted Laser Desorption Ionization Mass Spectrometry: A technique to map plant metabolites within tissues at high spatial resolution. J. Exp. Bot. 2007, 58, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Thunig, J.; Hansen, S.H.; Janfelt, C. Analysis of secondary plant metabolites by indirect desorption electrospray ionization imaging mass spectrometry. Anal. Chem. 2011, 83, 3256–3259. [Google Scholar] [CrossRef]
- Le Pogam, P.; Legouin, B.; Geairon, A.; Rogniaux, H.; Lohézic-Le Dévéhat, F.; Obermayer, W.; Boustie, J.; Le Lamer, A.-C. Spatial mapping of lichen specialized metabolites using LDI-MSI: Chemical ecology issues for Ophioparma ventosa. Sci. Rep. 2016, 6, 37807. [Google Scholar] [CrossRef]
- Gadea, A.; Charrier, M.; Fanuel, M.; Clerc, P.; Daugan, C.; Sauvager, A.; Rogniaux, H.; Boustie, J.; Le Lamer, A.-C.; Lohézic-Le Devehat, F. Overcoming deterrent metabolites by gaining essential nutrients: A lichen/snail case study. Phytochemistry 2019, 164, 86–93. [Google Scholar] [CrossRef]
- Gadea, A.; Le Pogam, P.; Biver, G.; Boustie, J.; Le Lamer, A.-C.; Le Dévéhat, F.; Charrier, M. Which specialized metabolites does the native Subantarctic Gastropod Notodiscus hookeri extract from the consumption of the lichens Usnea taylorii and Pseudocyphellaria crocata? Molecules 2017, 22, 425. [Google Scholar] [CrossRef]
- Maass, W.S.G. Pulvinamide and possible biosynthetic relationships with pulvinic acid. Phytochemistry 1970, 9, 2477–2481. [Google Scholar] [CrossRef]
- Maass, W.S.G. Lichen substances. V. Methylated derivatives of orsellinic acid, lecanoric acid, and gyrophoric acid from Pseudocyphellaria crocata. Can. J. Bot. 1975, 53, 1031–1039. [Google Scholar] [CrossRef]
- Maass, W.S.G. Lichen substances. IV. Incorporation of pulvinic-14C acids into calycin by the lichen Pseudocyphellaria crocata. Can. J. Biochem. 1970, 48, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Maass, W.S.G.; Neish, A.C. Lichen substances II: Biosynthesis of calycin and pulvinic dilactone by the lichen, Pseudocyphellaria crocata. Can. J. Bot. 1967, 45, 59–72. [Google Scholar] [CrossRef]
- Solem, A. The subantarctic land snail, Notodiscus hookeri (Reeve, 1854) (Pulmonata, Endodontidae). Proc. Malacol. Soc. Lond. 1968, 38, 251–265. [Google Scholar]
- Charrier, M.; Marie, A.; Guillaume, D.; Bédouet, L.; Le Lannic, J.; Roiland, C.; Berland, S.; Pierre, J.-S.; Le Floch, M.; Frenot, Y.; et al. Soil calcium availability influences shell ecophenotype formation in the Sub-Antarctic land snail, Notodiscus hookeri. PLoS ONE 2013, 8, e84527. [Google Scholar] [CrossRef] [PubMed]
- Gadea, A.; Le Lamer, A.-C.; Le Gall, S.; Jonard, C.; Ferron, S.; Catheline, D.; Ertz, D.; Le Pogam, P.; Boustie, J.; Lohézic-Le Dévéhat, F.; et al. Intrathalline metabolite profiles in the lichen Argopsis friesiana shape Gastropod grazing patterns. J. Chem. Ecol. 2018, 44, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Massé, L. Pseudocyphellaria crocata Vain, lichen nouveau pour l’île de la Possession (archipel Crozet, Terres Australes et Antarctiques Françaises) Ecologie sommaire et intérêt biogéographique. Comité Natl. Fr. Rech. Antarc. 1982, 51, 17–23. [Google Scholar]
- Galloway, D.J.; James, P.W. Nomenclatural Notes on Pseudocyphellaria in New Zealand. Lichenologist 1980, 12, 291–303. [Google Scholar] [CrossRef]
- Galloway, D.J.; Arvidsson, L. Studies in Pseudocyphellaria (lichens) II. Ecuadorean species. Lichenologist 1990, 22, 103–135. [Google Scholar] [CrossRef]
- Huneck, S.; Djerassi, C.; Becher, D.; Barber, M.; Von Ardenne, M.; Steinfelder, K.; Tümmler, R. Flechteninhaltsstoffe—XXXI: Massenspektrometrie und ihre anwendung auf strukturelle und streochemische probleme—CXXIII Massenspektrometrie von depsiden, depsidonen, depsonen, dibenzofuranen und diphenylbutadienen mit positiven und negativen ionen. Tetrahedron 1968, 24, 2707–2755. [Google Scholar] [CrossRef]
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular Imaging of Biological Samples: Localization of Peptides and Proteins Using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef]
- Parrot, D.; Papazian, S.; Foil, D.; Tasdemir, D. Imaging the Unimaginable: Desorption Electrospray Ionization – Imaging Mass Spectrometry (DESI-IMS) in Natural Product Research. Planta Med. 2018, 84, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Moncada, B.; Reidy, B.; Lücking, R. A phylogenetic revision of Hawaiian Pseudocyphellaria sensu lato (lichenized Ascomycota: Lobariaceae) reveals eight new species and a high degree of inferred endemism. Bryologist 2014, 117, 119–160. [Google Scholar] [CrossRef]
- Asplund, J. Snails avoid the medulla of Lobaria pulmonaria and L. scrobiculata due to presence of secondary compounds. Fungal Ecol. 2011, 4, 356–358. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Bidussi, M.; Solhaug, K.A.; Asplund, J.; Larsson, P. Seasonal and spatial variation in carbon based secondary compounds in green algal and cyanobacterial members of the epiphytic lichen genus Lobaria. Phytochemistry 2013, 94, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dudley, S.A.; Lechowicz, M.J. Losses of polyol through leaching in Subarctic lichens. Plant Physiol. 1987, 83, 813–815. [Google Scholar] [CrossRef] [PubMed]
- McCune, B.; Altermann, S. Letharia gracilis (Parmeliaceae), a New Species from California and Oregon. Bryologist 2009, 112, 375–378. [Google Scholar] [CrossRef]
- Moncada, B.; Lücking, R.; Betancourt-Macuase, L. Phylogeny of the Lobariaceae (lichenized Ascomycota: Peltigerales), with a reappraisal of the genus Lobariella. Lichenologist 2013, 45, 203–263. [Google Scholar] [CrossRef]
- Asplund, J.; Solhaug, K.A.; Gauslaa, Y. Fungal depsidones–An inducible or constitutive defence against herbivores in the lichen Lobaria pulmonaria? Basic Appl. Ecol. 2009, 10, 273–278. [Google Scholar] [CrossRef]
- Asplund, J.; Solhaug, K.A.; Gauslaa, Y. Optimal defense: Snails avoid reproductive parts of the lichen Lobaria scrobiculata due to internal defense allocation. Ecology 2010, 91, 3100–3105. [Google Scholar] [CrossRef]
- Boch, S.; Fischer, M.; Prati, D. To eat or not to eat—relationship of lichen herbivory by snails with secondary compounds and field frequency of lichens. J. Plant Ecol. 2015, 8, 642–650. [Google Scholar] [CrossRef]
- Delebassée, S.; Mambu, L.; Pinault, E.; Champavier, Y.; Liagre, B.; Millot, M. Cytochalasin E in the lichen Pleurosticta acetabulum. Anti-proliferative activity against human HT-29 colorectal cancer cells and quantitative variability. Fitoterapia 2017, 121, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Le Devehat, F.; Thüs, H.; Abasq, M.-L.; Delmail, D.; Boustie, J. Oxidative stress regulation in lichens and its relevance for survival in coastal habitats. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 467–503. [Google Scholar]
- Gauslaa, Y. Lichen palatability depends on investments in herbivore defence. Oecologia 2005, 143, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y. Mollusc grazing may constrain the ecological niche of the old forest lichen Pseudocyphellaria crocata. Plant Biol. 2008, 10, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Asplund, J. Chemical races of Lobaria pulmonaria differ in palatability to gastropods. Lichenologist 2011, 43, 491–494. [Google Scholar] [CrossRef]
- Lawrey, J.D. Vulpinic and pinastric acids as lichen antiherbivore compounds: Contrary evidence. Bryologist 1983, 86, 365–369. [Google Scholar] [CrossRef]
- Lücking, R.; Moncada, B.; McCune, B.; Farkas, E.; Goffinet, B.; Parker, D.; Chaves, J.L.; Lőkös, L.; Nelson, P.R.; Spribille, T.; et al. Pseudocyphellaria crocata (Ascomycota: Lobariaceae) in the Americas is revealed to be thirteen species, and none of them is P. crocata. Bryologist 2017, 120, 441–500. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual Computational Chemistry Laboratory–Design and Description. J. Comput. Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
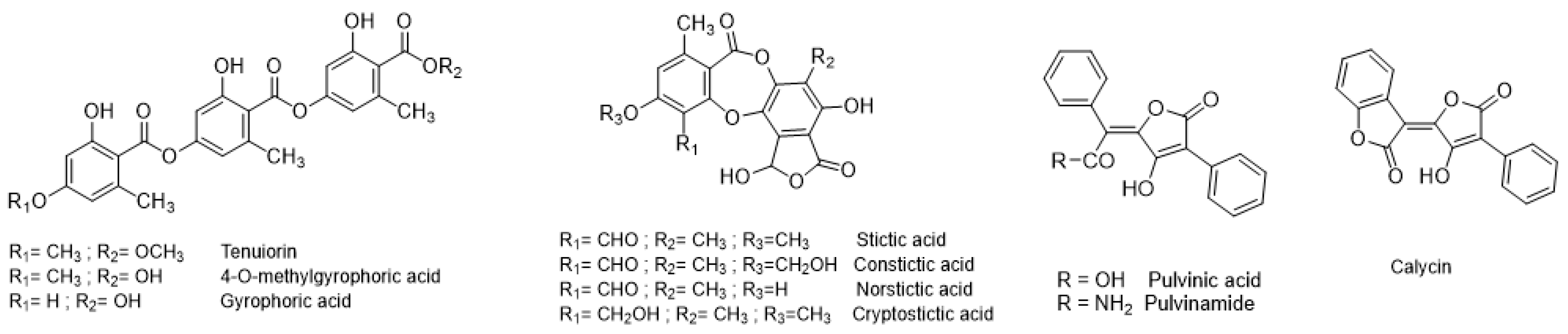
| Specialized Metabolites Described in P. crocata | Molecular Mass (g/mol) | LogP 1 | Water Solubility (× 104) | Levels of Specialized Metabolites in Thalli 2 (mg∙g−1 DM) | Levels of Specialized Metabolites in Apothecia 2 (mg∙g−1 DM) | Main MS Fragments m/z Expected in Negative Mode 3 | |
|---|---|---|---|---|---|---|---|
| Depsides | Tenuiorin | 496.471 | 4.26 | 0.10 | 56.03 ± 9.98 | 21.90 ± 1.48 | 149,167, 313, 331, 495 |
| Gyrophoric acid | 468.417 | 3.79 | 0.17 | 2.19 ± 0.31 | 3.23 ± 0.47 | 149, 167, 317, 467 | |
| 4-O-methylgyrophoric acid | 482.441 | 4.10 | 0.17 | 5.85 ± 1.13 | 6.62 ± 0.65 | 149, 167, 299, 331, 449, 481 | |
| Depsidones | Stictic acid | 386.312 | 1.67 | 3.46 | 3.84 ± 0.70 | 4.23 ± 0.35 | 285, 341, 385 |
| Norstictic acid | 372.285 | 1.64 | 6.91 | 2.96 ± 1.06 | 64.01 ± 11.68 | 327, 371 | |
| Constictic acid | 402.311 | 0.97 | 3.98 | 2.75 ± 0.46 | 2.14 ± 0.14 | 357, 401 | |
| Cryptostictic acid | 388.328 | 1.10 | 6.91 | 0.60 ± 0.07 | 1.07 ± 0.18 | 343, 387 | |
| Pulvinic acid derivatives | Calycin | 306,274 | 2.67 | 2.08 | 1.62 ± 0.20 | 0.51 ± 0.03 | 233, 305 |
| Pulvinic acid | 308.289 | 3.03 | 2.08 | 0.41 ± 0.06 | 0.35 ± 0.02 | 263, 307 | |
| Pulvinamide | 307.305 | 2.22 | 1.44 | 0.22 ± 0.02 | 0.18 ± 0.02 | 263, 306 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadea, A.; Fanuel, M.; Le Lamer, A.-C.; Boustie, J.; Rogniaux, H.; Charrier, M.; Lohézic-Le Devehat, F. Mass Spectrometry Imaging of Specialized Metabolites for Predicting Lichen Fitness and Snail Foraging. Plants 2020, 9, 70. https://doi.org/10.3390/plants9010070
Gadea A, Fanuel M, Le Lamer A-C, Boustie J, Rogniaux H, Charrier M, Lohézic-Le Devehat F. Mass Spectrometry Imaging of Specialized Metabolites for Predicting Lichen Fitness and Snail Foraging. Plants. 2020; 9(1):70. https://doi.org/10.3390/plants9010070
Chicago/Turabian StyleGadea, Alice, Mathieu Fanuel, Anne-Cécile Le Lamer, Joël Boustie, Hélène Rogniaux, Maryvonne Charrier, and Françoise Lohézic-Le Devehat. 2020. "Mass Spectrometry Imaging of Specialized Metabolites for Predicting Lichen Fitness and Snail Foraging" Plants 9, no. 1: 70. https://doi.org/10.3390/plants9010070
APA StyleGadea, A., Fanuel, M., Le Lamer, A.-C., Boustie, J., Rogniaux, H., Charrier, M., & Lohézic-Le Devehat, F. (2020). Mass Spectrometry Imaging of Specialized Metabolites for Predicting Lichen Fitness and Snail Foraging. Plants, 9(1), 70. https://doi.org/10.3390/plants9010070







