An Allelopathic Role for Garlic Root Exudates in the Regulation of Carbohydrate Metabolism in Cucumber in a Hydroponic Co-Culture System
Abstract
1. Introduction
2. Results
2.1. Plant Growth Parameters
2.2. Oxidative Damage Parameters
2.3. Protective Enzyme Activities
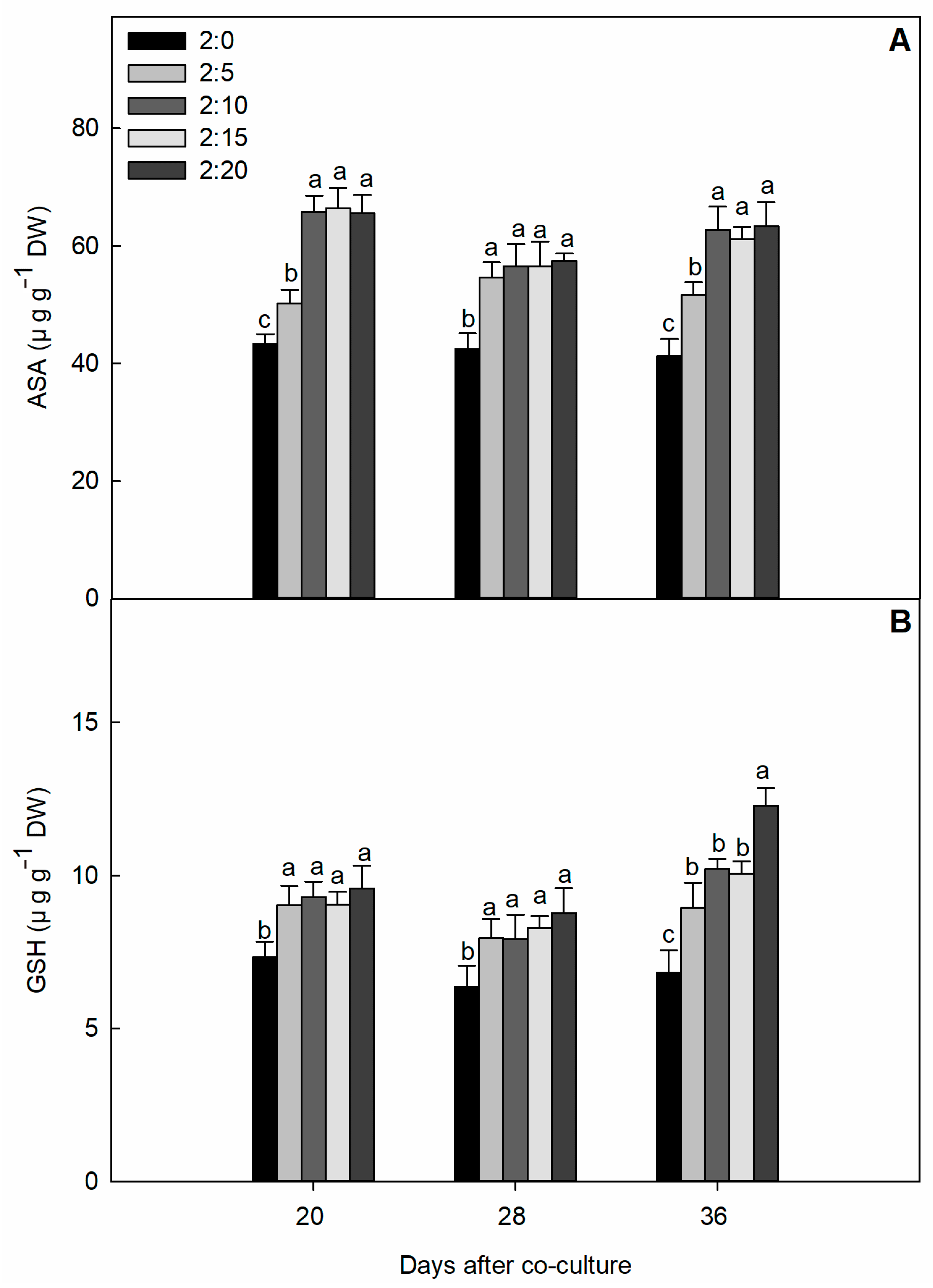
2.4. Non-Enzymatic Antioxidant Compound Content
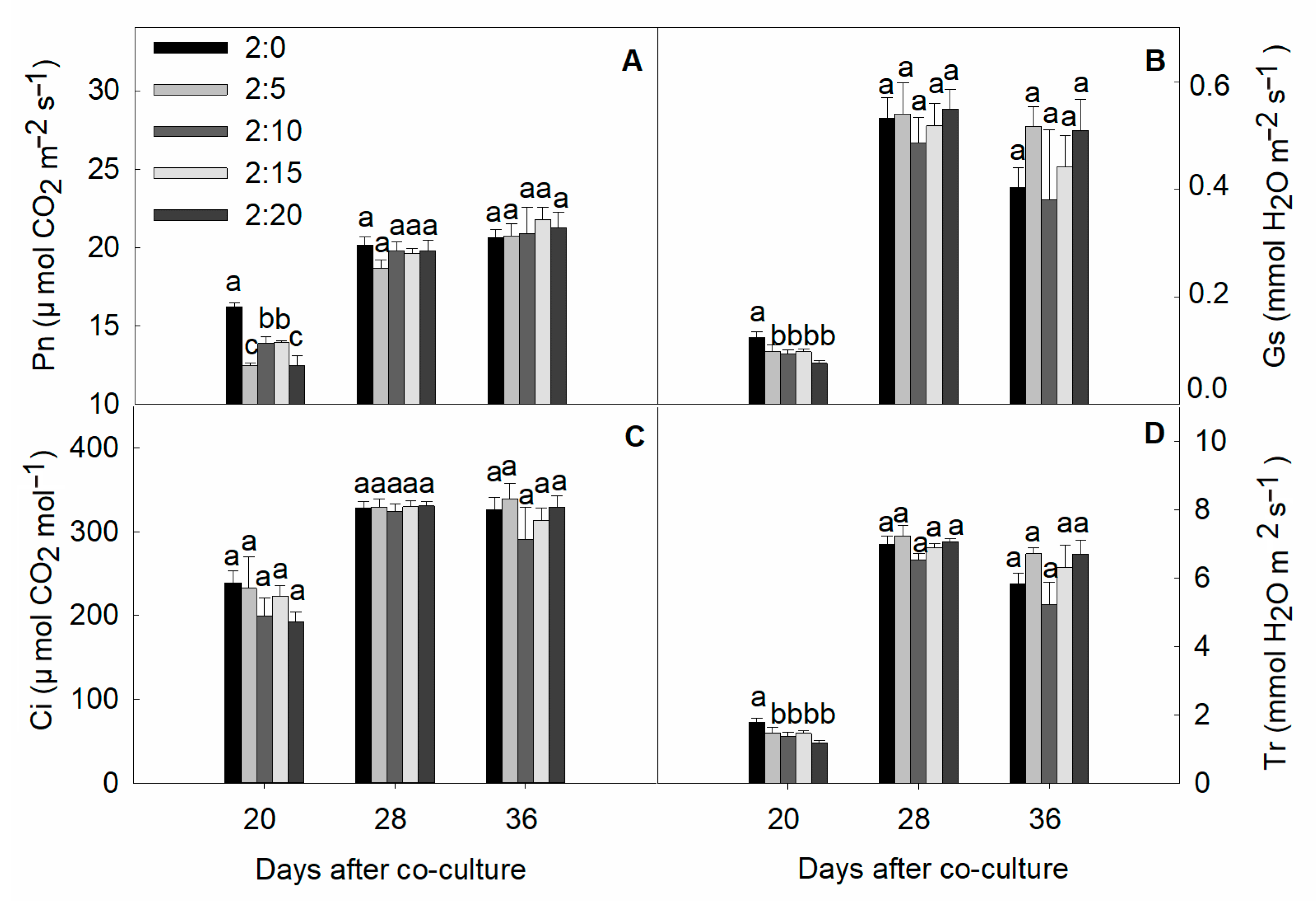
2.5. Gas Exchange Parameters
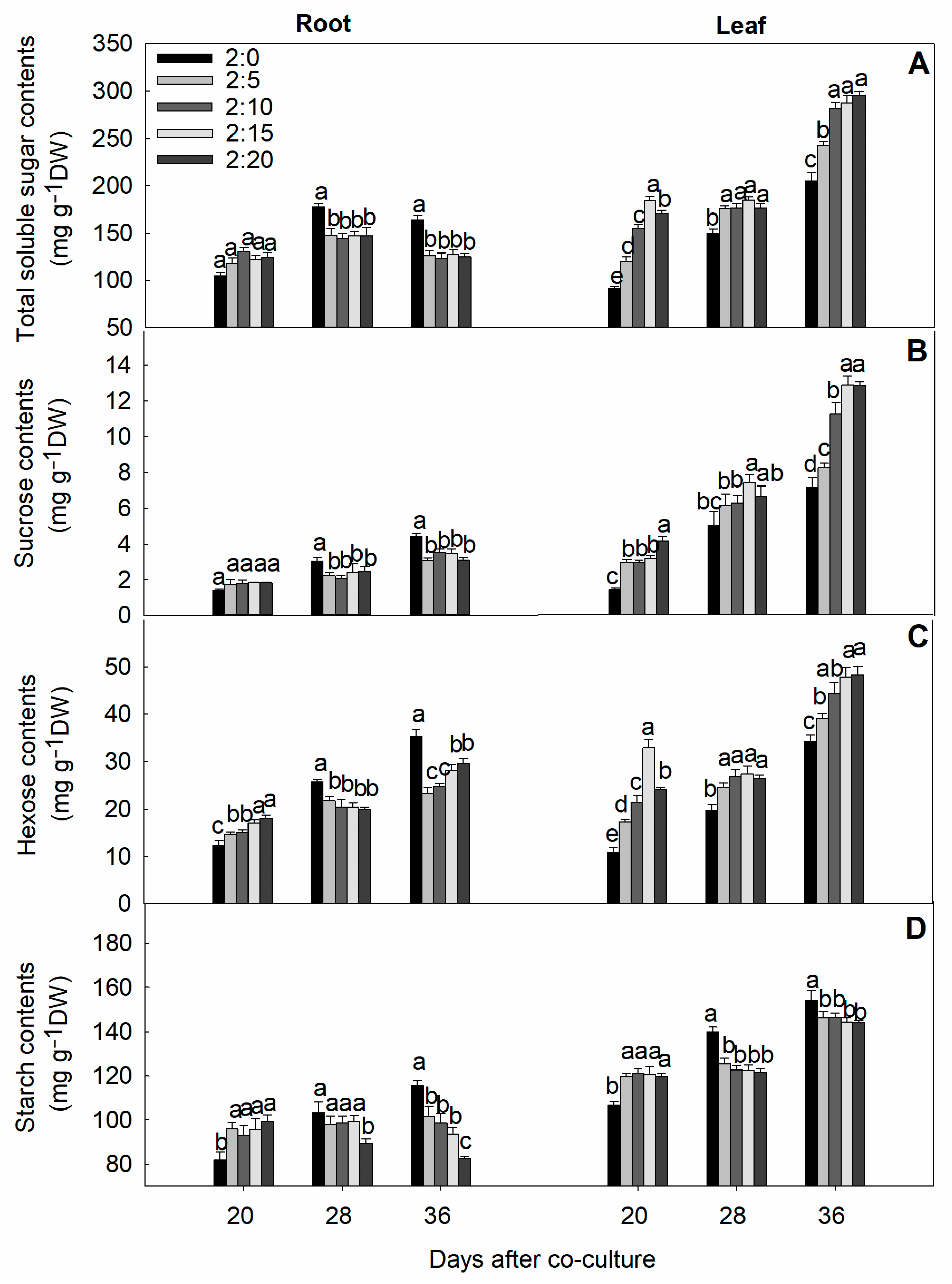
2.6. Soluble Sugar and Starch Contents
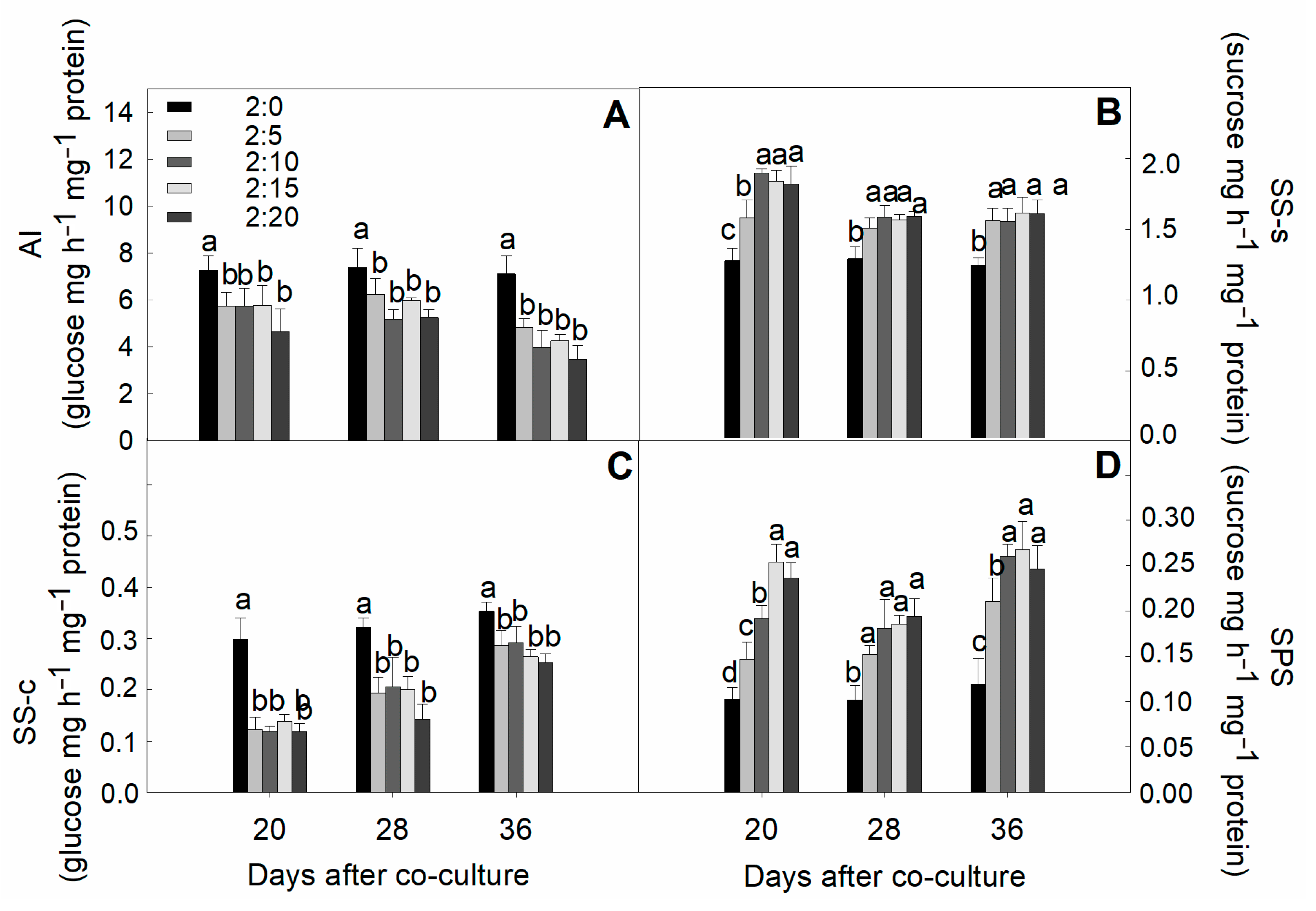
2.7. Activities of Sucrose-Metabolized Enzymes in Leaves
3. Discussion
3.1. Effect on Morphological Traits
3.2. Effect on the Antioxidant System
3.3. Effect on Photosynthetic Gas Exchange
3.4. Photosystem II (PSII) Inhibition: Limiting ROS Mediated Damage
3.5. Metabolism of Carbohydrates
4. Materials and Methods
4.1. Materials and Growth Conditions
4.2. Experimental Design
4.3. Measurements
4.3.1. Plant Growth
4.3.2. Oxidative Damage Parameters
4.3.3. Gas Exchange Measurements
4.3.4. Chlorophyll Fluorescence Parameters
4.3.5. Carbohydrate Content Measurements
4.3.6. Enzyme Activity of Carbohydrate Metabolism Measurements
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dayan, F.E.; Howell, J.; Weidenhamer, J.D. Dynamic root exudation of sorgoleone and its in planta mechanism of action. J. Exp. Bot. 2009, 60, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Yang, X.H.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Hejl, A.M.; Koster, K.L. Juglone disrupts root plasma membrane H+-ATPase activity and impairs water uptake, root respiration, and growth in soybean (Glycine max) and corn (Zea mays). J. Chem. Ecol. 2004, 30, 453–471. [Google Scholar] [CrossRef]
- Soltys, D.; Rudzińska-Langwald, A.; Gniazdowska, A.; Wiśniewska, A.; Bogatek, R. Inhibition of tomato (Solanum lycopersicum L.) root growth by cyanamide is due to altered cell division, phytohormone balance and expansin gene expression. Planta 2012, 236, 1629–1638. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. A chlorophyll fluorescence analysis of photosynthetic efficiency, quantum yield and photon energy dissipation in PSII antennae of Lactuca sativa L. leaves exposed to cinnamic acid. Plant Physiol. Biochem. 2011, 49, 1290–1298. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci. 2007, 4, 8–14. [Google Scholar] [CrossRef]
- Ye, S.F.; Zhou, Y.H.; Sun, Y.; Zou, L.Y.; Yu, J.Q. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot. 2006, 56, 255–262. [Google Scholar] [CrossRef]
- Czarnota, M.A.; Paul, R.N.; Dayan, F.E.; Nimbal, C.I.; Weston, L.A. Mode of Action, Localization of Production, Chemical Nature, and Activity of Sorgoleone: A Potent PSII Inhibitor in Sorghum spp. Root Exudates. Weed Technol. 2001, 15, 813–825. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Maheswari, M.; Shanker, A.K.; Shanker, C. Crop Stress and Its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Couee, I.; Sulmon, C.; Gouesbet, G.; El, A.A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, Y.; Xiao, C.L.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J. Exp. Bot. 2007, 58, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, X.; Xue, C. Effect of cinnamic acid on soil microbial characteristics in the cucumber rhizosphere. Eur. J. Soil Biol. 2009, 45, 356–362. [Google Scholar] [CrossRef]
- Xiao, X.M.; Cheng, Z.H.; Meng, H.W.; Liu, L.H.; Li, H.Z.; Dong, Y.X. Intercropping of Green Garlic (Allium sativum L.) Induces Nutrient Concentration Changes in the Soil and Plants in Continuously Cropped Cucumber (Cucumis sativus L.) in a Plastic Tunnel. PLoS ONE 2013, 8, e62173. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Z.; Meng, H.; Ahmad, I.; Zhao, H. Growth, yield and quality of spring tomato and physicochemical properties of medium in a tomato/garlic intercropping system under plastic tunnel organic medium cultivation. Sci. Hortic. 2014, 170, 159–168. [Google Scholar] [CrossRef]
- Han, X.; Cheng, Z.; Meng, H.; Yang, X.; Ahmad, I. Allelopathic effect of decomposed garlic (Allium sativum L.) Stalk on lettuce (L. Sativa Var. Crispa L.). Pak. J. Bot. 2013, 45, 225–233. [Google Scholar]
- Ding, H.; Cheng, Z.; Liu, M.; Hayat, S.; Feng, H. Garlic exerts allelopathic effects on pepper physiology in a hydroponic co-culture system. Biol. Open 2016, 5, 631–637. [Google Scholar] [CrossRef]
- Hayat, S.; Cheng, Z.H.; Ahmad, A.; Cheng, X.J.; Wang, M.Y. Garlic, from remedy to stimulant: Evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars; allicin containing aqueous garlic extracts trigger antioxidants in cucumber. Front. Plant Sci. 2016, 7, 1235. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y. Determination of root exudative phenolic compounds in hot pepper (Capsicum annuum L.) by polydimethylsiloxane tube microextraction after derivatization. Anal. Methods 2014, 6, 569–574. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y. Hollow fiber liquid-phase microextraction with in situ derivatization combined with gas chromatography-mass spectrometry for the determination of root exudate phenylamine compounds in hot pepper (Capsicum annuum L.). J. Agric. Food Chem. 2013, 61, 5494–5499. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z.; Meng, H.; Tang, X. The Garlic Allelochemical Diallyl Disulfide Affects Tomato Root Growth by Influencing Cell Division, Phytohormone Balance and Expansin Gene Expression. Front. Plant Sci. 2016, 7, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Cheng, Z.H. Comparative analysis of allelopathy and allelochemicals of the root exudates in garlic. J. Northwest A F Univ. Nat. Sci. Ed. 2012, 42, 116–120. [Google Scholar]
- Liu, S.H.; Liu, S.Q.; Zhang, Z.K.; Wei, H.; Huang, Z.J.; Zhang, Y. Inhibition Effect of Garlic Root Exudates on the Genus Allium. Sci. Agric. Sin. 2011, 44, 2625–2632. [Google Scholar]
- Cruz Ortega, R.; Ayala Cordero, G.; Anaya, A.L. Allelochemical stress produced by the aqueous leachate of Callicarpa acuminata: Effects on roots of bean, maize, and tomato. Physiol. Plant. 2002, 116, 20–27. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Dai, W.; Zhang, X. Oxidative damage and antioxidant responses in Microcystis aeruginosa exposed to the allelochemical berberine isolated from golden thread. J. Plant Physiol. 2011, 168, 639–643. [Google Scholar] [CrossRef]
- Lara-Nunez, A.; Romero-Romero, T.; Ventura, J.L.; Blancas, V.; Anaya, A.L.; Cruz-Ortega, R. Allelochemical stress causes inhibition of growth and oxidative damage in Lycopersicon esculentum Mill. Plant Cell Environ. 2006, 29, 2009–2016. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kaur, S.; Kohli, R.K. 2-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol. Biochem. 2006, 44, 819–827. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Wang, C.H.; Xiao, X.M.; Khan, M.A. Allelopathic effects of decomposing garlic stalk on some vegetable crops. Afr. J. Biotechnol. 2011, 10, 15514–15520. [Google Scholar]
- Catello, D.M.; Sebastiano, D.; Roberto, P.; Loreto, F.; Fuggi, A. Free amino acid and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. New Phytol. 2003, 158, 455–463. [Google Scholar]
- Mademba-Sy, F.; Bouchereau, A.; Larher, F.R. Proline accumulation in cultivated citrus and its relationship with salt tolerance. J. Horticult. Sci. Biotechnol. 2003, 78, 617–623. [Google Scholar] [CrossRef]
- Szekely, G.; Abraham, E.; Cseplo, A.; Rigo, G.; Zsigmond, L.; Csiszar, J.; Ayaydin, F.; Strizhov, N.; Jasik, J.; Schmelzer, E.; et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Lavanya, K.; Singh, H.P.; Kohli, R.K. Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul. 2007, 51, 119–128. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, F.; Liu, W.; Guo, J.; Wan, F. ρ-Cymene Inhibits Growth and Induces Oxidative Stress in Rice Seedling Plants. Weed Sci. 2012, 60, 564–570. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. Alpha-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Sgariglia, M.A.; Soberón, J.R.; Quiroga, E.N.; Vattuone, M.A. Role of sugarcane straw allelochemicals in the growth suppression of arrowleaf sida. Environ. Exp. Bot. 2007, 60, 495–503. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Desikan, R.; Hancock, J.; Neill, S. Reactive oxygen species as signalling molecules. In Biological Sciences Series; Smirnoff, N., Ed.; Blackwell: Oxford, UK, 2005; pp. 169–196. [Google Scholar]
- Yang, C.; Liu, S.; Zhou, S.; Wu, H.; Yu, J.; Xia, C. Allelochemical ethyl 2-methyl acetoacetate (EMA) induces oxidative damage and antioxidant responses in Phaeodactylum tricornutum. Pestic Biochem. Phys. 2011, 100, 93–103. [Google Scholar] [CrossRef]
- Chen, K.M.; Gong, H.J.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Up-regulation of glutathione metabolism and changes in redox status involved in adaptation of reed (Phragmites communis) ecotypes to drought-prone and saline habitats. J. Plant Physiol. 2003, 160, 293–301. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Chiapusio, G.; Reigosa, M.J. Benzoxazolin-2(3H)-one (BOA) induced changes in leaf water relations, photosynthesis and carbon isotope discrimination in Lactuca sativa. Plant Physiol. Biochem. 2011, 49, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, J.; Ye, J.; Li, G. Effects of phytotoxic extracts from peach root bark and benzoic acid on peach seedlings growth, photosynthesis, antioxidance and ultrastructure properties. Sci. Hortic. 2017, 215, 49–58. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Ye, S.F.; Zhang, M.F.; Hu, W.H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. [Google Scholar] [CrossRef]
- Cornic, G.; Briantais, J.M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 1991, 183, 178–184. [Google Scholar] [CrossRef]
- Munné Bosch, S.; Jubany Marí, T.; Alegre, L. Drought-induced senescence is characterized by a loss of antioxidant defences in chloroplasts. Plant Cell Environ. 2001, 24, 1319–1327. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Leu, E.; Krieger-Liszkay, A.; Goussias, C.; Gross, E. Polyphenolic Allelochemicals from the Aquatic Angiosperm Myriophyllum spicatum Inhibit Photosystem II. Plant Physiol. 2002, 130, 2011–2018. [Google Scholar] [CrossRef]
- Uddin, M.; Park, K.; Han, S.; Pyon, J.; Park, S. Effects of sorgoleone allelochemical on chlorophyll fluorescence and growth inhibition in weeds. Allelopath. J. 2012, 30, 61–70. [Google Scholar]
- Sanchez-Moreiras, A.M.; Oliveros-Bastidas, A.; Reigosa, M.J. Reduced photosynthetic activity is directly correlated with 2-(3H)-benzoxazolinone accumulation in lettuce leaves. J. Chem. Ecol. 2010, 36, 205–209. [Google Scholar] [CrossRef]
- Qian, H.; Xu, X.; Chen, W.; Jiang, H.; Jin, Y.; Liu, W.; Fu, Z. Allelochemical stress causes oxidative damage and inhibition of photosynthesis in Chlorella vulgaris. Chemosphere 2009, 75, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Lelong, A.; Haberkorn, H.; Le Goic, N.; Hegaret, H.; Soudant, P. A new insight into allelopathic effects of Alexandrium minutum on photosynthesis and respiration of the diatom Chaetoceros neogracile revealed by photosynthetic-performance analysis and flow cytometry. Microb. Ecol. 2011, 62, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Zhang, H.; Lyu, S.S.; Du, G.D.; Wang, X.Q.; Wu, C.H.; Lyu, D.G. Effects of exogenous phenolic acids on photosystem functions and photosynthetic electron transport rate in strawberry leaves. Photosynthetica 2018, 56, 616–622. [Google Scholar] [CrossRef]
- Zhang, K.M.; Shen, Y.; Zhou, X.Q.; Fang, Y.; Liu, Y.; Lena, Q.M.; Ahmad, A. Photosynthetic electrontransfer reactions in the gametophyte of Pteris multifida reveal the presence of allelopathic interference from the invasive plant species Bidens pilosa. J. Photochem. Photobiol. B Biol. 2016, 158, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T. Source-sink regulation by sugar and stress. Curr. Opin. Plant. Biol. 1999, 2, 198–206. [Google Scholar] [CrossRef]
- Morsy, M.R.; Jouve, L.; Hausman, J.F.; Hoffmann, L.; Stewart, J.M. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J. Plant Physiol. 2007, 164, 157–167. [Google Scholar] [CrossRef]
- Arbona, V.; Marco, A.J.; Iglesias, D.J.; López-Climent, M.F.; Talon, M.; Gómez-Cadenas, A. Carbohydrate depletion in roots and leaves of salt-stressed potted Citrus clementina L. Plant Growth Regul. 2005, 46, 153–160. [Google Scholar] [CrossRef]
- Macías, F.A.; Oliveros-Bastidas, A.; Marín, D.; Chinchilla, N.; Castellano, D.; Molinillo, J.M.G. Evidence for an allelopathic interaction between rye and wild oats. J. Agric. Food Chem. 2014, 62, 9450–9457. [Google Scholar] [CrossRef]
- Hegab, M.M.; Gabr, M.A.; Al-Wakeel, S.A.M.; Hamed, B.A. Allelopathic Potential of Eucalyptus rostrata Leaf Residue on Some Metabolic Activities of Zea mays L. Univers. J. Plant Sci. 2016, 4, 11–21. [Google Scholar]
- Yuan, Y.; Zhong, M.; Shu, S.; Du, N.; He, L.; Yuan, L.; Sun, J.; Guo, S. Effects of Exogenous Putrescine on Leaf Anatomy and Carbohydrate Metabolism in Cucumber (Cucumis sativus L.) Under Salt Stress. J. Plant Growth Regul. 2015, 34, 451–464. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; Gonzalez, J.A.; Hilal, M.; Prado, F.E. Soluble sugars—Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Nayer, M.; Heidari, R. Drought-induced Accumulation of Soluble Sugars and Proline in Two Maize Varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Ahmadi, A.; Baker, D.A. The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat. Plant Growth Regul. 2001, 35, 81–91. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Suwa, R.; Fujimaki, S.; Suzui, N.; Kawachi, N.; Ishii, S.; Sakamoto, K.; Nguyen, N.T.; Saneoka, H.; Mohapatra, P.K.; Moghaieb, R.E.; et al. Use of positron-emitting tracer imaging system for measuring the effect of salinity on temporal and spatial distribution of 11C tracer and coupling between source and sink organs. Plant Sci. 2008, 175, 210–216. [Google Scholar] [CrossRef]
- Peng, J.; Liu, J.; Zhang, L.; Luo, J.; Dong, H.; Ma, Y.; Zhao, X.; Chen, B.; Sui, N.; Zhou, Z.; et al. Effects of Soil Salinity on Sucrose Metabolism in Cotton Leaves. PLoS ONE 2016, 11, e156241. [Google Scholar] [CrossRef]
- Ferrarese, M.D.L.L.; de Souza, N.E.; Rodrigues, J.D.; Ferrarese-Filho, O. Carbohydrate and lipid status in soybean roots influenced by ferulic acid uptake. Acta Physiol. Plant 2001, 23, 421–427. [Google Scholar] [CrossRef]
- Bu, R.; Xie, J.; Yu, J.; Liao, W.; Xiao, X.; Lv, J.; Wang, C.; Ye, J.; Calderon-Urrea, A. Autotoxicity in cucumber (Cucumis sativus L.) seedlings is alleviated by silicon through an increase in the activity of antioxidant enzymes and by mitigating lipid peroxidation. J. Plant Biol. 2016, 59, 247–259. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, J.; Feng, R.; Jia, J.; Han, W.; Gong, H. The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant Soil 2016, 406, 231–249. [Google Scholar] [CrossRef]
- Entiana, K.D.; Barnettb, J.A. Regulation of sugar utilization by Saccharomyces cerevisiae. Trends Biochem. Sci. 1992, 17, 506–510. [Google Scholar] [CrossRef]
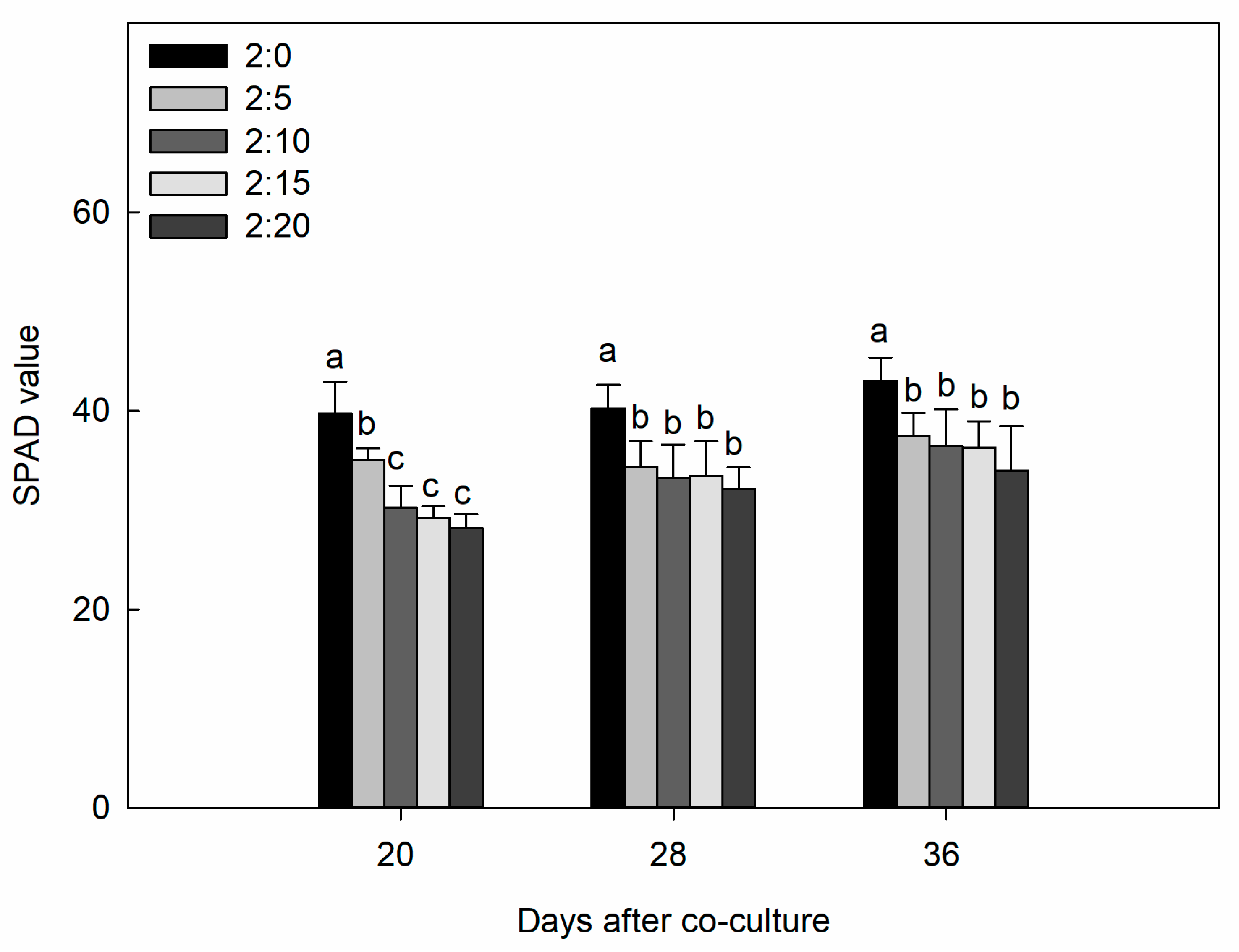
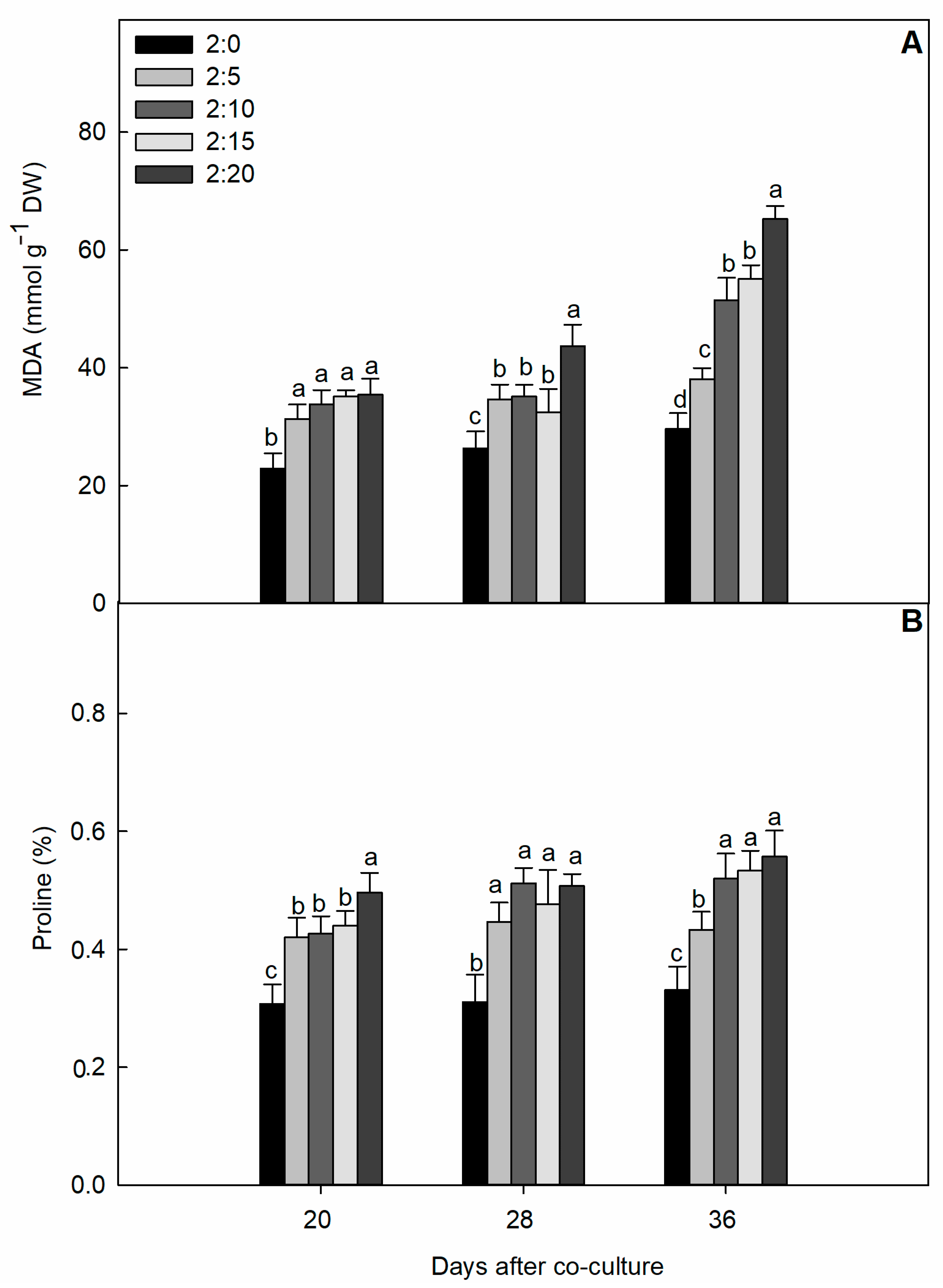
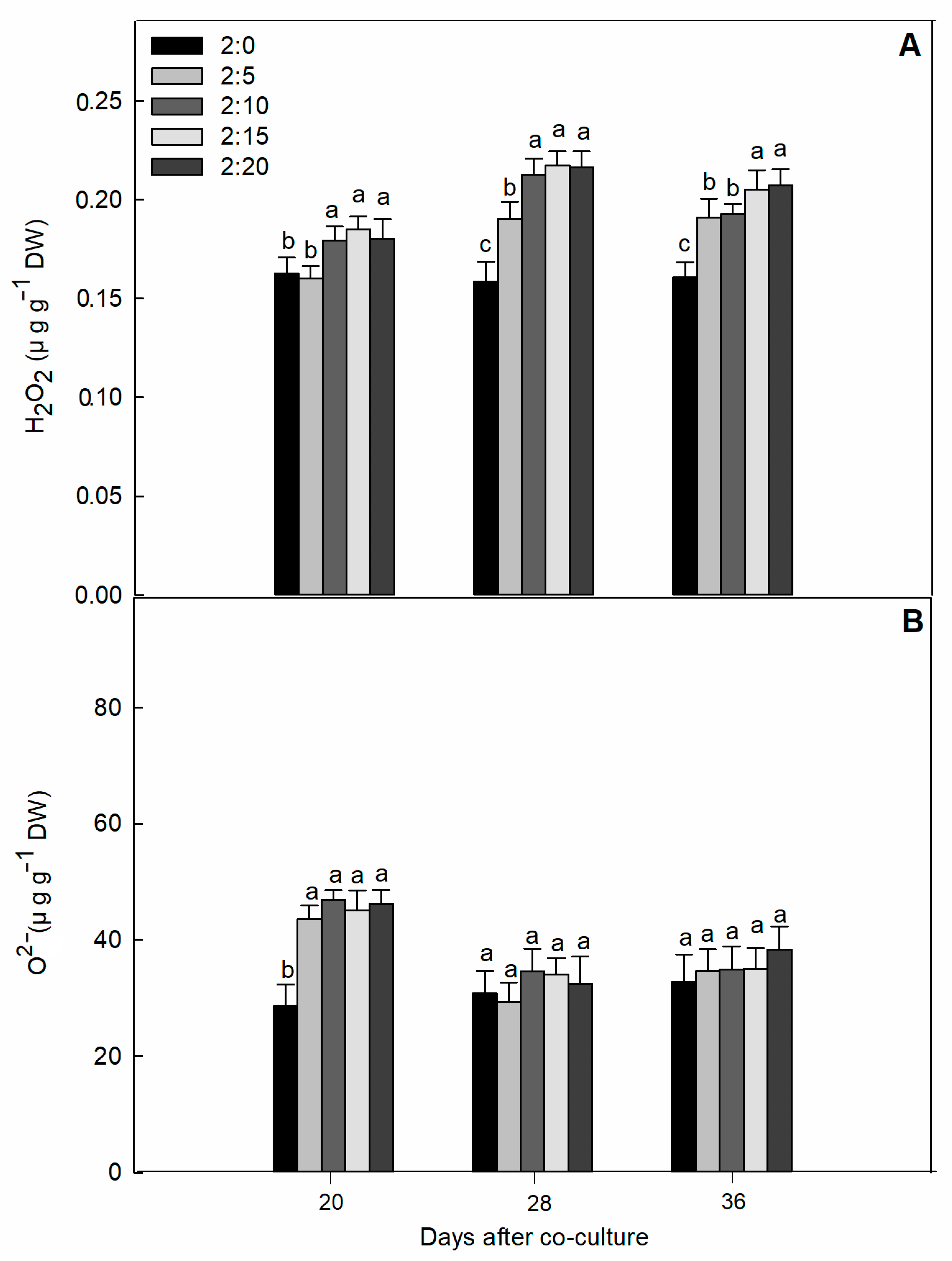
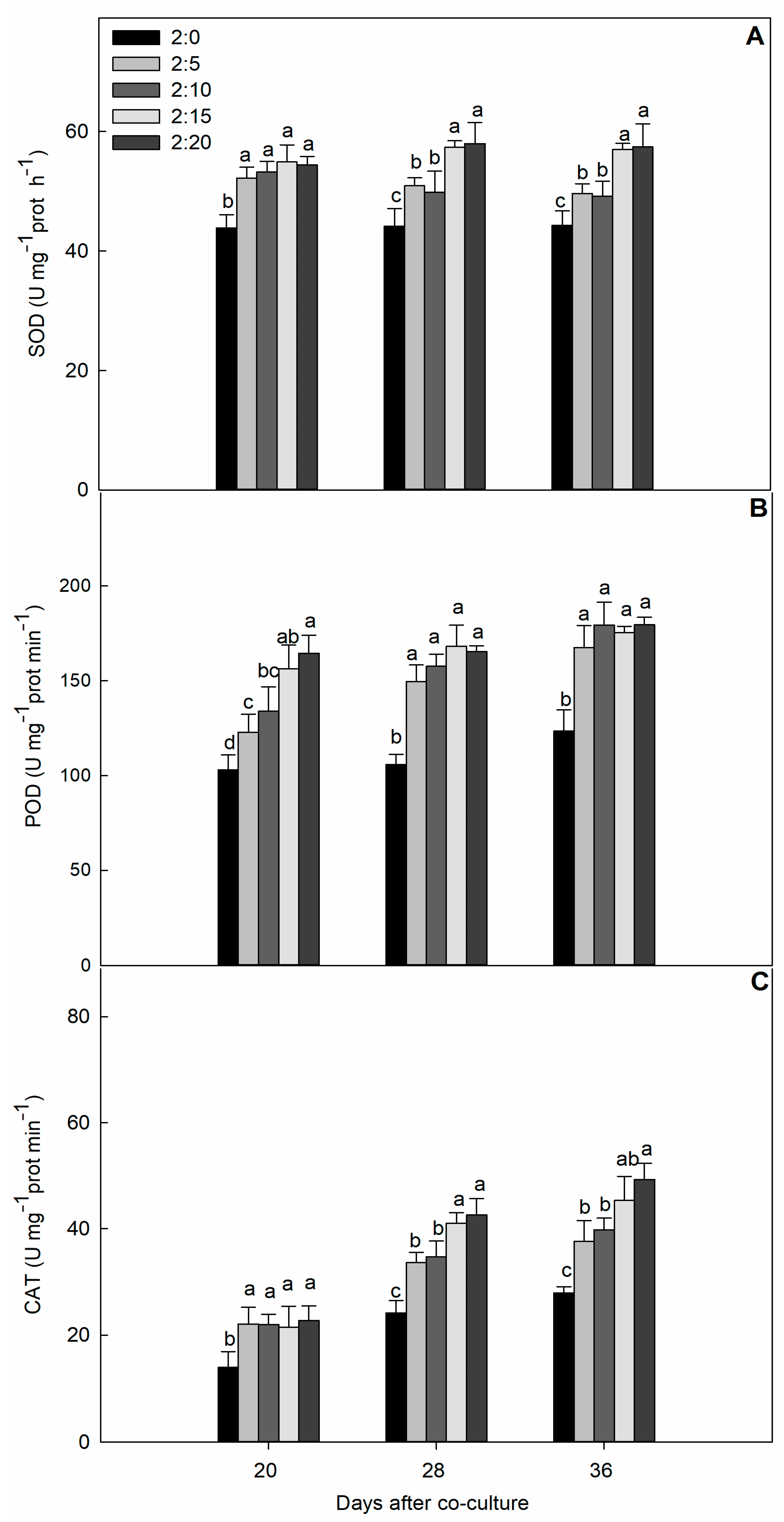
| C/G Ratios | Increase of Shoot Dry Mass Per Day (g·plant−1·day−1) | Increase of Root DRY Mass Per Day (g·plant−1·day−1) | Increase of Total Dry Mass Per Day (g·plant−1·day−1) | Increase of Leaf Area Per Day (cm2·plant−1·day−1) |
|---|---|---|---|---|
| 2:0 | 1.021 ± 0.052 a | 0.135 ± 0.016 a | 1.156 ± 0.026 a | 12.911 ± 0.217 a |
| 2:5 | 0.930 ± 0.027 b | 0.125 ± 0.028 ab | 1.055 ± 0.018 ab | 10.875 ± 0.202 b |
| 2:10 | 0.852 ± 0.023 bc | 0.109 ± 0.033 b | 0.961 ± 0.033 b | 9.672 ± 0.352 c |
| 2:15 | 0.825 ± 0.017 c | 0.114 ± 0.026 b | 0.939 ± 0.021 bc | 10.014 ± 0.110 c |
| 2:20 | 0.792 ± 0.036 c | 0.117 ± 0.017 b | 0.909 ± 0.037 c | 9.688 ± 0.177 c |
| Days after Co-Culture | C/G Ratio | Fv/Fm | Fv’/Fm’ | ΦPSII | qP | NPQ |
|---|---|---|---|---|---|---|
| 20 | 2:0 | 0.759 ± 0.013 a | 0.692 ± 0.005 a | 0.614 ± 0.011 a | 0.888 ± 0.017 a | 0.230 ± 0.012 b |
| 2:5 | 0.705 ± 0.005 b | 0.614 ± 0.007 b | 0.572 ± 0.016 b | 0.749 ± 0.019 b | 0.323 ± 0.027 a | |
| 2:10 | 0.711 ± 0.008 b | 0.632 ± 0.044 b | 0.531 ± 0.056 b | 0.727 ± 0.044 b | 0.335 ± 0.030 a | |
| 2:15 | 0.714 ± 0.014 b | 0.625 ± 0.006 b | 0.578 ± 0.020 b | 0.756 ± 0.024 b | 0.355 ± 0.022 a | |
| 2:20 | 0.700 ± 0.016 b | 0.622 ± 0.023 b | 0.509 ± 0.025 c | 0.781 ± 0.066 b | 0.372 ± 0.027 a | |
| 28 | 2:0 | 0.746 ± 0.012 a | 0.671 ± 0.037 a | 0.573 ± 0.023 a | 0.853 ± 0.030 a | 0.244 ± 0.025 a |
| 2:5 | 0.736 ± 0.019 a | 0.645 ± 0.023 a | 0.596 ± 0.053 a | 0.806 ± 0.058 a | 0.224 ± 0.049 a | |
| 2:10 | 0.765 ± 0.025 a | 0.692 ± 0.031 a | 0.621 ± 0.038 a | 0.897 ± 0.020 a | 0.249 ± 0.038 a | |
| 2:15 | 0.760 ± 0.014 a | 0.681 ± 0.054 a | 0.603 ± 0.054 a | 0.884 ± 0.018 a | 0.265 ± 0.029 a | |
| 2:20 | 0.753 ± 0.038 a | 0.659 ± 0.049 a | 0.580 ± 0.047 a | 0.880 ± 0.017 a | 0.263 ± 0.063 a | |
| 36 | 2:0 | 0.772 ± 0.008 a | 0.673 ± 0.030 a | 0.591 ± 0.045 a | 0.875 ± 0.033 a | 0.386 ± 0.035 a |
| 2:5 | 0.741 ± 0.031 a | 0.654 ± 0.027 a | 0.554 ± 0.026 a | 0.823 ± 0.052 a | 0.403 ± 0.057 a | |
| 2:10 | 0.782 ± 0.007 a | 0.678 ± 0.021 a | 0.577 ± 0.047 a | 0.848 ± 0.043 a | 0.425 ± 0.047 a | |
| 2:15 | 0.772 ± 0.003 a | 0.672 ± 0.013 a | 0.537 ± 0.030 a | 0.906 ± 0.058 a | 0.396 ± 0.091 a | |
| 2:20 | 0.770 ± 0.005 a | 0.672 ± 0.024 a | 0.555 ± 0.032 a | 0.884 ± 0.019 a | 0.399 ± 0.123 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Ali, A.; Cheng, Z. An Allelopathic Role for Garlic Root Exudates in the Regulation of Carbohydrate Metabolism in Cucumber in a Hydroponic Co-Culture System. Plants 2020, 9, 45. https://doi.org/10.3390/plants9010045
Ding H, Ali A, Cheng Z. An Allelopathic Role for Garlic Root Exudates in the Regulation of Carbohydrate Metabolism in Cucumber in a Hydroponic Co-Culture System. Plants. 2020; 9(1):45. https://doi.org/10.3390/plants9010045
Chicago/Turabian StyleDing, Haiyan, Ahmad Ali, and Zhihui Cheng. 2020. "An Allelopathic Role for Garlic Root Exudates in the Regulation of Carbohydrate Metabolism in Cucumber in a Hydroponic Co-Culture System" Plants 9, no. 1: 45. https://doi.org/10.3390/plants9010045
APA StyleDing, H., Ali, A., & Cheng, Z. (2020). An Allelopathic Role for Garlic Root Exudates in the Regulation of Carbohydrate Metabolism in Cucumber in a Hydroponic Co-Culture System. Plants, 9(1), 45. https://doi.org/10.3390/plants9010045






