Studying the Influence of Apple Peel Polyphenol Extract Fortification on the Characteristics of Probiotic Yoghurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Peel Polyphenol Extract
2.2. Quality Characteristics of APPE
2.3. TPC and TFC of APPE
2.4. Preparation of Probiotic Yoghurt
2.5. Microbiological Analysis
2.6. Physicochemical Analysis
2.7. Sensory Evaluation
2.8. Data Analysis
3. Results and Discussion
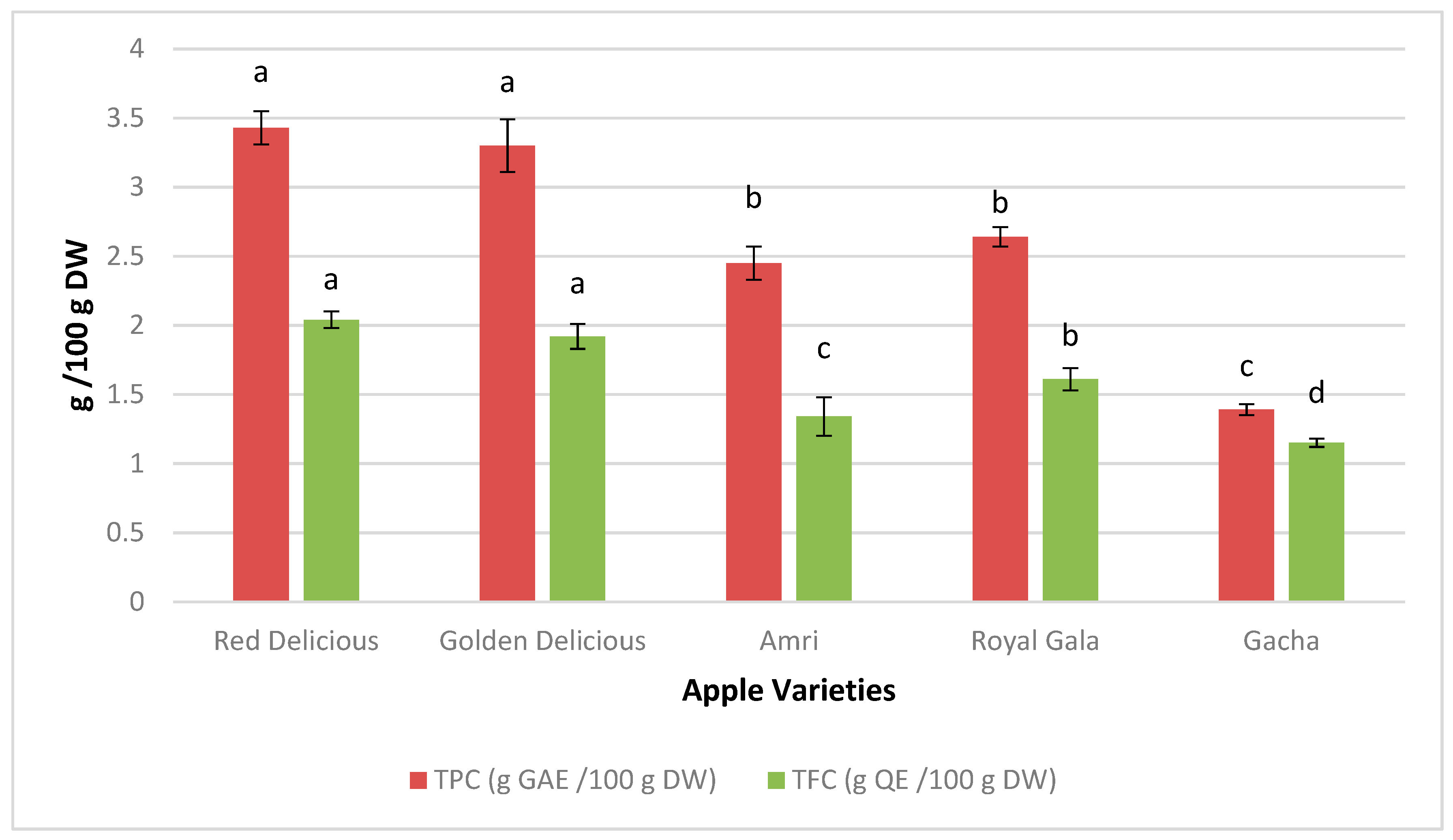
3.1. Quality Characteristics of APPE
3.2. Microbiological Characteristics of Probiotic Yoghurt
3.3. Chemical Characteristics of Probiotic Yoghurt
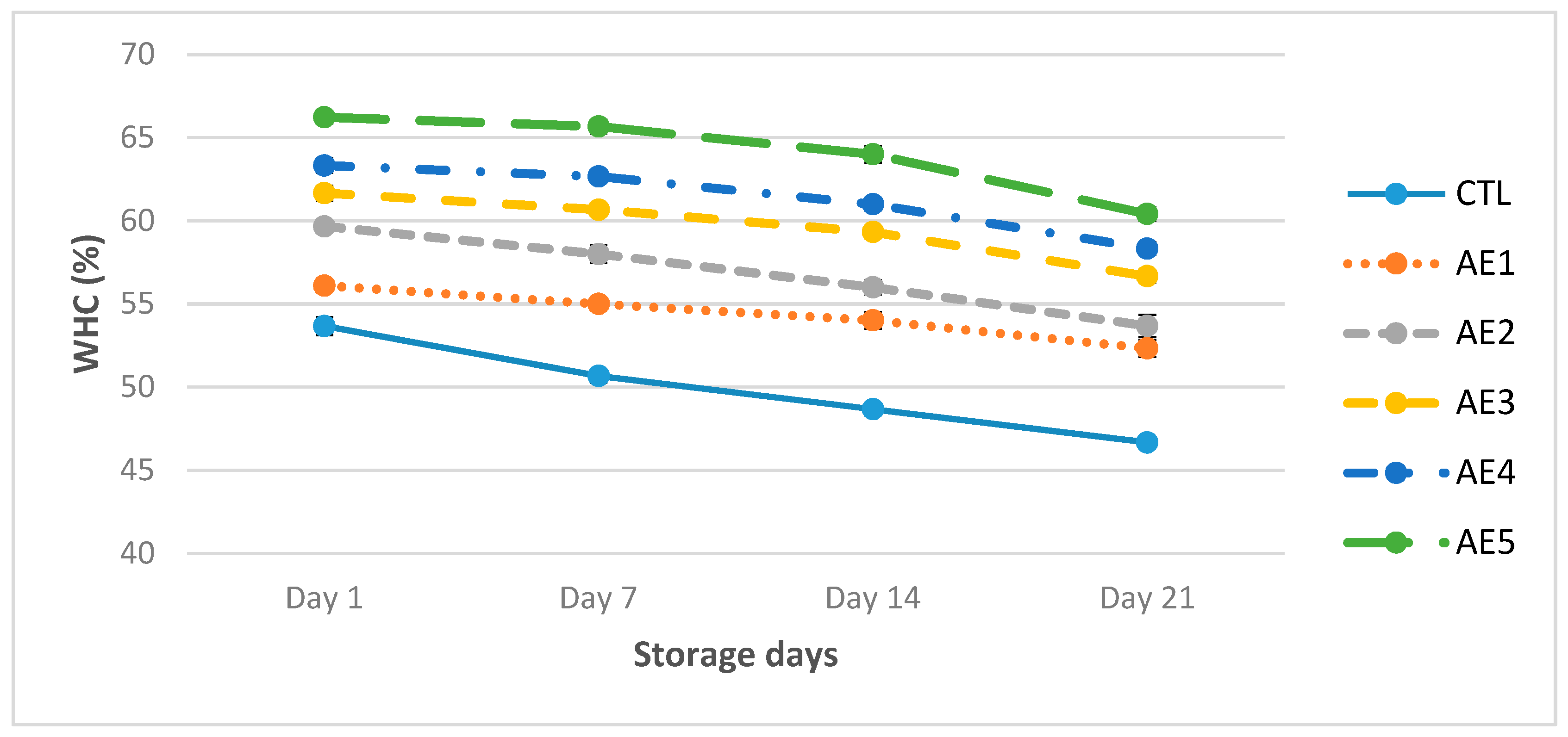
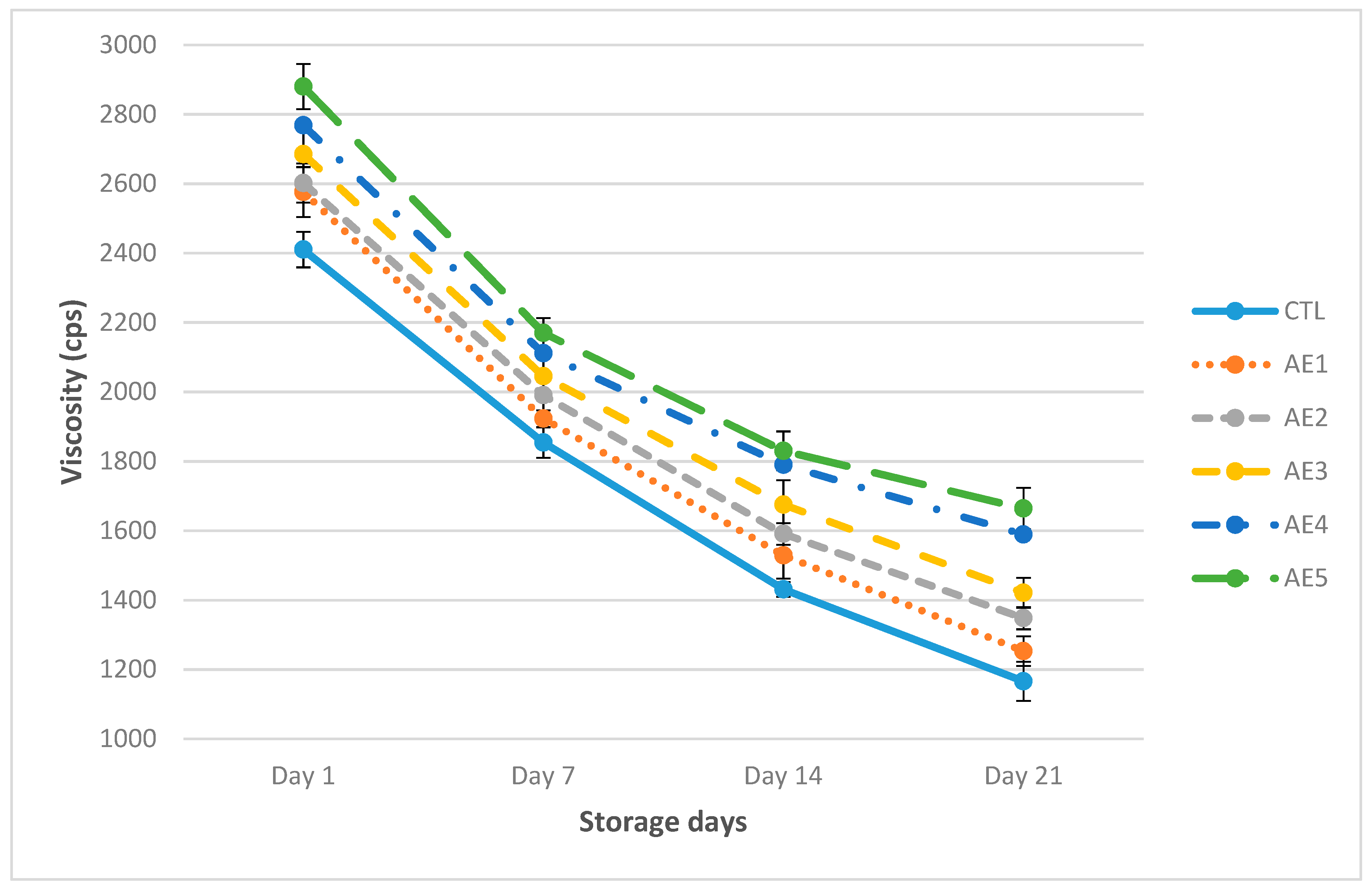
3.4. Physical Characteristics of Probiotic Yoghurt
3.5. Total Phenolic Contents (TPC) and Total Favonoid Content (TFC)
3.6. Antioxidant Activity
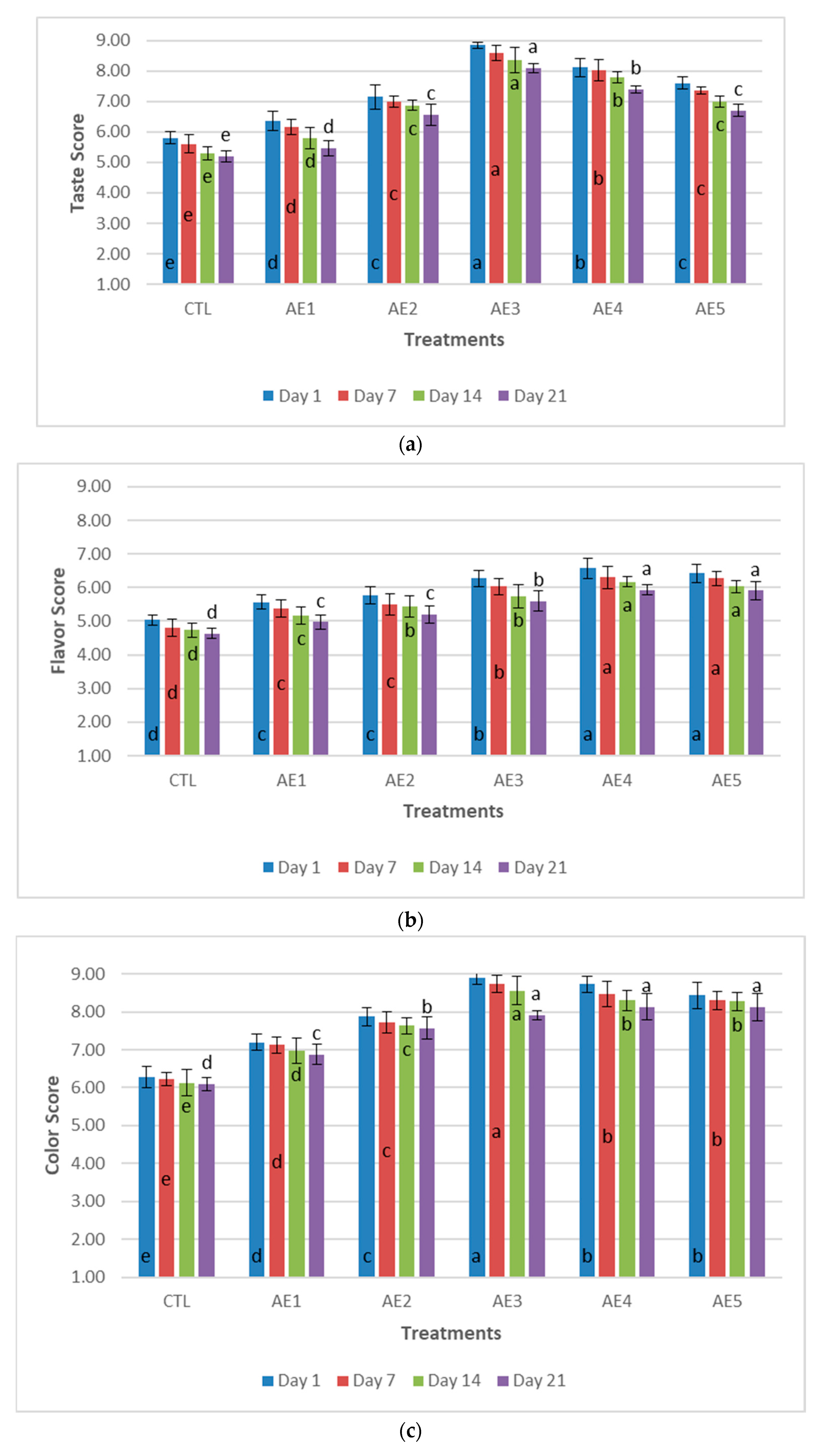
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, Y.; Oral, C.; Rodolfo, M.N. Assessing the demand for a functional food product: Is there cannibalization in the orange juice category? Agric. Resour. Econ. Rev. 2009, 38, 153–165. [Google Scholar] [CrossRef]
- Aurelia, P.; Capurso, L.; Castellazzi, A.M.; Clerici, M.; Giovanninie, M.; Morelli, L.; Poli, A.; Pregliasco, F.; Salvini, F.; Zuccotti, G.V. Probiotics and health: An evidence-based review. J. Pharm. Res. 2011, 63, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Effect of dairy probiotic combinations on in vitro gastrointestinal tolerance, intestinal epithelial cell adhesion and cytokine secretion. J. Funct. Foods 2014, 8, 18–25. [Google Scholar] [CrossRef]
- Fazilah, N.F.; Ariff, A.B.; Khayat, M.E.; Rios-Solis, L.; Halim, M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 2018, 48, 387–399. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Alenisan, M.A.; Alqattan, H.H.; Tolbah, L.S.; Shori, A.B. Antioxidant properties of dairy products fortified with natural additives: A review. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 24, 101–106. [Google Scholar] [CrossRef]
- Fernandez, A.; Barreiro-de, A.M.; Vallejo, N.; Iglesias, M.; Carmona, A.; Gonzalez-Portela, C.; Lorenzo, A.; Domínguez-Muñoz, J.E. Complementary and alternative medicine in inflammatory bowel disease patients: Frequency and risk factors. Dig. Liver Dis. 2012, 44, 904–908. [Google Scholar] [CrossRef]
- McGhie, T.K.; Hunt, M.; Barnett, L.E. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apple grown in New Zealand. J. Agric. Food Chem. 2005, 53, 3065–3070. [Google Scholar] [CrossRef]
- PES. Pakistan Economic Survey 2018–19; Economic Adviser’s Wing, Finance Division, Government of Pakistan: Islamabad, Pakistan, 2018; p. 31. [Google Scholar]
- Manzoor, M.; Anwar, F.; Saari, N.; Ashraf, M. Variations of antioxidant characteristics and mineral contents in pulp and peel of different apple (Malus domestica Borkh.) cultivars from Pakistan. Molecules 2012, 17, 390–407. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Markowski, J.; Kosmala, M.; Król, B.; Płocharski, W. Apple pomace as a potential source of nutraceutical products. Pol. J. Food Nutr. Sci. 2007, 57, 291–295. [Google Scholar]
- Gill, C.O. Microbiology of frozen foods. In Handbook of Frozen Food Processing and Packaging; Da-Wen, B.S., Ed.; CRC: Boca Raton, FL, USA, 2006; pp. 85–100. [Google Scholar]
- Koren, E.; Kohen, R.; Ovadia, H.; Ginsburg, I. Bacteria coated by polyphenols acquire potent oxidant scavenging capacities. Exp. BioMed. 2009, 234, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Shinde, T.S.; Brooks, J.D.; Sun-Waterhouse, D. Preparation and use of apple skin polyphenol extracts in milk: Enhancement of the viability and adhesion of probiotic Lactobacillus acidophilus (ATCC 1643) bacteria. Int. J. Food Sci. Technol. 2015, 50, 1303–1310. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Luberriaga, C.; Jin, D.; Wibisono, R.; Wadhwa, S.; Waterhouse, G. Juices, fibres and skin waste extracts from white, pink or red-fleshed apple genotypes as potential food ingredients. Food Bioprocess Technol. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; Vargas Mde, L.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt Science & Technology, 3rd ed.; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- IDF. General Standard of Identity for Fermented Milks, Standard No. 163; International Dairy Federation: Brussels, Belgium, 1992. [Google Scholar]
- Vasiljevic, T.; Kealy, T.; Mishra, V.K. Effects beta-glucan addition to a probiotic containing yogurt. J. Food Sci. 2007, 72, C405–C411. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists, AOAC, Int.: Rockville, MD, USA, 2016. [Google Scholar]
- Donkor, O.N.; Nilmini, S.L.I.; Stolic, P.; Vasiljevic, T.; Shah, N.P. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int. Dairy J. 2007, 17, 657–665. [Google Scholar] [CrossRef]
- Remeuf, F.; Mohammed, S.; Sodini, I.; Tissier, P. Preliminary observations on the effects of milk fortification and heating on microstructure and physical properties of stirred yoghurt. Int. Dairy J. 2003, 13, 773–782. [Google Scholar] [CrossRef]
- Khanahmadi, M.; Rezazadeh, S.H.; Taran, M. In vitro antimicrobial and antioxidant properties of Smyrnium cordifolium boiss (Umberlliferae) extract. Asian J. Plant Sci. 2010, 9, 99–103. [Google Scholar]
- Rogers, L. Sensory Panel Management: A Practical Handbook for Recruitment, Training and Performance; Woodhead Publishing Series: Duxford, UK, 2018. [Google Scholar]
- SAS. SAS/Stat User’s Guide: Statistics; Version 9.1; SAS Institute Inc.: Cary, NC, USA, 2002–2003.
- Can-Cauich, C.A.; Sauri-Duch, E.; Betancur-Ancon, D.; Chel-Guerrero, L.; González-Aguilar, G.A.; Cuevas-Glory, L.F.; Pérez-Pacheco, E.; Moo-Huchin, V.M. Tropical fruit peel powders as functional ingredients: Evaluation of their bioactive compounds and antioxidant activity. J. Funct. Foods 2017, 37, 501–506. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Effects of adding apple polyphenols before and after fermentation on the properties of drinking yoghurt. Food Bioprocess Technol. 2012, 5, 2674–2686. [Google Scholar] [CrossRef]
- Robinson, R.K. Dairy Microbiology Handbook, 3rd ed.; John Wiley and Sons Inc.: New York, NY, USA, 2002; pp. 441–453. [Google Scholar]
- Soccol, C.R.; Vandenberghe, L.P.D.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.; Pandey, A.; Thomaz-Soccol, V. The potential of probiotics: A review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of vegetable origin: A new alternative for the consumption of probiotic bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Sohail, A.; Turner, M.S.; Coombes, A.; Bhandari, B. The viability of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM following double encapsulation in alginate and maltodextrin. Food Bioprocess Technol. 2013, 6, 2763–2769. [Google Scholar] [CrossRef]
- Gurkan, H.; Osman, S.B.; Adnan, A.H. Influence of purple basil extract (Ocimum basilicum L.) on chemical composition, rheology and antioxidant activity of set-yoghurt. Mljekarstvo J. Dairy Prod Process. Improv. 2019, 69, 42–52. [Google Scholar] [CrossRef]
- Tura, D.; Robards, K. Sample handling strategies for the determination of biophenols in food and plants. J. Chromatogr. A 2002, 975, 71–93. [Google Scholar] [CrossRef]
- Renan, M.; Guyomarćh, F.; Arnoult-Delest, V.; Páquet, D.; Brulé, G.; Famelart, M.H. Rheological properties of stirred yoghurt as affected by gel pH on stirring, storage temperature and pH changes after stirring. Int. Dairy J. 2009, 19, 142–148. [Google Scholar] [CrossRef]
- Ramchandran, L.; Shah, N.P. Characterization of functional, biochemical and textural properties of symbiotic low fat yoghurts during refrigerated storage. Lebenson Wiss Technol. 2010, 43, 819–827. [Google Scholar] [CrossRef]
- Sodini, I.; Montella, J.; Tong, P.S. Physical properties of yoghurt fortified with various commercial whey proteins concentrates. J. Sci. Food Agric. 2005, 85, 853–859. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E. Emulsifying properties of whey protein-dextran conjugates at low pH and different salt concentrations. Colloids Surf. B Biointerfaces 2003, 31, 125–132. [Google Scholar] [CrossRef]
- Lucey, J.A.; Singh, H. Formation and physical properties of acid milk gels: A review. Food Rev. Int. 1998, 7, 529–542. [Google Scholar] [CrossRef]
- Lee, S.J.; Hwang, J.H.; Lee, S.; Ahn, J.; Kwak, H.S. Property changes and cholesterol-lowering effects in evening primrose oil enriched and cholesterol reduced yogurt. Int. J. Dairy Technol. 2007, 60, 22–30. [Google Scholar] [CrossRef]
- Supavititpatana, P.; Wirjantoro, T.I.; Raviyan, P. Characteristics and shelf-life of corn milk yogurt. Chiang Mai University. J. Nat. Sci. 2010, 9, 133–149. [Google Scholar]
- Aguirre-Ezkauriatza, E.J.; Galarza-González, M.G.; Uribe-Bujanda, A.I.; Ríos-Licea, M.; López Pacheco, F.; Hernández Brenes, C.M. Effect of mixing during fermentation in yoghurt manufacturing. J. Dairy Sci. 2008, 91, 4454–4465. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.S.; Zahoor, T.; Iqbal, Z.; Ihsan-ul-Haq, A.A. Effect of storage on the rheological and sensory characteristics of cow and buffalo milk yoghurt. Pak. J. Food Sci. 2012, 22, 61–70. [Google Scholar]
- Adesegun, S.A.; Elechi, N.A.; Coker, H.A.B. Antioxidant activities of methanolic extract of Sapium elliticum. Pak. J. Biol. Sci. 2008, 11, 453–457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chinnici, F.; Bendini, A.; Galani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. golden delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin—Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Schaich, K.M. Lipid Oxidation: A Chemical Stabilization Challenge for Packaging. In The Wiley Encyclopedia of Packaging Technology, 3rd ed.; Yam, K.L., Ed.; John Wiley and Sons Inc.: New York, NY, USA, 2009; pp. 659–667. [Google Scholar]
- Ayar, A.; Gurlin, E. Production and sensory, textural, physicochemical properties of flavored spreadable yoghurt. Life Sci. J. 2014, 11, 58–65. [Google Scholar]
- Bakirci, I.; Kavaz, A. An investigation of some properties of banana yogurts made with commercial ABT-2 starter culture during storage. Int. J. Dairy Technol. 2008, 61, 270–276. [Google Scholar] [CrossRef]

| Viable Count | Treatment | Storage Period | |||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | ||
| Streptococcus thermophilus (log cfu/mL) | CTL | 10.63 ± 0.49 Aa | 9.09 ± 0.02 Fb | 8.41 ± 0.06 Fc | 7.78 ± 0.05 Fd |
| AE1 | 10.50 ± 0.46 Aa | 9.45 ± 0.02 Eb | 9.02 ± 0.07 Ec | 8.83 ± 0.09 Ed | |
| AE2 | 10.53 ± 0.50 Aa | 9.80 ± 0.05 Db | 9.42 ± 0.02 Dc | 9.05 ± 0.04 Dd | |
| AE3 | 10.61 ± 0.34 Aa | 10.07 ± 0.03 Cb | 9.88 ± 0.09 Cc | 9.37 ± 0.05 Cd | |
| AE4 | 10.65 ± 0.34 Aa | 10.35 ± 0.03 Bb | 10.08 ± 0.08 Bc | 9.86 ± 0.12 Bd | |
| AE5 | 10.61 ± 0.15 Aa | 10.56 ± 0.07 Aa | 10.43 ± 0.02 Aa | 10.14 ± 0.11 Ab | |
| Lactobacillus bulgaricus (log cfu/mL) | CTL | 10.24 ± 0.29 Aa | 9.10 ± 0.12 Fb | 8.67 ± 0.21 Fc | 7.60 ± 0.08 Fd |
| AE1 | 10.14 ± 0.45 Aa | 9.37 ± 0.04 Eb | 8.83 ± 0.20 Ec | 8.24 ± 0.02 Ed | |
| AE2 | 10.22 ± 0.45 Aa | 9.55 ± 0.10 Db | 9.31 ± 0.01 Dc | 8.87 ± 0.09 Dd | |
| AE3 | 10.35 ± 0.22 Aa | 9.89 ± 0.10 Cb | 9.69 ± 0.11 Cc | 9.30 ± 0.04 Cd | |
| AE4 | 10.38 ± 0.20 Aa | 10.10 ± 0.06 Bb | 9.84 ± 0.17 Bc | 9.64 ± 0.10 Bd | |
| AE5 | 10.37 ± 0.39 Aa | 10.34 ± 0.59 Aa | 10.29 ± 0.03 Aa | 10.01 ± 0.11 Ab | |
| Lactobacillus acidophilus (log cfu/mL) | CTL | 11.14 ± 0.24 Aa | 9.85 ± 0.13 Fb | 8.67 ± 0.21 Fc | 8.07 ± 0.13 Fd |
| AE1 | 11.17 ± 0.15 Aa | 9.98 ± 0.10 Eb | 9.18 ± 0.10 Ec | 8.93 ± 0.13 Ed | |
| AE2 | 11.22 ± 0.22 Aa | 10.09 ± 0.17 Db | 9.46 ± 0.02 Dc | 9.15 ± 0.05 Dd | |
| AE3 | 11.24 ± 0.20 Aa | 10.37 ± 0.04 Cb | 10.03 ± 0.09 Cc | 9.64 ± 0.16 Cd | |
| AE4 | 11.20 ± 0.30 Aa | 10.68 ± 0.18 Bb | 10.39 ± 0.05 Bc | 10.12 ± 0.07 Bd | |
| AE5 | 11.16 ± 0.15 Aa | 11.08 ± 0.17 Aa | 10.77 ± 0.26 Ab | 10.41 ± 0.04 Ac | |
| Bifidobacterium lactis (log cfu/mL) | CTL | 10.71 ± 0.17 Aa | 9.39 ± 0.03 Fb | 8.37 ± 0.10 Fc | 7.81 ± 0.15 Fd |
| AE1 | 10.68 ± 0.54 Aa | 9.79 ± 0.14 Eb | 9.42 ± 0.10 Ec | 9.19 ± 0.07 Ed | |
| AE2 | 10.75 ± 0.10 Aa | 10.03 ± 0.08 Db | 9.80 ± 0.07 Dc | 9.60 ± 0.18 Dd | |
| AE3 | 10.68 ± 0.59 Aa | 10.19 ± 0.11 Cb | 10.04 ± 0.05 Cc | 9.87 ± 0.12 Cd | |
| AE4 | 10.76 ± 0.24 Aa | 10.40 ± 0.03 Bb | 10.18 ± 0.09 Bc | 10.05 ± 0.05 Bd | |
| AE5 | 10.80 ± 0.41 Aa | 10.76 ± 0.13 Aa | 10.36 ± 0.23 Ab | 10.23 ± 0.05 Ac | |
| Composition | Treatment | Storage Period | |||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | ||
| pH | CTL | 4.56 ± 0.01 Aa | 4.41 ± 0.03 Ab | 4.28 ± 0.03 Ac | 4.19 ± 0.02 Ad |
| AE1 | 4.51 ± 0.01 Aa | 4.40 ± 0.04 Ab | 4.24 ± 0.03 Ac | 4.13 ± 0.03 Ad | |
| AE2 | 4.47 ± 0.03 Aa | 4.36 ± 0.04 Ab | 4.22 ± 0.01 Ac | 4.04 ± 0.02 Ad | |
| AE3 | 4.46 ± 0.01 Aa | 4.33 ± 0.03 Ab | 4.14 ± 0.04 Ac | 4.03 ± 0.02 Ad | |
| AE4 | 4.40 ± 0.02 Aa | 4.31 ± 0.06 Ab | 4.19 ± 0.03 Ac | 4.12 ± 0.05 Ad | |
| AE5 | 4.36 ± 0.01 Aa | 4.33 ± 0.04 Aa | 4.14 ± 0.04 Ab | 4.02 ± 0.08 Ac | |
| Acidity (%) | CTL | 0.82 ± 0.08 Aa | 0.92 ± 0.06 Aa | 1.06 ± 0.04 Ab | 1.09 ± 0.06 Ab |
| AE1 | 0.79 ± 0.06 Aa | 0.92 ± 0.07 Ab | 1.10 ± 0.02 Ac | 1.11 ± 0.04 Ac | |
| AE2 | 0.90 ± 0.08 Aa | 1.00 ± 0.04 Aa | 1.15 ± 0.03 Ab | 1.18 ± 0.03 Ab | |
| AE3 | 0.91 ± 0.06 Aa | 1.00 ± 0.06 Aa | 1.15 ± 0.04 Ab | 1.20 ± 0.03 Ab | |
| AE4 | 0.95 ± 0.04 Aa | 1.05 ± 0.04 Aa | 1.18 ± 0.03 Ab | 1.22 ± 0.02 Ab | |
| AE5 | 1.01 ± 0.03 Aa | 1.10 ± 0.02 Aa | 1.24 ± 0.02 Ab | 1.25 ± 0.04 Ab | |
| Total Solids (%) | CTL | 14.48 ± 0.02 Ba | 14.45 ± 0.07 Ba | 14.47 ± 0.03 Ba | 14.44 ± 0.03 Ba |
| AE1 | 14.56 ± 0.06 Ba | 14.60 ± 0.05 Ba | 14.63 ± 0.03 Ba | 14.61 ± 0.03 Ba | |
| AE2 | 14.66 ± 0.06 Aa | 14.70 ± 0.03 Aa | 14.73 ± 0.05 Aa | 14.76 ± 0.05 Aa | |
| AE3 | 14.78 ± 0.09 Aa | 14.79 ± 0.02 Aa | 14.84 ± 0.08 Aa | 14.86 ± 0.04 Aa | |
| AE4 | 14.77 ± 0.22 Aa | 14.82 ± 0.07 Aa | 14.85 ± 0.01 Aa | 14.86 ± 0.03 Aa | |
| AE5 | 14.79 ± 0.11 Aa | 14.82 ± 0.02 Aa | 14.96 ± 0.02 Aa | 14.93 ± 0.08 Aa | |
| Composition | Treatment | Storage Period | |||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | ||
| Total Phenolic Contents (g GAE/100g DW) | CTL | 1.48 ± 0.91 Fc | 2.21 ± 0.17 Fb | 2.46 ± 0.06 Fa | 2.10 ± 0.16 Eb |
| AE1 | 3.54 ± 0.08 Ec | 3.97 ± 0.08 Eb | 4.34 ± 0.05 Ea | 4.01 ± 0.04 Db | |
| AE2 | 4.76 ± 0.45 Dc | 5.23 ± 0.05 Db | 5.86 ± 0.15 Da | 5.54 ± 0.21 Ca | |
| AE3 | 6.11 ± 0.20 Cc | 6.73 ± 0.17 Cb | 7.34 ± 0.17 Ca | 7.06 ± 0.32 Ba | |
| AE4 | 7.45 ± 0.12 Bc | 8.04 ± 0.11 Bb | 8.85 ± 0.23 Ba | 8.63 ± 0.28 Ba | |
| AE5 | 8.94 ± 0.16 Ab | 9.35 ± 0.16 Aa | 9.71 ± 0.21 Aa | 9.52 ± 0.17 Aa | |
| Total Flavonoid Contents (g QE/100g DW) | CTL | 0.07 ± 0.09 Ea | 0.07 ± 0.31 Da | 0.06 ± 0.34 Da | 0.04 ± 0.13 Da |
| AE1 | 0.59 ± 0.06 Da | 0.55 ± 0.20 Ca | 0.52 ± 0.04 Ca | 0.48 ± 0.15 Ca | |
| AE2 | 0.71 ± 0.16 Ca | 0.66 ± 0.18 Ca | 0.61 ± 0.07 Ca | 0.59 ± 0.21 Ca | |
| AE3 | 0.97 ± 0.30 Ca | 0.93 ± 0.15 Ba | 0.89 ± 0.16 Ba | 0.84 ± 0.14 Ba | |
| AE4 | 1.18 ± 0.24 Ba | 1.14 ± 0.12 Aa | 1.09 ± 0.13 Ba | 1.04 ± 0.13 Aa | |
| AE5 | 1.35 ± 0.15 Aa | 1.29 ± 0.07 Aa | 1.23 ± 0.31 Aa | 1.18 ± 0.11 Aa | |
| DPPH Inhibition (%) | CTL | 27.13 ± 1.53 Ca | 24.67 ± 2.08 Db | 29.21 ± 0.33 Da | 27.12 ± 0.58 Ca |
| AE1 | 39.21 ± 3.30 Ba | 34.70 ± 1.00 Ca | 28.21 ± 1.00 Db | 26.83 ± 1.53 Cb | |
| AE2 | 46.67 ± 1.53 Ba | 41.30 ± 2.65 Ba | 36.70 ± 4.00 Cb | 31.28 ± 3.21 Bb | |
| AE3 | 51.27 ± 1.15 Ba | 47.97 ± 1.15 Ba | 42.53 ± 1.53 Bb | 37.02 ± 2.08 Bb | |
| AE4 | 59.96 ± 2.52 Aa | 55.67 ± 1.53 Aa | 49.65 ± 1.00 Bb | 42.03 ± 4.04 Ab | |
| AE5 | 67.10 ± 2.00 Aa | 61.77 ± 0.58 Ab | 58.31 ± 0.58 Ab | 47.02 ± 2.31 Ac | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Khalique, A.; Shahid, M.Q.; Ahid Rashid, A.; Faiz, F.; Ikram, M.A.; Ahmed, S.; Imran, M.; Khan, M.A.; Nadeem, M.; et al. Studying the Influence of Apple Peel Polyphenol Extract Fortification on the Characteristics of Probiotic Yoghurt. Plants 2020, 9, 77. https://doi.org/10.3390/plants9010077
Ahmad I, Khalique A, Shahid MQ, Ahid Rashid A, Faiz F, Ikram MA, Ahmed S, Imran M, Khan MA, Nadeem M, et al. Studying the Influence of Apple Peel Polyphenol Extract Fortification on the Characteristics of Probiotic Yoghurt. Plants. 2020; 9(1):77. https://doi.org/10.3390/plants9010077
Chicago/Turabian StyleAhmad, Ishtiaque, Anjum Khalique, Muhammad Qamar Shahid, Abdul Ahid Rashid, Furukh Faiz, Muhammad Asim Ikram, Sheraz Ahmed, Muhammad Imran, Muhammad Asif Khan, Muhammad Nadeem, and et al. 2020. "Studying the Influence of Apple Peel Polyphenol Extract Fortification on the Characteristics of Probiotic Yoghurt" Plants 9, no. 1: 77. https://doi.org/10.3390/plants9010077
APA StyleAhmad, I., Khalique, A., Shahid, M. Q., Ahid Rashid, A., Faiz, F., Ikram, M. A., Ahmed, S., Imran, M., Khan, M. A., Nadeem, M., Afzal, M. I., Umer, M., Kaleem, I., Shahbaz, M., & Rasool, B. (2020). Studying the Influence of Apple Peel Polyphenol Extract Fortification on the Characteristics of Probiotic Yoghurt. Plants, 9(1), 77. https://doi.org/10.3390/plants9010077







