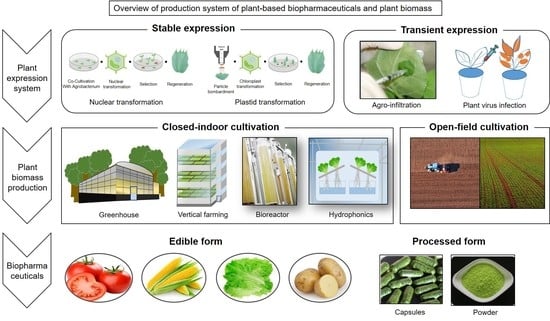
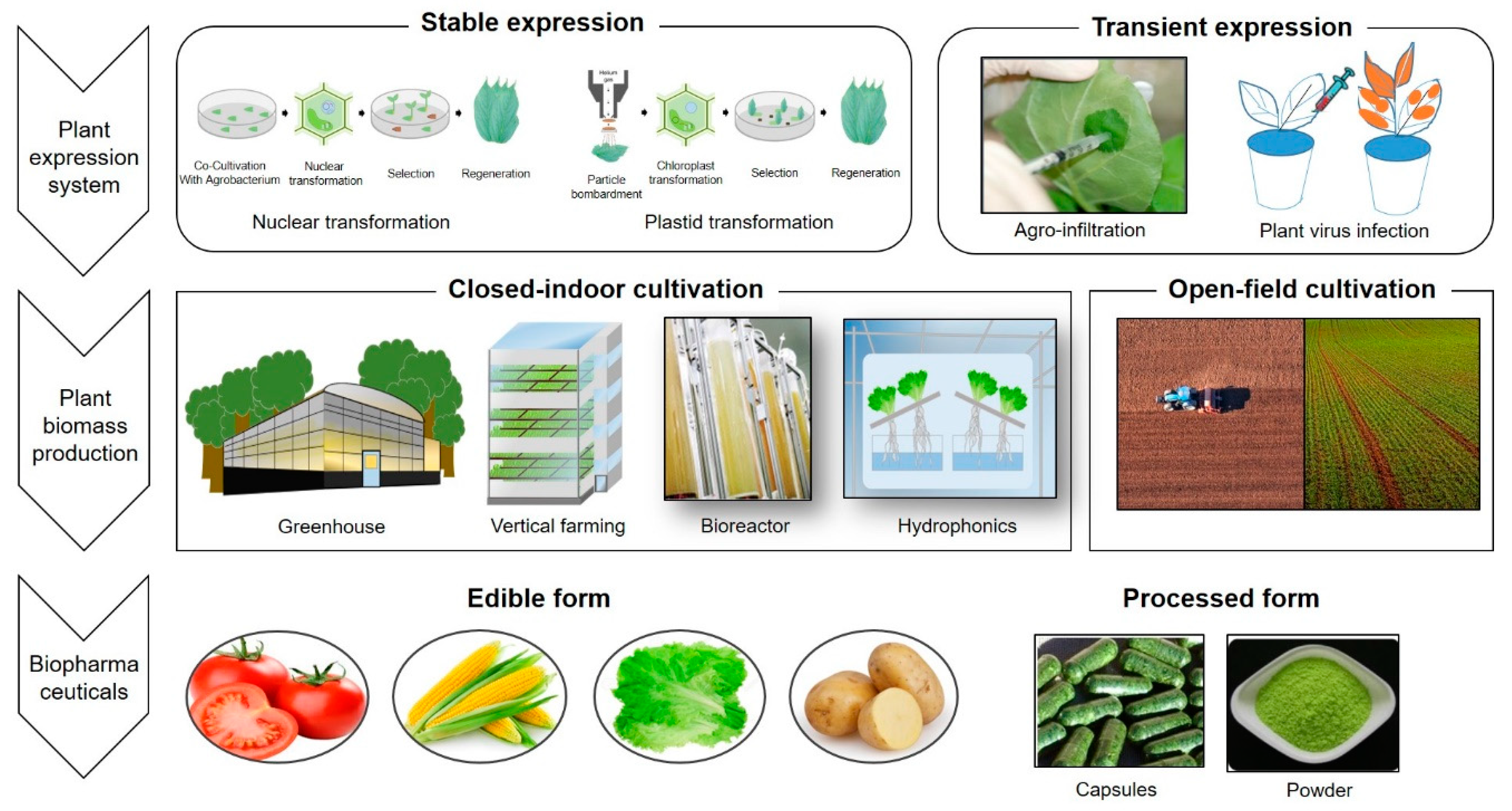
Development of Systems for the Production of Plant-Derived Biopharmaceuticals
Abstract
1. Introduction
2. Production Systems of Target Proteins of Interests
2.1. A “Stable” Expression Platform Using Agrobacterium-Mediated Transformation
2.2. Cell Cultures in “Stable” Systems
2.3. Transient Expression Systems
3. Plant Biomass Production Systems
3.1. Open-Field Cultivation
3.2. Closed Culture Systems
3.2.1. Greenhouse Systems
3.2.2. Bioreactor Culture Systems
3.2.3. Hydroponic Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fischer, R.; Liao, Y.-C.; Hoffmann, K.; Schillberg, S.; Emans, N. Molecular farming of recombinant antibodies in plants. Biol. Chem. 1999, 380, 825–839. [Google Scholar] [CrossRef]
- Fischer, R.; Drossard, J.; Commandeur, U.; Schillberg, S.; Emans, N. Towards molecular farming in the future: Moving from diagnostic protein and antibody production in microbes to plants. Biotechnol. Appl. Biochem. 1999, 30, 101–108. [Google Scholar] [PubMed]
- Fischer, R.; Schillberg, S. Molecular Farming: Plant-Made Pharmaceuticals and Technical Proteins; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Stoger, E.; Ma, J.K.; Fischer, R.; Christou, P. Sowing the seeds of success: Pharmaceutical proteins from plants. Curr. Opin. Biotechnol. 2005, 16, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.C.; Drake, P.M.; Christou, P. Genetic modification: The production of recombinant pharmaceutical proteins in plants. Nat. Rev. Genet. 2003, 4, 794. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kim, B.-G.; Kim, T.-G.; Kang, T.-J.; Yang, M.-S. Expression of a cholera toxin b subunit in transgenic lettuce (Lactuca sativa L.) using agrobacterium-mediated transformation system. Plant Cell Tissue Organ Cult. 2006, 87, 203–210. [Google Scholar] [CrossRef]
- Mohebodini, M.; Jalali-Javaran, M.; Alizadeh, H.; Mahboudi, F.; Yarbakht, M. Agrobacterium-mediated transformation of lettuce (Lactuca sativa L.) to express igg-binding protein a and human pro-insulin as a fusion protein. J. Hortic. Sci. Biotechnol. 2014, 89, 719–725. [Google Scholar] [CrossRef]
- Xu, J.; Dolan, M.C.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Green factory: Plants as bioproduction platforms for recombinant proteins. Biotechnol. Adv. 2012, 30, 1171–1184. [Google Scholar] [CrossRef]
- Faye, L.; Gomord, V. Success stories in molecular farming—A brief overview. Plant Biotechnol. J. 2010, 8, 525–528. [Google Scholar] [CrossRef]
- Huang, J.; Nandi, S.; Wu, L.; Yalda, D.; Bartley, G.; Rodriguez, R.; Lonnerdal, B.; Huang, N. Expression of natural antimicrobial human lysozyme in rice grains. Mol. Breed. 2002, 10, 83–94. [Google Scholar] [CrossRef]
- Nandi, S.; Suzuki, Y.A.; Huang, J.; Yalda, D.; Pham, P.; Wu, L.; Bartley, G.; Huang, N.; Lönnerdal, B. Expression of human lactoferrin in transgenic rice grains for the application in infant formula. Plant Sci. 2002, 163, 713–722. [Google Scholar] [CrossRef]
- Abiri, R.; Valdiani, A.; Maziah, M.; Shaharuddin, N.A.; Sahebi, M.; Yusof, Z.N.B.; Atabaki, N.; Talei, D. A critical review of the concept of transgenic plants: Insights into pharmaceutical biotechnology and molecular farming. Curr. Issues Mol. Biol. 2015, 18, 21–42. [Google Scholar] [PubMed]
- Horn, M.; Woodard, S.; Howard, J. Plant molecular farming: Systems and products. Plant Cell Rep. 2004, 22, 711–720. [Google Scholar] [PubMed]
- Youm, J.W.; Jeon, J.H.; Kim, H.; Kim, Y.H.; Ko, K.; Joung, H.; Kim, H. Transgenic tomatoes expressing human beta-amyloid for use as a vaccine against alzheimer’s disease. Biotechnol. Lett. 2008, 30, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, A.; Zhao, L.; Shen, G.; Cui, L.; Tang, K. Expression of thymosin α1 concatemer in transgenic tomato (Solanum lycopersicum) fruits. Biotechnol. Appl. Biochem. 2009, 52, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Ruhlman, T.; Verma, D.; Samson, N.; Daniell, H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010, 152, 2088–2104. [Google Scholar] [CrossRef]
- Oey, M.; Lohse, M.; Kreikemeyer, B.; Bock, R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009, 57, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Datta, R.; Varma, S.; Gray, S.; Lee, S.-B. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat. Biotechnol. 1998, 16, 345. [Google Scholar] [CrossRef]
- Floss, D.M.; Mockey, M.; Zanello, G.; Brosson, D.; Diogon, M.; Frutos, R.; Bruel, T.; Rodrigues, V.; Garzon, E.; Chevaleyre, C. Expression and immunogenicity of the mycobacterial ag85b/esat-6 antigens produced in transgenic plants by elastin-like peptide fusion strategy. BioMed Res. Int. 2010, 2010, 274346. [Google Scholar] [CrossRef]
- Lakshmi, P.S.; Verma, D.; Yang, X.; Lloyd, B.; Daniell, H. Low cost tuberculosis vaccine antigens in capsules: Expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro. PLoS ONE 2013, 8, e54708. [Google Scholar] [CrossRef]
- Uvarova, E.A.; Belavin, P.A.; Permyakova, N.V.; Zagorskaya, A.A.; Nosareva, O.V.; Kakimzhanova, A.A.; Deineko, E.V. Oral immunogenicity of plant-made mycobacterium tuberculosis esat6 and cfp10. BioMed Res. Int. 2013, 2013, 8. [Google Scholar] [CrossRef]
- Daniell, H.; Lee, S.-B.; Panchal, T.; Wiebe, P.O. Expression of the native cholera toxin b subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 2001, 311, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Koya, V.; Moayeri, M.; Leppla, S.H.; Daniell, H. Plant-based vaccine: Mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect. Immun. 2005, 73, 8266–8274. [Google Scholar] [CrossRef] [PubMed]
- Arlen, P.A.; Singleton, M.; Adamovicz, J.J.; Ding, Y.; Davoodi-Semiromi, A.; Daniell, H. Effective plague vaccination via oral delivery of plant cells expressing f1-v antigens in chloroplasts. Infect. Immun. 2008, 76, 3640–3650. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.T.; Xiao, Y.; Weldon, W.C.; Oberste, S.M.; Chumakov, K.; Daniell, H. Cold chain and virus-free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes. Plant Biotechnol. J. 2016, 14, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shanmugaraj, B.; Daniell, H. Expression and functional evaluation of biopharmaceuticals made in plant chloroplasts. Curr. Opin. Chem. Biol. 2017, 38, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Michoux, F.; Lössl, A.G.; Nixon, P.J. Challenges and perspectives in commercializing plastid transformation technology. J. Exp. Bot. 2016, 67, 5945–5960. [Google Scholar] [CrossRef]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in humans of an edible vaccine for hepatitis b. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar] [CrossRef]
- Nochi, T.; Takagi, H.; Yuki, Y.; Yang, L.; Masumura, T.; Mejima, M.; Nakanishi, U.; Matsumura, A.; Uozumi, A.; Hiroi, T. Rice-based mucosal vaccine as a global strategy for cold-chain-and needle-free vaccination. Proc. Natl. Acad. Sci. USA 2007, 104, 10986–10991. [Google Scholar] [CrossRef]
- Tokuhara, D.; Álvarez, B.; Mejima, M.; Hiroiwa, T.; Takahashi, Y.; Kurokawa, S.; Kuroda, M.; Oyama, M.; Kozuka-Hata, H.; Nochi, T. Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J. Clin. Investig. 2013, 123, 3829–3838. [Google Scholar] [CrossRef]
- Sijmons, P.C.; Dekker, B.M.; Schrammeijer, B.; Verwoerd, T.C.; Van Den Elzen, P.J.; Hoekema, A. Production of correctly processed human serum albumin in transgenic plants. Biotechnology (N. Y.) 1990, 8, 217. [Google Scholar] [CrossRef]
- Barta, A.; Sommergruber, K.; Thompson, D.; Hartmuth, K.; Matzke, M.A.; Matzke, A.J. The expression of a nopaline synthase—Human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol. Biol. 1986, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.A.; Hong, L.M.; Trombly, D.M.; Xie, Q.; Jackman, A.P. Production of human α-1-antitrypsin from transgenic rice cell culture in a membrane bioreactor. Biotechnol. Prog. 2005, 21, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, X.; Luo, D.; Yu, W. Efficient production of a bioactive bevacizumab monoclonal antibody using the 2a self-cleavage peptide in transgenic rice callus. Front. Plant Sci. 2016, 7, 1156. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-L.; Lin, Y.-L.; Lee, Y.-L.; Yang, N.-S.; Chan, M.-T. Expression of bioactive human interferon-gamma in transgenic rice cell suspension cultures. Transgenic Res. 2004, 13, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, P.D.; Schäfer, H.; Ramamoorthy, S.; Wink, M. Biologically active recombinant human erythropoietin expressed in hairy root cultures and regenerated plantlets of Nicotiana tabacum L. PLoS ONE 2017, 12, e0182367. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-F.; Liu, Y.-K.; Lu, C.-A.; Hsieh, S.-L.; Yu, S.-M. Production of human serum albumin by sugar starvation induced promoter and rice cell culture. Transgenic Res. 2005, 14, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-S.; Yu, H.-Y.; Chung, N.-D.; Shin, Y.-J.; Kwon, T.-H.; Yang, M.-S. Production of functional recombinant bovine trypsin in transgenic rice cell suspension cultures. Protein Expr. Purif. 2011, 76, 121–126. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, C.-I.; Park, M.-Y.; Jung, H.-S.; Ryu, W.-S.; Lim, S.-M.; Tan, H.-K.; Kwon, T.-H.; Yang, M.-S.; Kim, D.-I. Production and characterization of human ctla4ig expressed in transgenic rice cell suspension cultures. Protein Expr. Purif. 2007, 51, 293–302. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Li, Y.-T.; Lu, C.-F.; Huang, L.-F. Enhancement of recombinant human serum albumin in transgenic rice cell culture system by cultivation strategy. New Biotechnol. 2015, 32, 328–334. [Google Scholar] [CrossRef]
- Shin, Y.J.; Hong, S.Y.; Kwon, T.H.; Jang, Y.S.; Yang, M.S. High level of expression of recombinant human granulocyte-macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol. Bioeng. 2003, 82, 778–783. [Google Scholar] [CrossRef]
- Kim, T.-G.; Baek, M.-Y.; Lee, E.-K.; Kwon, T.-H.; Yang, M.-S. Expression of human growth hormone in transgenic rice cell suspension culture. Plant Cell Rep. 2008, 27, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.-D.; Kim, N.-S.; Jang, S.-H.; Oh, S.-M.; Jang, S.-H.; Kim, T.-G.; Jang, Y.-S.; Yang, M.-S. Production of functional human vascular endothelial growth factor165 in transgenic rice cell suspension cultures. Enzym. Microb. Technol. 2014, 63, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Kim, S.-H.; Kim, N.-S.; Huy, N.-X.; Choi, Y.-S.; Lee, J.-Y.; Jang, Y.-S.; Yang, M.-S.; Kim, T.-G. Production of monoclonal antibody against fima protein from Porphyromonas gingivalis in rice cell suspension culture. Plant Cell Tissue Organ Cult. 2014, 118, 293–304. [Google Scholar] [CrossRef]
- Islam, M.R.; Kim, N.-S.; Jung, J.-W.; Kim, H.-B.; Han, S.-C.; Yang, M.-S. Spontaneous pepsin c-catalyzed activation of human pepsinogen c in transgenic rice cell suspension culture: Production and characterization of human pepsin c. Enzym. Microb. Technol. 2018, 108, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.J.; Joensuu, J.J.; Menassa, R.; Brandle, J.E. Induction of protein body formation in plant leaves by elastin-like polypeptide fusions. BMC Biol. 2009, 7, 48. [Google Scholar] [CrossRef]
- Kaldis, A.; Ahmad, A.; Reid, A.; McGarvey, B.; Brandle, J.; Ma, S.; Jevnikar, A.; Kohalmi, S.E.; Menassa, R. High-level production of human interleukin-10 fusions in tobacco cell suspension cultures. Plant Biotechnol. J. 2013, 11, 535–545. [Google Scholar] [CrossRef]
- Joseph, M.; Ludevid, M.D.; Torrent, M.; Rofidal, V.; Tauzin, M.; Rossignol, M.; Peltier, J.-B. Proteomic characterisation of endoplasmic reticulum-derived protein bodies in tobacco leaves. BMC Plant Biol. 2012, 12, 36. [Google Scholar] [CrossRef]
- Torrent, M.; Llop-Tous, I.; Ludevid, M.D. Protein body induction: A new tool to produce and recover recombinant proteins in plants. In Recombinant Proteins from Plants; Springer: Berlin, Germany, 2009; pp. 193–208. [Google Scholar]
- Reuter, L.J.; Bailey, M.J.; Joensuu, J.J.; Ritala, A. Scale-up of hydrophobin-assisted recombinant protein production in tobacco by-2 suspension cells. Plant Biotechnol. J. 2014, 12, 402–410. [Google Scholar] [CrossRef]
- Joensuu, J.J.; Conley, A.J.; Lienemann, M.; Brandle, J.E.; Linder, M.B.; Menassa, R. Hydrophobin fusions for high-level transient protein expression and purification in Nicotiana benthamiana. Plant Physiol. 2010, 152, 622–633. [Google Scholar] [CrossRef]
- Gutiérrez, S.P.; Saberianfar, R.; Kohalmi, S.E.; Menassa, R. Protein body formation in stable transgenic tobacco expressing elastin-like polypeptide and hydrophobin fusion proteins. BMC Biotechnol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Huang, Z.; Phoolcharoen, W.; Lai, H.; Piensook, K.; Cardineau, G.; Zeitlin, L.; Whaley, K.J.; Arntzen, C.J.; Mason, H.S.; Chen, Q. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol. Bioeng. 2010, 106, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-B.; Lee, J.; Kang, S.; Kim, M.; Mason, H.S.; Jeon, J.-H.; Kim, H.-S. Overexpression and self-assembly of virus-like particles in Nicotiana benthamiana by a single-vector DNA replicon system. Appl. Microbiol. Biotechnol. 2014, 98, 8281–8290. [Google Scholar] [CrossRef] [PubMed]
- Fujiuchi, N.; Matsuda, R.; Matoba, N.; Fujiwara, K. Effect of nitrate concentration in nutrient solution on hemagglutinin content of Nicotiana benthamiana leaves in a viral vector-mediated transient gene expression system. Plant Biotechnol. 2014, 31, 207–211. [Google Scholar] [CrossRef]
- Fujiuchi, N.; Matsuda, R.; Matoba, N.; Fujiwara, K. Removal of bacterial suspension water occupying the intercellular space of detached leaves after agroinfiltration improves the yield of recombinant hemagglutinin in a Nicotiana benthamiana transient gene expression system. Biotechnol. Bioeng. 2016, 113, 901–906. [Google Scholar] [CrossRef]
- Fujiuchi, N.; Matsuda, R.; Matoba, N.; Fujiwara, K. Effects of plant density on recombinant hemagglutinin yields in an agrobacterium-mediated transient gene expression system using Nicotiana benthamiana plants. Biotechnol. Bioeng. 2017, 114, 1762–1770. [Google Scholar] [CrossRef]
- Moon, K.-B.; Jeon, J.-H.; Lee, W.-S.; Kim, H. Transient erythropoietin overexpression with cucumber mosaic virus suppressor 2b in Nicotiana benthamiana. Hortic. Environ. Biotechnol. 2012, 53, 434–440. [Google Scholar] [CrossRef]
- Merlin, M.; Gecchele, E.; Arcalis, E.; Remelli, S.; Brozzetti, A.; Pezzotti, M.; Avesani, L. Enhanced gad65 production in plants using the magnicon transient expression system: Optimization of upstream production and downstream processing. Biotechnol. J. 2016, 11, 542–553. [Google Scholar] [CrossRef]
- Testa, D.; Liao, M.-J.; Ferencz-Biro, K.; Rashidbaigi, A.; DiPaola, M. Composition Containing Human Alpha Interferon Species Proteins and Method for Use Thereof. U.S. Patent 5,676,942, 14 October 1997. [Google Scholar]
- Huang, T.-K.; McDonald, K.A. Bioreactor engineering for recombinant protein production in plant cell suspension cultures. Biochem. Eng. J. 2009, 45, 168–184. [Google Scholar] [CrossRef]
- Xu, J.; Ge, X.; Dolan, M.C. Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol. Adv. 2011, 29, 278–299. [Google Scholar] [CrossRef]
- Vermij, P.; Waltz, E. Usda approves the first plant-based vaccine. Nat. Biotechnol. 2006, 24, 234. [Google Scholar]
- Mor, T.S. Molecular pharming’s foot in the fda’s door: Protalix’s trailblazing story. Biotechnol. Lett. 2015, 37, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Tello-Olea, M.A. Carrot cells: A pioneering platform for biopharmaceuticals production. Mol. Biotechnol. 2015, 57, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-A.; Lim, E.-K.; Yu, S.-M. Sugar response sequence in the promoter of a rice α-amylase gene serves as a transcriptional enhancer. J. Biol. Chem. 1998, 273, 10120–10131. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Schillberg, S.; Hellwig, S.; Twyman, R.M.; Drossard, J. Gmp issues for recombinant plant-derived pharmaceutical proteins. Biotechnol. Adv. 2012, 30, 434–439. [Google Scholar] [CrossRef]
- Sparrow, P.; Broer, I.; E Hood, E.; Eversole, K.; Hartung, F.; Schiemann, J. Risk assessment and regulation of molecular farming-a comparison between Europe and US. Curr. Pharm. Des. 2013, 19, 5513–5530. [Google Scholar] [CrossRef]
- Pogue, G.P.; Vojdani, F.; Palmer, K.E.; Hiatt, E.; Hume, S.; Phelps, J.; Long, L.; Bohorova, N.; Kim, D.; Pauly, M. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol. J. 2010, 8, 638–654. [Google Scholar] [CrossRef]
- Fujiuchi, N.; Matoba, N.; Matsuda, R. Environment control to improve recombinant protein yields in plants based on agrobacterium-mediated transient gene expression. Front. Bioeng. Biotechnol. 2016, 4, 23. [Google Scholar] [CrossRef]
- Twyman, R.M.; Schillberg, S.; Fischer, R. Optimizing the yield of recombinant pharmaceutical proteins in plants. Curr. Pharm. Des. 2013, 19, 5486–5494. [Google Scholar] [CrossRef]
- Stoger, E.; Sack, M.; Perrin, Y.; Vaquero, C.; Torres, E.; Twyman, R.M.; Christou, P.; Fischer, R. Practical considerations for pharmaceutical antibody production in different crop systems. Mol. Breed. 2002, 9, 149–158. [Google Scholar] [CrossRef]
- Sala, F.; Rigano, M.M.; Barbante, A.; Basso, B.; Walmsley, A.M.; Castiglione, S. Vaccine antigen production in transgenic plants: Strategies, gene constructs and perspectives. Vaccine 2003, 21, 803–808. [Google Scholar] [CrossRef]
- Twyman, R.M.; Stoger, E.; Schillberg, S.; Christou, P.; Fischer, R. Molecular farming in plants: Host systems and expression technology. TRENDS Biotechnol. 2003, 21, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Devos, Y.; Reheul, D.; Schrijver, A.D.; Cors, F.; Moens, W. Management of herbicide-tolerant oilseed rape in europe: A case study on minimizing vertical gene flow. Environ. Biosaf. Res. 2004, 3, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.E.; Witcher, D.R.; Maddock, S.; Meyer, T.; Baszczynski, C.; Bailey, M.; Flynn, P.; Register, J.; Marshall, L.; Bond, D. Commercial production of avidin from transgenic maize: Characterization of transformant, production, processing, extraction and purification. Mol. Breed. 1997, 3, 291–306. [Google Scholar] [CrossRef]
- Federation of American Scientists (FAS). The Prodigene Incident. Available online: https://fas.org/biosecurity/education/dualuse-agriculture/2.-agricultural-biotechnology/prodigene-incident.html (accessed on 24 December 2019).
- Boothe, J.; Nykiforuk, C.; Kuhlman, P.; Whelan, H.; Pollock, W.; Clark, S.; Yuan, S.; Kumar, R.; Murray, E.; Visser, F. Analytical characterization, safety and clinical bioequivalence of recombinant human insulin from transgenic plants. In 69th Scientific Sessions; Abstract; American Diabetes Association: Arlington County, VA, USA, 2009; Volume 5. [Google Scholar]
- Breyer, D.; Goossens, M.; Herman, P.; Sneyers, M. Biosafety considerations associated with molecular farming in genetically modified plants. J. Med. Plants Res. 2009, 3, 825–838. [Google Scholar]
- McPherson, M.A.; Yang, R.-C.; Good, A.G.; Nielson, R.L.; Hall, L.M. Potential for seed-mediated gene flow in agroecosystems from transgenic safflower (Carthamus tinctorius L.) intended for plant molecular farming. Transgenic Res. 2009, 18, 281–299. [Google Scholar] [CrossRef]
- Ruf, S.; Karcher, D.; Bock, R. Determining the transgene containment level provided by chloroplast transformation. Proc. Natl. Acad. Sci. USA 2007, 104, 6998–7002. [Google Scholar] [CrossRef]
- Svab, Z.; Maliga, P. Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc. Natl. Acad. Sci. USA 2007, 104, 7003–7008. [Google Scholar] [CrossRef]
- Arlen, P.A.; Falconer, R.; Cherukumilli, S.; Cole, A.; Cole, A.M.; Oishi, K.K.; Daniell, H. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol. J. 2007, 5, 511–525. [Google Scholar] [CrossRef]
- Zavaleta, N.; Figueroa, D.; Rivera, J.; Sánchez, J.; Alfaro, S.; Lönnerdal, B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in peruvian children with acute diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 258–264. [Google Scholar] [CrossRef]
- MacManus, C.F.; Collins, C.B.; Nguyen, T.T.; Alfano, R.W.; Jedlicka, P.; de Zoeten, E.F. Ven-120, a recombinant human lactoferrin, promotes a regulatory t cell [treg] phenotype and drives resolution of inflammation in distinct murine models of inflammatory bowel disease. J. Crohn’s Colitis 2017, 11, 1101–1112. [Google Scholar] [CrossRef]
- Kozai, T.; Afreen, F.; Zobayed, S.M. Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Farran, I.; Río-Manterola, F.; Íñiguez, M.; Gárate, S.; Prieto, J.; Mingo-Castel, A.M. High-density seedling expression system for the production of bioactive human cardiotrophin-1, a potential therapeutic cytokine, in transgenic tobacco chloroplasts. Plant Biotechnol. J. 2008, 6, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Ho, L. The contribution of plant physiology in glasshouse tomato soilless culture. In South Pacific Soilless Culture Conference-SPSCC 648; ISHS: Leuven, Belgium, 2003; pp. 19–25. [Google Scholar]
- Jones, J.B., Jr. Tomato Plant Culture: In the Field, Greenhouse, and Home Garden; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kubota, C.; Thomson, C.A.; Wu, M.; Javanmardi, J. Controlled environments for production of value-added food crops with high phytochemical concentrations: Lycopene in tomato as an example. HortScience 2006, 41, 522–525. [Google Scholar] [CrossRef]
- Pniewski, T.; Czyż, M.; Wyrwa, K.; Bociąg, P.; Krajewski, P.; Kapusta, J. Micropropagation of transgenic lettuce containing hbsag as a method of mass-scale production of standardised plant material for biofarming purposes. Plant Cell Rep. 2017, 36, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Pujol, M.; Ramírez, N.I.; Ayala, M.; Gavilondo, J.V.; Valdés, R.; Rodríguez, M.; Brito, J.; Padilla, S.; Gómez, L.; Reyes, B. An integral approach towards a practical application for a plant-made monoclonal antibody in vaccine purification. Vaccine 2005, 23, 1833–1837. [Google Scholar] [CrossRef]
- Paul, M.; Reljic, R.; Klein, K.; Drake, P.M.; van Dolleweerd, C.; Pabst, M.; Windwarder, M.; Arcalis, E.; Stoger, E.; Altmann, F. Characterization of a plant-produced recombinant human secretory IgA with broad neutralizing activity against HIV. mAbs 2014, 6, 1585–1597. [Google Scholar] [CrossRef]
- Magnusdottir, A.; Vidarsson, H.; Björnsson, J.M.; Örvar, B.L. Barley grains for the production of endotoxin-free growth factors. Trends Biotechnol. 2013, 31, 572–580. [Google Scholar] [CrossRef]
- Haberlandt, G. Experiments on the culture of isolated plant cells. Bot. Rev. 1969, 35, 68–88. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef]
- Holland, T.; Buyel, J.F. Bioreactor-based production of glycoproteins in plant cell suspension cultures. In Recombinant Glycoprotein Production; Springer: Berlin, Germany, 2018; pp. 129–146. [Google Scholar]
- Trexler, M.M.; McDonald, K.A.; Jackman, A.P. A cyclical semicontinuous process for production of human α1-antitrypsin using metabolically induced plant cell suspension cultures. Biotechnol. Prog. 2005, 21, 321–328. [Google Scholar] [CrossRef]
- Barretto, S.; Michoux, F.; Nixon, P.J. Temporary immersion bioreactors for the contained production of recombinant proteins in transplastomic plants. In Recombinant Proteins from Plants; Springer: Berlin, Germany, 2016; pp. 149–160. [Google Scholar]
- Michoux, F.; Ahmad, N.; McCarthy, J.; Nixon, P.J. Contained and high-level production of recombinant protein in plant chloroplasts using a temporary immersion bioreactor. Plant Biotechnol. J. 2011, 9, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Michoux, F.; Ahmad, N.; Hennig, A.; Nixon, P.J.; Warzecha, H. Production of leafy biomass using temporary immersion bioreactors: An alternative platform to express proteins in transplastomic plants with drastic phenotypes. Planta 2013, 237, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-J.; Lee, W.-S.; Choi, E.-G.; Kim, J.-W.; Kim, B.-G.; Yang, M.-S. Mass production of somatic embryos expressing Escherichia coli heat-labile enterotoxin b subunit in siberian ginseng. J. Biotechnol. 2006, 121, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Týcová, A.; Piernikarczyk, R.J.; Kugler, M.; Lipovová, P.; Podzimek, T.; Steger, G.; Matoušek, J. A 5′ p degradation hot spot influences molecular farming of anticancerogenic nuclease tbn1 in tobacco cells. Plant Cell Tissue Organ Cult. 2016, 127, 347–358. [Google Scholar] [CrossRef]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Very-large-scale production of antibodies in plants: The biologization of manufacturing. Biotechnol. Adv. 2017, 35, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.; Kaiser, S.; Lombriser, R.; Eibl, D. Disposable bioreactors: The current state-of-the-art and recommended applications in biotechnology. Appl. Microbiol. Biotechnol. 2010, 86, 41–49. [Google Scholar] [CrossRef]
- Eibl, R.; Eibl, D. Design of bioreactors suitable for plant cell and tissue cultures. Phytochem. Rev. 2008, 7, 593–598. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Eibl, R.; Zhong, J.-J. Hosting the plant cells in vitro: Recent trends in bioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 3787–3800. [Google Scholar] [CrossRef]
- Raven, N.; Schillberg, S.; Rasche, S. Plant cell-based recombinant antibody manufacturing with a 200 L orbitally shaken disposable bioreactor. In Recombinant Proteins from Plants; Springer: Berlin, Germany, 2016; pp. 161–172. [Google Scholar]
- Raven, N.; Rasche, S.; Kuehn, C.; Anderlei, T.; Klöckner, W.; Schuster, F.; Henquet, M.; Bosch, D.; Büchs, J.; Fischer, R. Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol. Bioeng. 2015, 112, 308–321. [Google Scholar] [CrossRef]
- Corbin, J.M.; Hashimoto, B.I.; Karuppanan, K.; Kyser, Z.R.; Wu, L.; Roberts, B.A.; Noe, A.R.; Rodriguez, R.L.; McDonald, K.A.; Nandi, S. Semicontinuous bioreactor production of recombinant butyrylcholinesterase in transgenic rice cell suspension cultures. Front. Plant Sci. 2016, 7, 412. [Google Scholar] [CrossRef]
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.; Munigunti, R.K.; White, E.L.; Wilkerson, D.C.; Wong, K.Y.I.; Ly, L.H.; Marcel, S. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol. J. 2015, 13, 1180–1190. [Google Scholar] [CrossRef]
- Matsuda, R.; Kubota, C.; Alvarez, M.L.; Cardineau, G.A. Biopharmaceutical protein production under controlled environments: Growth, development, and vaccine productivity of transgenic tomato plants grown hydroponically in a greenhouse. HortScience 2009, 44, 1594–1599. [Google Scholar]
- Matsuda, R.; Kubota, C.; Alvarez, M.L.; Cardineau, G.A. Effect of high electrical conductivity of hydroponic nutrient solution on vaccine protein content in transgenic tomato. HortTechnology 2012, 22, 362–367. [Google Scholar] [CrossRef]
- Matsuda, R.; Kubota, C.; Alvarez, M.L.; Cardineau, G.A. Determining the optimal timing of fruit harvest in transgenic tomato expressing f1-v, a candidate subunit vaccine against plague. HortScience 2010, 45, 347–351. [Google Scholar] [CrossRef]
- Su, J.; Zhu, L.; Sherman, A.; Wang, X.; Lin, S.; Kamesh, A.; Norikane, J.H.; Streatfield, S.J.; Herzog, R.W.; Daniell, H. Low cost industrial production of coagulation factor ix bioencapsulated in lettuce cells for oral tolerance induction in hemophilia b. Biomaterials 2015, 70, 84–93. [Google Scholar] [CrossRef]
- Kashima, K.; Yuki, Y.; Mejima, M.; Kurokawa, S.; Suzuki, Y.; Minakawa, S.; Takeyama, N.; Fukuyama, Y.; Azegami, T.; Tanimoto, T. Good manufacturing practices production of a purification-free oral cholera vaccine expressed in transgenic rice plants. Plant Cell Rep. 2016, 35, 667–679. [Google Scholar] [CrossRef]
- Tabayashi, N.; Matsumura, T. Forefront study of plant biotechnology for practical use: Development of oral drug for animal derived from transgenic strawberry. Soc. Biotechnol. J. Japn 2014, 92, 537–539. [Google Scholar]
- McCormick, A.; Reddy, S.; Reinl, S.; Cameron, T.; Czerwinkski, D.; Vojdani, F.; Hanley, K.; Garger, S.; White, E.; Novak, J. Plant-produced idiotype vaccines for the treatment of non-hodgkin’s lymphoma: Safety and immunogenicity in a phase i clinical study. Proc. Natl. Acad. Sci. USA 2008, 105, 10131–10136. [Google Scholar] [CrossRef]
| Expression System | Host Plant | Protein Yield | Target | Material | Binary Vector/Agrobacterium Strain | Ref. |
|---|---|---|---|---|---|---|
| Agrobacterium-mediated transformation | Lettuce | 0.24%(w/w, TSP) | cholera toxin B subunit (CTB) | Cotyledons | pMYO114/LBA4404 | [6] |
| Lettuce | 0.13%(w/w, TSP) | pro-insulin (Pins) | Cotyledons | pCAMINS/LBA4404 | [7] | |
| Tomato | 6 ug/g fresh weight | thymosin α1 | Cotyledons/hypocotyls | PG-pRD12-4×Tα1/EHA105 | [15] | |
| Tobacco | 4% of TSP | TBAg-ELP | Leaf disk | pCB301/C58C1 | [19] | |
| Carrot | 0.056% of TSP | ESAT6 | Zygotic embryos | pBI121/- | [21] | |
| Carrot | 0.002% of TSP | CFP10 | Zygotic embryos | pBI121/- | [21] | |
| Potato | 8.5 μg/g FW | HBsAg | Leaf disk | pHB114/LBA4404 | [28] | |
| Rice | 0.15% of seed weight | CTB | Seed | pGPTV-35S-HPT/LBA4404 | [29] | |
| Rice | 11.9% of total protein | ARP1 | Seed | pZH2Bik45G1B/- | [30] | |
| Microprojectile-mediated transformation | Rice | 0.6% (w/w, 45% of TSP) | Glutelin1 (Gt-1) | Embryogenic callus | pAPI134/- | [10] |
| Rice | 0.5% (w/w, 25% of TSP) | hLF | Embryogenic callus | pCRGT1/- | [11] | |
| Lettuce | >72% of TSP | CTB-Pins | Plastid | pBSSK+/- | [16] | |
| Tobacco | >70% of TSP | plyGBS | Plastid | pRB95/- | [17] | |
| Tobacco | - | EPSPS | Plastid | pZS-RD/- | [18] | |
| Tobacco | 7.5% of TSP | CTB | Plastid | pLsDV/- | [20] | |
| Lettuce | 0.75% of TP | CTB | Plastid | pLsDV/- | [20] | |
| Tobacco | 4.1% of TSP | CTB | Plastid | pLD-CtV2/- | [22] | |
| Tobacco | 14.2% of TSP | Protective antigen | Plastid | pLD-ctv/- | [23] | |
| Tobacco | 3.68% of TSP | F1-V | Plastid | pLDS-F1V/- | [24] | |
| Tobacco | 4–5% of total leaf protein | CTB-VP1 | Plastid | pGEM-T/- | [25] | |
| Cell and tissue culture | Tobacco | 0.25 ug/mg protein | Human serum albumin (HSA) | Leaf disc | pMOG18/GV2260 | [31] |
| Sunflower | 0.02% of hGH transcripts | human growth hormone (hGH) | Callus | pRK290/A208 | [32] | |
| Rice | 10% of TSP | α-1-antitrypsin | Callus | pAPI73/- | [33] | |
| Rice | 242.8 mg/kg FW | mAb | Callus | pUN1390/EHA105 | [34] | |
| Rice | 699.79 ng/g FW | Interferon-gamma | Callus | pBS3S/LBA4404 | [35] | |
| Tobacco | 185.48 pg/g FW | rhEPO | Hairy root | pK7WG2D/A. rhizogenes | [36] | |
| Rice | 76.5 mg/L | HSA | Callus | pBluescript SKII+/EHA105 | [37] | |
| Rice | 15 mg/L | Trypsin | Callus | pMYT111/LBA4404 | [38] | |
| Rice | 31.4 mg/L | human cytotoxic T-lymphocyte antigen 4-immunoglobulin | Callus | pMYN409/- | [39] | |
| Rice | 45 mg/L | HSA | Callus | pA3HSA/EHA105 | [40] | |
| Rice | 73 mg/g cells | human granulocyte-macrophage colony stimulating factor | Callus | pMYN24/- | [41] | |
| Rice | 57 mg/L | hGH | Callus | pMYN449/- | [42] | |
| Rice | 19 mg/L | rhVEGF165 | Callus | pMYD171/- | [43] | |
| Rice | 17.3 mg/L | FimA mAb | Callus | pMYV657/- | [44] | |
| Rice | 18 mg/L | human pepsinogen C | Callus | pMYD213/- | [45] | |
| Tobacco | 11% of TSP | Elastin-like polypeptides (ELPs) | Leaves | pCaMterX/EHA105 | [46] | |
| Tobacco | 6.42 mg/kg FW | ELPs | BY-2 cells | pCaMterX/EHA105 | [47] | |
| Tobacco | - | Zein -derived peptides | Leaves | pC2300/EHA105 | [48] | |
| Tobacco | - | Zein -derived peptides | Leaves | pBin19/EHA105 | [49] | |
| Tobacco | 0.30 ± 0.018 g/L | Hydrophobins | BY-2 cells | pCaMterX/EHA105 | [50] | |
| Tobacco | 5.0 mg/g FW | Hydrophobins-GFP | BY-2 cells | pCaMterX/EHA105 | [51] | |
| Tobacco | 0.2% of TSP | ELP/HFBI | Leaves | pCaMterX/- | [52] | |
| Transient expression system | Tobacco | 0.5 mg/g FW | mAb | Leaves | pBY/LBA4404 | [53] |
| Tobacco | 1.0 mg/g FW | BMVCP/CMVCP/MRFVCP | Leaves | pBYR2fp/GV3101 | [54] | |
| Tobacco | >1.0 mg/g FW | Hemagglutinin (HA) | Leaves | pNM216/GV3101::pMP90 | [55] | |
| Tobacco | 846 ug/g FW | Hemagglutinin (HA) | Leaves | pNM216/GV3101::pMP90 | [56] | |
| Tobacco | 215 ug/g fresh mass | Hemagglutinin (HA) | Leaves | pNM216/GV3101::pMP90 | [57] | |
| Tobacco | 2.0 ug/mg TSP | rhEPO | Leaves | pEG101/EHA105 | [58] | |
| Tobacco | 226.9 µg/g FW | human glutamic acid decarboxylase | Leaves | pK7WG2/EHA105 | [59] |
| Expression System | Advantages | Disadvantages |
|---|---|---|
| Production System of Target Proteins | ||
| Nuclear stable transformation |
|
|
| Plastid stable transformation |
|
|
| Transient expression |
|
|
| Plant Biomass Production Systems | ||
| Open field cultivation |
|
|
| Closed culture |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, K.-B.; Park, J.-S.; Park, Y.-I.; Song, I.-J.; Lee, H.-J.; Cho, H.S.; Jeon, J.-H.; Kim, H.-S. Development of Systems for the Production of Plant-Derived Biopharmaceuticals. Plants 2020, 9, 30. https://doi.org/10.3390/plants9010030
Moon K-B, Park J-S, Park Y-I, Song I-J, Lee H-J, Cho HS, Jeon J-H, Kim H-S. Development of Systems for the Production of Plant-Derived Biopharmaceuticals. Plants. 2020; 9(1):30. https://doi.org/10.3390/plants9010030
Chicago/Turabian StyleMoon, Ki-Beom, Ji-Sun Park, Youn-Il Park, In-Ja Song, Hyo-Jun Lee, Hye Sun Cho, Jae-Heung Jeon, and Hyun-Soon Kim. 2020. "Development of Systems for the Production of Plant-Derived Biopharmaceuticals" Plants 9, no. 1: 30. https://doi.org/10.3390/plants9010030
APA StyleMoon, K.-B., Park, J.-S., Park, Y.-I., Song, I.-J., Lee, H.-J., Cho, H. S., Jeon, J.-H., & Kim, H.-S. (2020). Development of Systems for the Production of Plant-Derived Biopharmaceuticals. Plants, 9(1), 30. https://doi.org/10.3390/plants9010030