Comparison of the Androgenic Response of Spring and Winter Wheat (Triticum aestivum L.)
Abstract
1. Introduction
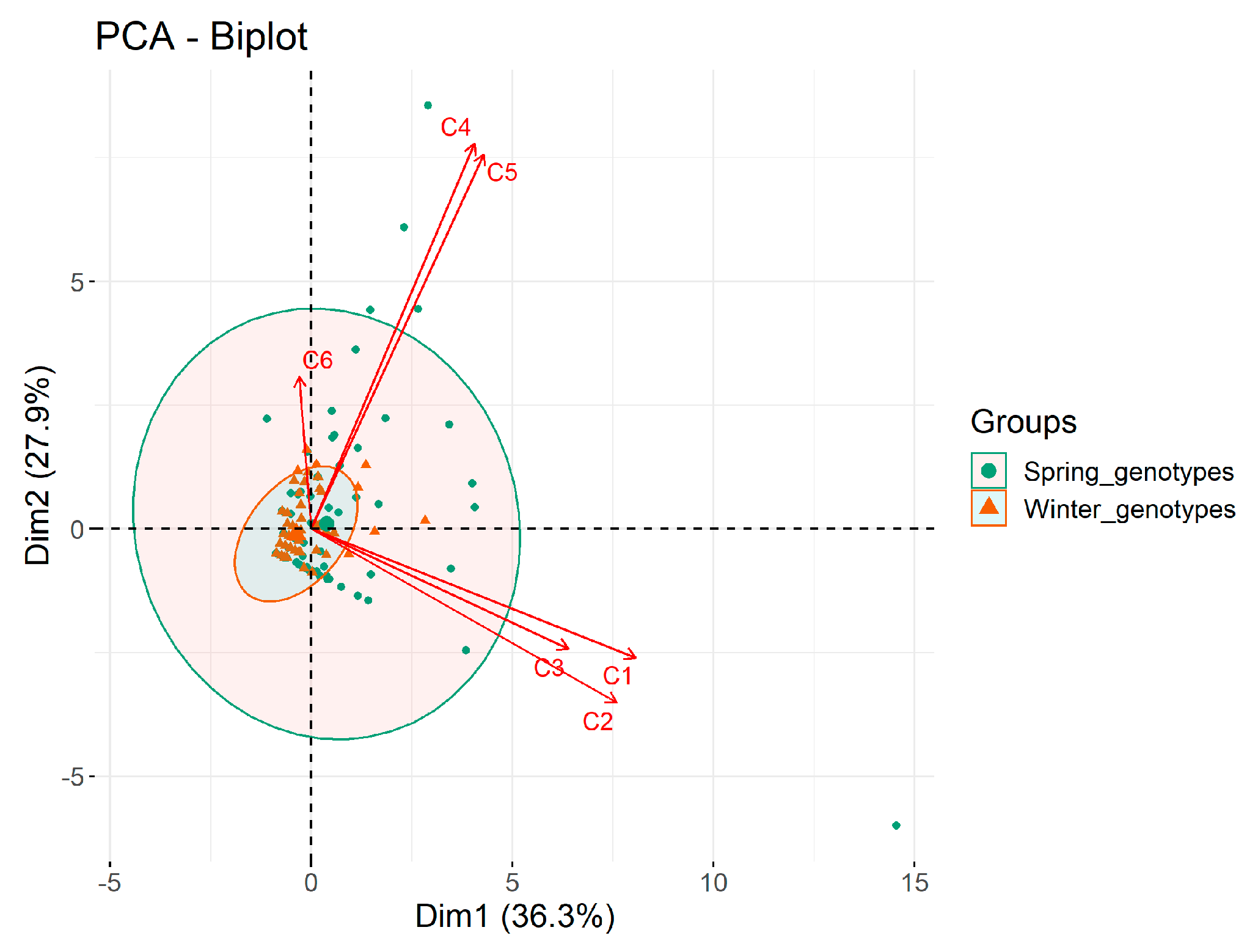
2. Results
2.1. Induction of Androgenesis
2.2. Regeneration of Green Plants
2.3. Regeneration of Albino Plants
3. Material and Methods
3.1. Plant Material
3.2. Initial Treatment of Donor Plants
3.3. Preparation and Course of In Vitro Cultures
3.4. Data Analysis
4. Discussion
5. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Touraev, A.; Indrianto, S.; Wratschko, I.; Vincente, O.; Heberle-Bors, E. Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperature. Sex. Plant Reprod. 1996, 9, 209–215. [Google Scholar] [CrossRef]
- Testillano, P.S. Microspore embryogenesis: Targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J. Exp. Bot. 2019, 70, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Oleszczuk, S.; Zimny, A.; Czaplicki, A.; Zimny, J. Androgenic capability among genotypes of winter and spring barley. Plant Breed. 2015, 134, 668–674. [Google Scholar] [CrossRef]
- Grauda, D.; Lepse, N.; Strazdiņa, V.; Kokina, I.; Lapiņa, L.; Miķelsone, A.; Lubinskis, L.; Rashal, I. Obtaining of doubled haploid lines by anther culture method for the Latvian wheat breeding. Agron. Res. 2010, 8, 545–552. [Google Scholar]
- Osadchaya, T.S.; Pershina, L.A.; Trubacheeva, N.V.; Belan, I.A.; Rosseeva, L.P.; Devyatkina, E.P. Androgenetic ability in euplasmic lines of common wheat and alloplasmic recombinant lines (H. vulgare)—T. aestivum carrying 1RS.1BL and 7DL−7Ai translocations and development of double haploid lines. Rus. J. Genet. Appl. Res. 2015, 5, 174–181. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Britt, A.B.; Tripathi, L.; Sharma, S.; Upadhyaya, H.D.; Ortiz, R. Haploids: Constraints and opportunities in plant breeding. Biotechnol. Adv. 2015, 33, 812–829. [Google Scholar] [CrossRef]
- Dunwell, J.M. Haploids in flowering plants: Origins and exploitation. Plant Biotechnol. J. 2010, 8, 377–424. [Google Scholar] [CrossRef]
- Lantos, C.; Bóna, L.; Boda, K.; Pauk, J. Comparative analysis of in vitro anther- and isolated microspore culture in hexaploid Triticale (X Triticosecale Wittmack) for androgenic parameters. Euphytica 2014, 197, 27–37. [Google Scholar] [CrossRef]
- Dağüstü, N. Diallel analysis of anther culture response in wheat (Triticum aestivum L.). Afr. J. Biotechnol. 2008, 7, 3419–3423. [Google Scholar]
- Chaudhary, H.K.; Dhaliwal, I.; Singh, S.; Sethi, G.S. Genetics of androgenesis in winter and spring wheat genotypes. Euphytica 2003, 132, 311–319. [Google Scholar] [CrossRef]
- Grauda, D.; Miķelsone, A.; Lisina, N.; Žagata, K.; Ornicāns, R.; Fokina, O.; Lapiņa, L.; Rashal, I. Anther culture effectiveness in producing doubled haploids of cereals. Proc. Latv. Acad. Sci. Sect. B 2014, 68, 142–147. [Google Scholar]
- Sharma, S.; Sethi, G.S.; Chaudhary, H.K. Influence of winter and spring wheat genetic backgrounds on haploid induction parameters and trait correlations in the wheat x maize system. Euphytica 2005, 144, 199–205. [Google Scholar] [CrossRef]
- Kim, K.M.; Baenziger, P.S. A simple wheat haploid and doubled haploid production system using anther culture. In Vitro Cell. Dev. Biol. Plant 2005, 41, 22–27. [Google Scholar] [CrossRef]
- Cistue, L.; Soriano, M.; Castillo, A.M.; Valles, M.P.; Sanz, J.M.; Echavarri, B. Production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep. 2006, 25, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, B.; Szakacs, E.; Karsai, I.; Bedö, Z. In vitro androgenesis of wheat: From fundamentals to practical application. Euphytica 2001, 119, 211–216. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y. Preliminary study on production of height of pollen H2 generation in winter wheat grown in the field. Acta Agron. Sin. 1983, 9, 283–284. [Google Scholar]
- Weigt, D.; Nawracała, J.; Popowska, D.; Nijak, K. Examination of ability to androgenesis of spring wheat genotypes resistant to Fusarium. BTa JBCBB 2012, 93, 116–122. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Weigt, D.; Kiel, A.; Nawracała, J.; Tomkowiak, A.; Kurasiak-Popowska, D.; Siatkowski, I.; Ługowska, B. Obtaining doubled haploid lines of the Lr19 gene using anther cultures of winther wheat genotypes. BTa JBCBB 2016, 97, 285–293. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.4.0; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 20 May 2019).
- Zamani, I.; Kovacs, G.; Gouli-Vavdinoudi, E.; Roupakias, D.G.; Barnabas, B. Regeneration of fertile doubled haploid plants from colchicine-supplemented media in wheat anther culture. Plant Breed. 2000, 119, 461–465. [Google Scholar] [CrossRef]
- Immonen, S.; Robinson, J. Stress treatments and ficoll for improving green plant regeneration in triticale anther culture. Plant Sci. 2000, 150, 77–84. [Google Scholar] [CrossRef]
- Marciniak, K.; Kaczmarek, Z.; Adamski, T.; Surma, M. The anther culture response of Triticale line x tester progenies. Cell. Mol. Biol. Lett. 2003, 8, 343–351. [Google Scholar] [PubMed]
- Ponitka, A.; Ślusarkiewicz-Jarzina, A. The effect of liquid and solid medium on production of winter triticale (× Triticosecale Wittm.) anther-derived embryos and plants. Cereal Res. Commun. 2007, 35, 15–22. [Google Scholar] [CrossRef]
- Ślusarkiewicz-Jarzina, A.; Ponitka, A. Doubled haploid production of winter and spring triticale hybrids using colchicine in anther culture. Bull. Ihar 2015, 276, 57–67. [Google Scholar]
- Ślusarkiewicz-Jarzina, A.; Pudelska, H.; Woźna, J.; Pniewski, T. Improved production of doubled haploids of winter and spring triticale hybrids via combination of colchicine treatments on anthers and regenerated plants. J. Appl. Genet. 2017, 58, 287–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, M.Y.; Konzak, C.F. Effect of 2,4 dichlorophenoxyacetic acid on callus induction and plant regeneration in anther culture of wheat (Triticum aestivum L.). Plant Cell Rep. 1999, 19, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Kruglova, N.; Abramov, S.N. The Induction of Androgenesis in vitro in Spring Soft Wheat. Balance of Exogenous and Endogenous Phytohormones. Biol. Bull. 2001, 28, 25–30. [Google Scholar] [CrossRef]
- Ponitka, A.; Ślusarkiewicz-Jarzina, A. Regeneration of oat androgenic plants in relation to induction media and culture conditions of embryo-like structures. Acta Soc. Bot. Pol. 2011, 78, 209–213. [Google Scholar] [CrossRef]
- Seldimirova, O.A.; Zaytsev, D.Y.; Galin, I.R.; Kruglova, N.N. Phytohormonal regulation of in vitro formation of wheat androgenic structures. Res. Result Physiol. Ser. 2016, 1, 1–6. [Google Scholar] [CrossRef][Green Version]
- Makowska, K.; Oleszczuk, S. Albinism in barley androgenesis. Plant Cell Rep. 2014, 33, 385–392. [Google Scholar] [CrossRef]
- Krzewska, M.; Czyczyło-Mysza, I.; Dubas, E.; Gołębiowska-Pikania, G.; Żur, I. Identification of QTLs associated with albino plant formation and some new facts concerning green versus albino ratio determinants in triticale (x Triticosecale Wittm.) anther culture. Euphytica 2015, 206, 263–278. [Google Scholar] [CrossRef]
- Weigt, D.; Kiel, A.; Nawracała, J.; Pluta, M.; Łacka, A. Solid-stemmed spring wheat cultivars give better androgenic response than hollow-stemmed cultivars in anther culture. In Vitro Cell. Dev. Biol. Plant 2016, 52, 619–625. [Google Scholar] [CrossRef] [PubMed][Green Version]
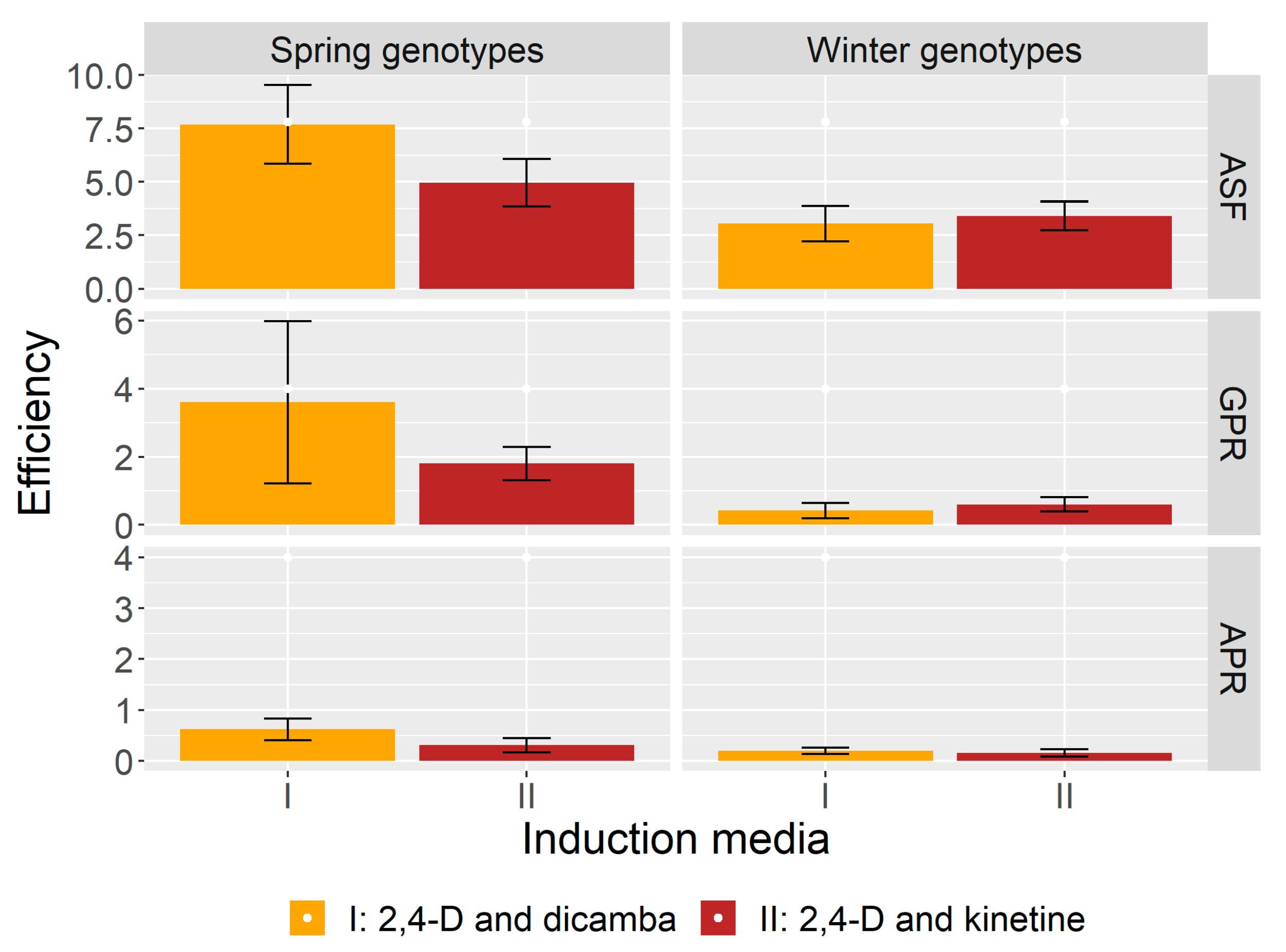
| Phenotype | Genotype | ASF | GPR | APR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | Avg. | I | II | Avg. | I | II | Avg. | ||
| Spring | Tybalt | 2.7 | 1.7 | 2.2 | 2.3 | 0.0 | 1.2 | 0.0 | 0.3 | 0.2 |
| Rescue | 0.7 | 3.3 | 2.0 | 0.0 | 2.7 | 1.3 | 0.7 | 0.0 | 0.3 | |
| Fortuna | 4.0 | 0.0 | 2.0 | 1.3 | 0.0 | 0.7 | 0.7 | 2.0 | 1.3 | |
| Leda Collection A47 | 5.3 | 0.7 | 3.0 | 1.3 | 0.7 | 1.0 | 2.7 | 0.0 | 1.3 | |
| Ostka Smolicka | 8.0 | 1.3 | 4.7 | 0.0 | 1.3 | 0.7 | 0.0 | 0.0 | 0.0 | |
| Ruzynska II | 4.0 | 8.0 | 6.0 | 2.7 | 6.7 | 4.7 | 0.0 | 0.0 | 0.0 | |
| Carola | 4.7 | 3.3 | 4.0 | 0.0 | 2.7 | 1.3 | 1.3 | 0.7 | 1.0 | |
| AC Abbey | 24.7 | 6.0 | 15.3 | 36.7 | 3.3 | 20.0 | 2.0 | 0.0 | 1.0 | |
| Tioga | 23.3 | 13.3 | 18.3 | 1.3 | 3.3 | 2.3 | 0.7 | 0.0 | 0.3 | |
| Parabola | 3.3 | 4.7 | 4.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.3 | |
| Chinook | 5.3 | 11.3 | 8.3 | 0.0 | 3.3 | 1.7 | 0.0 | 0.7 | 0.3 | |
| Glenman | 10.7 | 10.0 | 10.3 | 0.7 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | |
| Arabella | 5.0 | 1.3 | 3.2 | 0.7 | 1.7 | 1.2 | 1.0 | 0.3 | 0.7 | |
| Sawtana | 10.0 | 8.7 | 9.3 | 4.0 | 1.3 | 2.7 | 0.0 | 0.0 | 0.0 | |
| Sumai 3 | 3.7 | 0.7 | 2.2 | 3.0 | 0.0 | 1.5 | 0.3 | 0.0 | 0.2 | |
| Mean | 7.7 | 4.9 | 6.3 | 3.6 | 1.8 | 2.7 | 0.6 | 0.3 | 0.5 | |
| Winter | Greer | 2.3 | 1.7 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| KS96WGRC36 | 0.7 | 0.0 | 0.3 | 0.3 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | |
| Wichita | 3.0 | 5.0 | 4.0 | 0.0 | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 | |
| Geneva | 1.3 | 0.7 | 1.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.3 | 0.3 | |
| Freedom | 1.3 | 2.7 | 2.0 | 0.0 | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 | |
| Lr19 | 1.3 | 1.3 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Augusta | 0.3 | 0.7 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Century | 1.7 | 2.3 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Antelope | 6.7 | 5.7 | 6.2 | 0.3 | 0.3 | 0.3 | 0.7 | 0.3 | 0.5 | |
| Agrus | 3.7 | 2.3 | 3.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.2 | |
| Clark | 1.0 | 3.7 | 2.3 | 0.3 | 1.0 | 0.7 | 0.3 | 1.0 | 0.7 | |
| Ozon | 6.0 | 5.3 | 5.7 | 2.0 | 2.0 | 2.0 | 0.0 | 0.3 | 0.2 | |
| Hondia | 12.3 | 10.0 | 11.2 | 3.0 | 2.3 | 2.7 | 0.7 | 0.0 | 0.3 | |
| Karl 92 | 0.7 | 4.3 | 2.5 | 0.0 | 1.3 | 0.7 | 0.3 | 0.0 | 0.2 | |
| Tam 107 | 3.3 | 5.3 | 4.3 | 0.3 | 1.3 | 0.8 | 0.3 | 0.3 | 0.3 | |
| Mean | 3.0 | 3.4 | 3.2 | 0.4 | 0.6 | 0.5 | 0.2 | 0.1 | 0.2 | |
| I Induction Medium (2.4-D and Dicamba)—The Right-Sided Wilcoxon Test | ||
| Parameters | The Value of Wilcoxon’s Statistics | p-Value |
| ASF (no of androgenic structures/100 plated anthers) | 177.0 | 0.004 ** |
| GPR (no of green plants regeneration/100 plated anthers) | 160.0 | 0.020 * |
| APR (no of albino plants regeneration/100 plated anthers) | 140.5 | 0.109 |
| II Induction Medium (2.4-D and Kinetine)—The Right-Sided Wilcoxon Test | ||
| Parameters | The Value of Wilcoxon’s Statistics | p-Value |
| ASF (no of androgenic structures/100 plated anthers) | 128.5 | 0.260 |
| GPR (no of green plants regeneration/100 plated anthers) | 154.5 | 0.040 * |
| APR (no of albino plants regeneration/100 plated anthers) | 126.0 | 0.265 |
| Phenotype | Genotype | Source | Pedigree |
|---|---|---|---|
| Spring | Tybalt | NSGC | ZE 95-2355/Chablis |
| Rescue | NSGC | Apex/S-615 | |
| Fortuna | NSGC | Kenya 58/Newthatch//Frontana/3/Rescue/Chinook | |
| Leda Collection A47 | NSGC | Leda/Hybrid 46 | |
| Ostka Smolicka | PBS | Palermo/KOC 2926/92 | |
| Ruzynska II | NSGC | Unknown-Solid-stemmed-Variety | |
| Carola | LIPK | Capega/Garant | |
| AC Abbey | AAFC | BW608/93464//BW591 | |
| Tioga | NSGC | Fortuna/3/ND 4/Rescue//II-50-17/51-3349 | |
| Parabola | MPB | Torka /Henika//Candeza. | |
| Chinook | NSGC | Thatcher/S615-11 | |
| Glenman | NSGC | Tezanos Pintos Precoz/Sonora 64 (208774C-1R8M)//Fortuna | |
| Arabella | DPB | Leiffer/Batuta | |
| Sawtana | NSGC | Rescue//Mida/Cadet | |
| Sumai 3 | NSGC | Funo/Taiwan Xiaomai | |
| Winter | Greer | NSGC | WA 4765//Burt/PI 178383 |
| KS96WGRC36 | NSGC | TAM 107/4/TA 870 | |
| Wichita | NSGC | Early Blackhull/Tenmarq | |
| Geneva | NSGC | Ross wheat (Heine’s VII)/3/(NY5207aB-2B-34) Burt//Genesee/CI 12658/4/Genesee | |
| Freedom | NSGC | GR-876/OH-217 | |
| Lr19 | NSGC | Thatcher*6/Agropyron elongatum | |
| Augusta | NSGC | B2747 (Genessee/Redcoat) //Yorkstar | |
| Century | NSGC | Payne//TAM W-101/Amigo | |
| Antelope | NSGC | Pronghorn/Arlin | |
| Agrus | NSGC | Trumbull/Agropyron elongatum/4/Fultz sel./3/Trumbull//Hope/Hussar | |
| Clark | NSGC | Beau//65256A1-8-1/67137B5-16/4/Sullivan/3/Beau//5517B8-5-3-3 /Logan; 65256A1-8-1 = Caldwell sib | |
| Ozon | DPB | LP-296-4-96/Tambor//Denver | |
| Hondia | DPB | CHS38337/KOC1284/97 | |
| Karl | NSGC | Plainsman V/3/Kaw/Atlas 50//Parker*5/Agent | |
| Tam 107 | NSGC | TAM 105*4/Amigo |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigt, D.; Kiel, A.; Siatkowski, I.; Zyprych-Walczak, J.; Tomkowiak, A.; Kwiatek, M. Comparison of the Androgenic Response of Spring and Winter Wheat (Triticum aestivum L.). Plants 2020, 9, 49. https://doi.org/10.3390/plants9010049
Weigt D, Kiel A, Siatkowski I, Zyprych-Walczak J, Tomkowiak A, Kwiatek M. Comparison of the Androgenic Response of Spring and Winter Wheat (Triticum aestivum L.). Plants. 2020; 9(1):49. https://doi.org/10.3390/plants9010049
Chicago/Turabian StyleWeigt, Dorota, Angelika Kiel, Idzi Siatkowski, Joanna Zyprych-Walczak, Agnieszka Tomkowiak, and Michał Kwiatek. 2020. "Comparison of the Androgenic Response of Spring and Winter Wheat (Triticum aestivum L.)" Plants 9, no. 1: 49. https://doi.org/10.3390/plants9010049
APA StyleWeigt, D., Kiel, A., Siatkowski, I., Zyprych-Walczak, J., Tomkowiak, A., & Kwiatek, M. (2020). Comparison of the Androgenic Response of Spring and Winter Wheat (Triticum aestivum L.). Plants, 9(1), 49. https://doi.org/10.3390/plants9010049






