Plant-Unique cis/trans Isomerism of Long-Chain Base Unsaturation is Selectively Required for Aluminum Tolerance Resulting from Glucosylceramide-Dependent Plasma Membrane Fluidity
Abstract
1. Introduction
2. Results
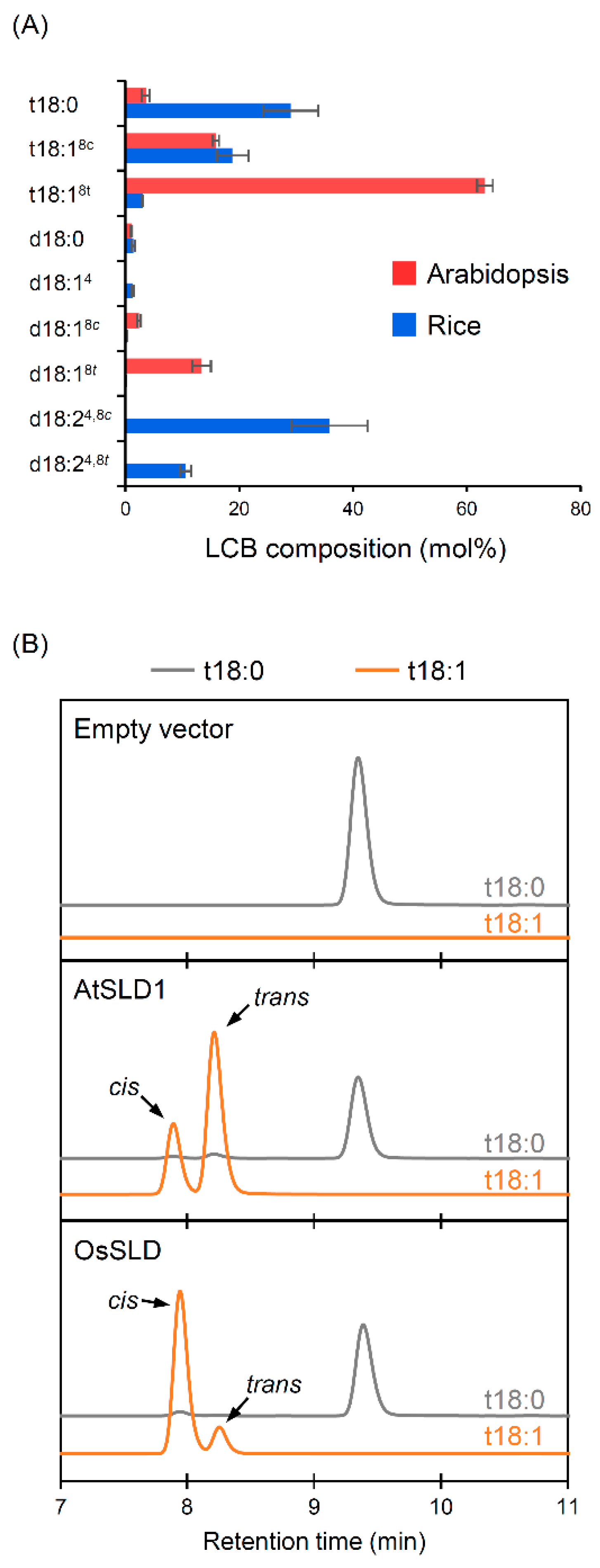
2.1. Different Isomer Productivity of Arabidopsis and Rice SLDs
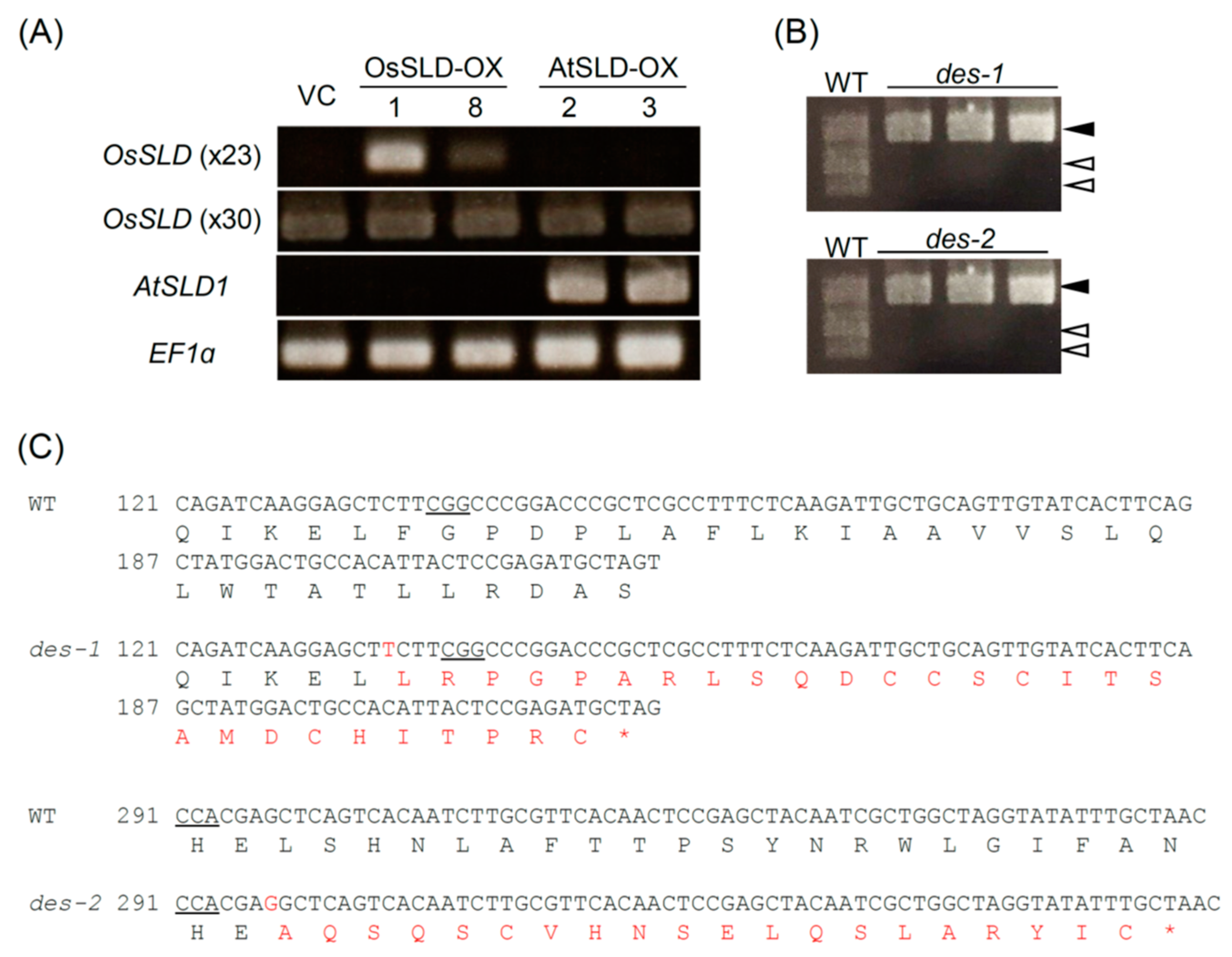
2.2. Genetic Modification of the LCB Composition in Rice
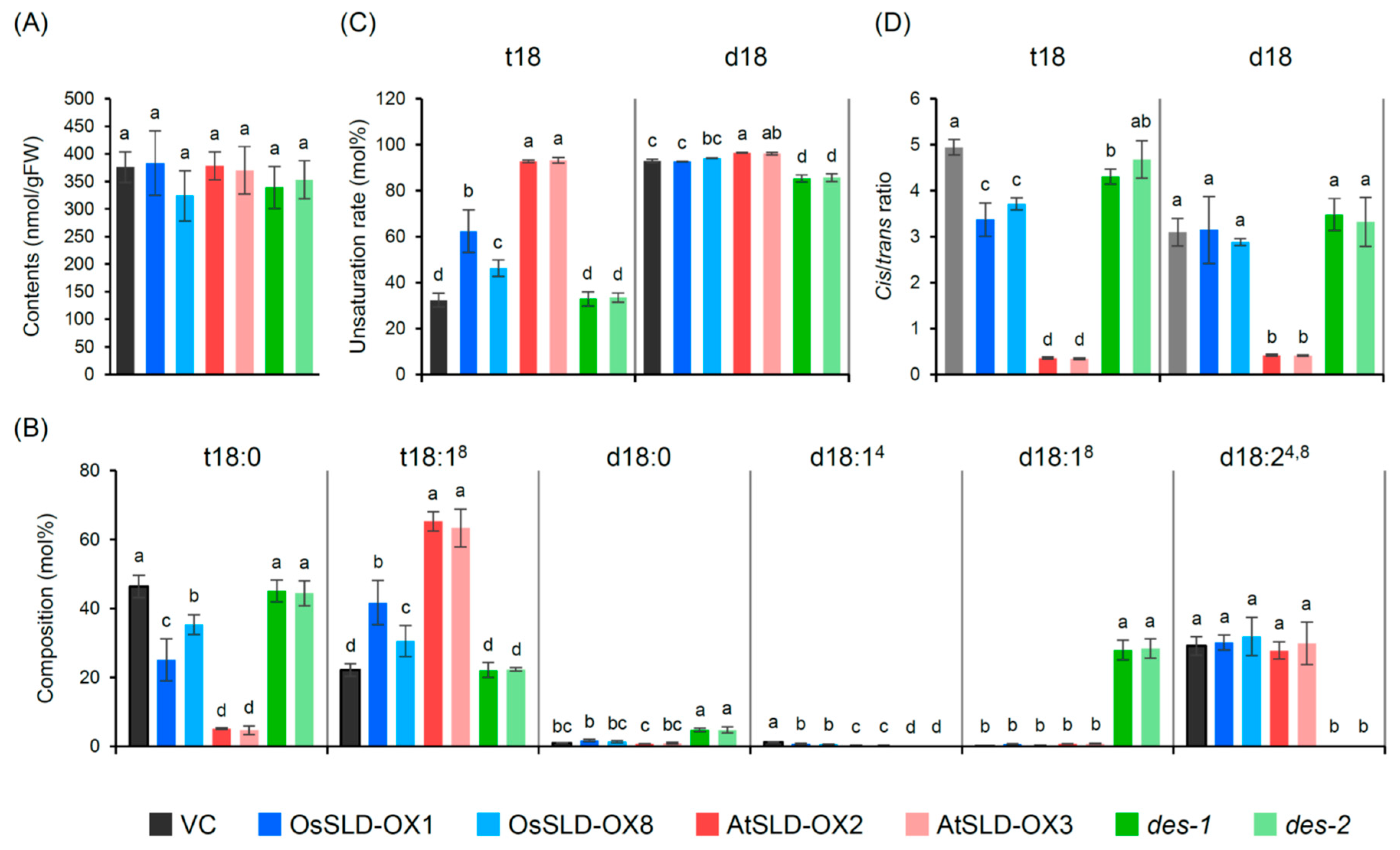
2.3. Alteration of LCB Profiles in Transgenic Rice
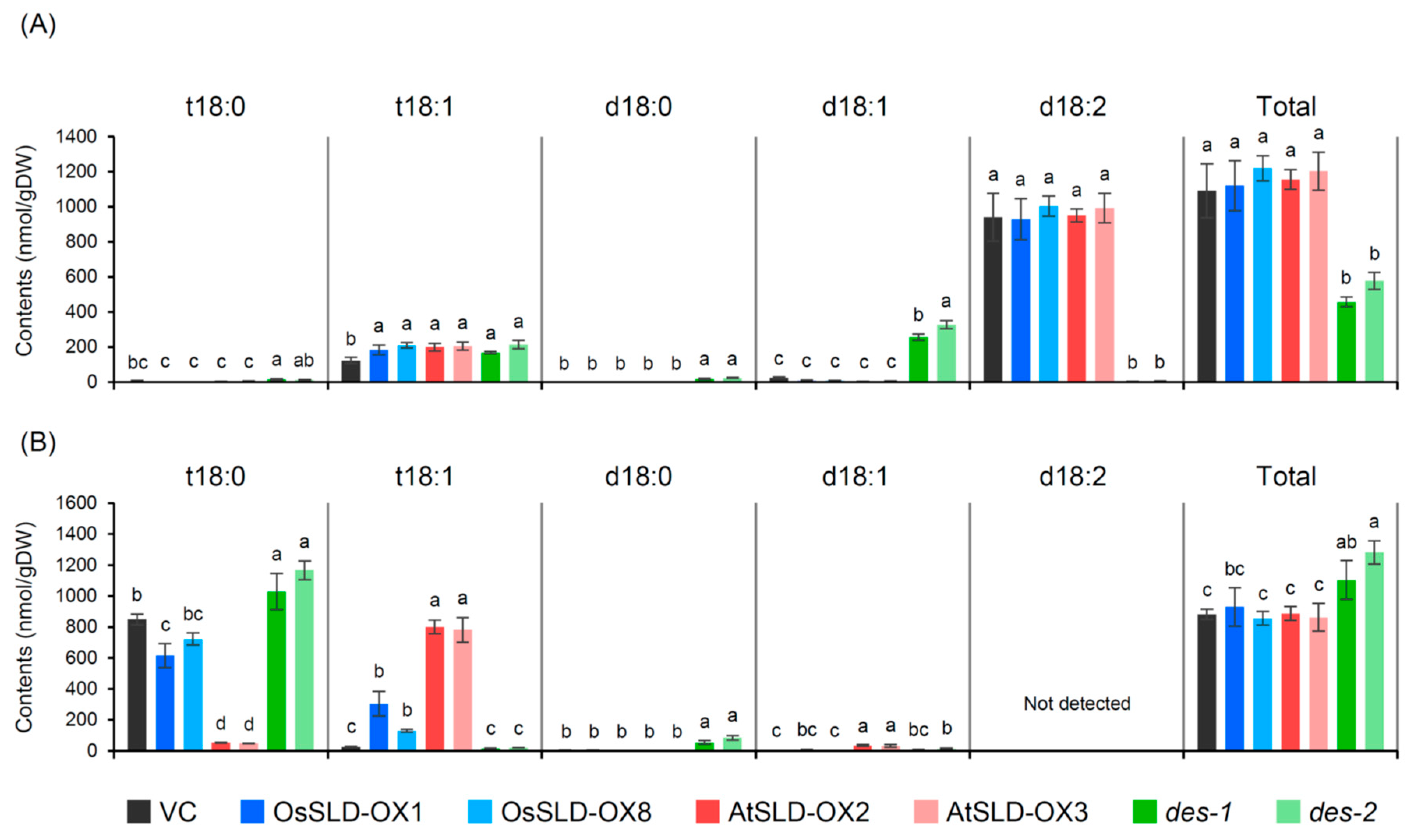
2.4. Characterization of the Sphingolipidomic Profile of Transgenic Rice Root Tissue
2.5. Imaging Analysis of the Fluidity of the Plasma Membrane
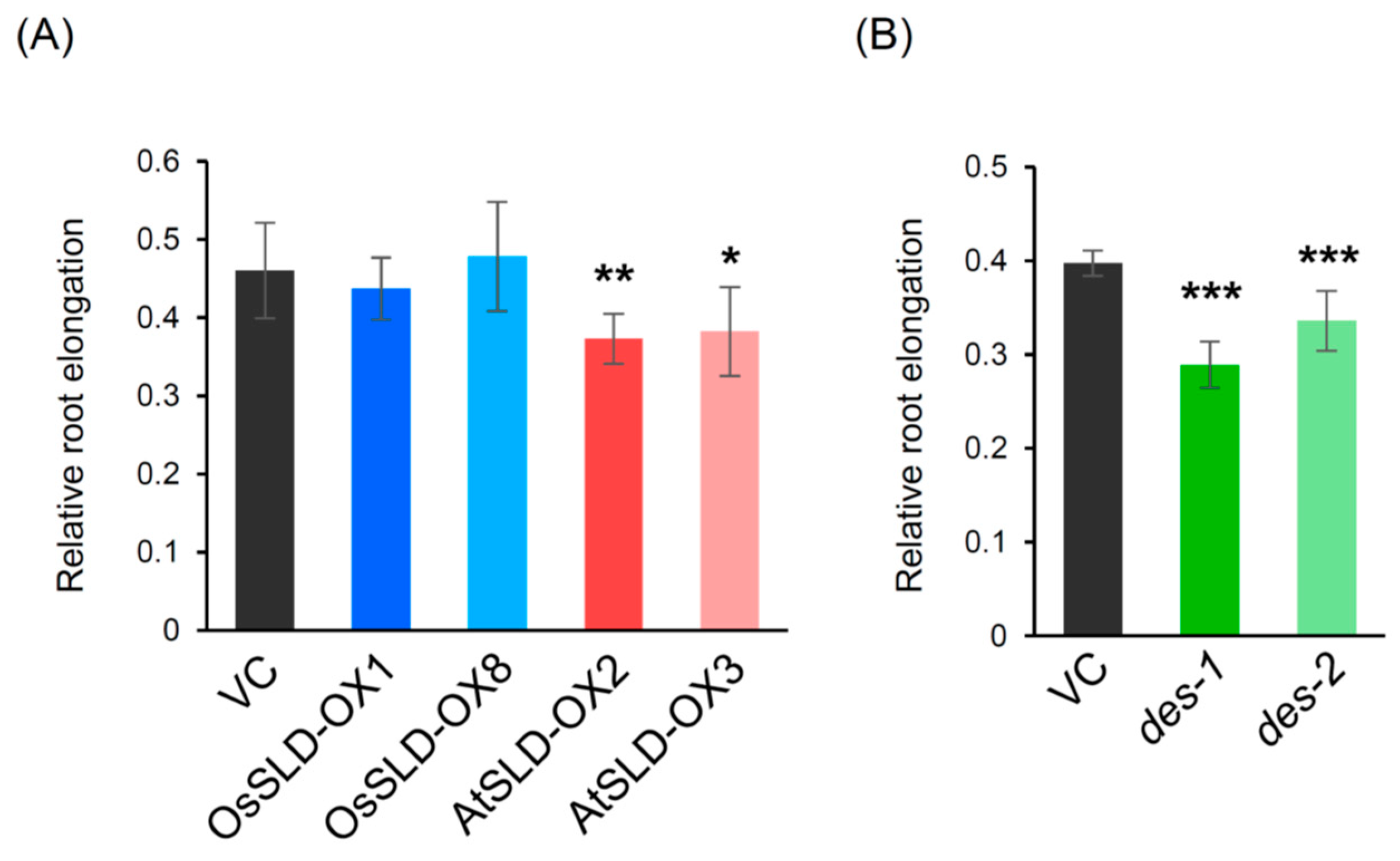
2.6. Aluminum Tolerance Assay
3. Discussion
3.1. Metabolic Effects of the Genetic Modification of LCB Unsaturation on the Sphingolipidome in Rice
3.2. Relationship between LCB Unsaturation, Membrane Fluidity, and Aluminum Tolerance
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. LCB Analysis
4.3. Yeast Expression Assay
4.4. Transgenic Rice
4.5. RT-PCR
4.6. Sphingolipidomic Analysis
4.7. Measurement of Membrane Fluidity
4.8. Aluminum Tolerance Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sperling, P.; Heinz, E. Plant sphingolipids: Structural diversity, biosynthesis, first genes and functions. Biochim. Biophys. Acta 2003, 1632, 1–15. [Google Scholar] [CrossRef]
- Dunn, T.M.; Lynch, D.V.; Michaelson, L.V.; Napier, J.A. A post-genomic approach to understanding sphingolipid metabolism in Arabidopsis thaliana. Ann. Bot. 2004, 93, 483–497. [Google Scholar] [CrossRef]
- Markham, J.E.; Jaworski, J.G. Rapid measurement of sphingolipids from Arabidopsis thaliana by reversed-phase high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1304–1314. [Google Scholar] [CrossRef]
- Cassim, M.A.; Gouguet, P.; Gronnier, J.; Laurent, N.; Germain, V.; Grison, M.; Boutté, Y.; Gerbeau-Pissot, P.; Simon-Plas, F.; Mongrand, S. Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res. 2019, 73, 1–27. [Google Scholar] [CrossRef]
- Chen, M.; Markham, J.E.; Cahoon, E.B. Sphingolipid Δ8 unsaturation is important for glucosylceramides biosynthesis and low-temperature performance in Arabidopsis. Plant J. 2012, 69, 769–781. [Google Scholar] [CrossRef]
- Dutilleul, C.; Benhassaine-Kesri, G.; Demandre, C.; Rézé, N.; Launay, A.; Pelletier, S.; Renou, J.P.; Zachowski, A.; Baudouin, E.; Guillas, I. Phytosphingosine-phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. New Phytol. 2012, 194, 181–191. [Google Scholar] [CrossRef]
- Nagano, M.; Ishikawa, T.; Ogawa, Y.; Iwabuchi, M.; Nakasone, A.; Shimamoto, K.; Uchimiya, H.; Kawai-Yamada, M. Arabidopsis Bax inhibitor-1 promotes sphingolipid synthesis during cold stress by interacting with ceramide-modifying enzymes. Planta 2014, 240, 77–89. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Fu, X.; Mei, X.; Cheng, S.; Liao, Y.; Deng, R.; Xu, X.; Jiang, Y.; Duan, X.; et al. The sphingolipid biosynthetic enzyme Sphingolipid Δ8 desaturase is important for chilling resistance of tomato. Sci. Rep. 2016, 6, 38742. [Google Scholar] [CrossRef]
- De Silva, K.; Laska, B.; Brown, C.; Sederoff, H.W.; Khodakovskaya, M. Arabidopsis thaliana calcium-dependent lipid-binding protein (AtCLB): A novel repressor of abiotic stress response. J. Exp. Bot. 2011, 62, 2679–2689. [Google Scholar] [CrossRef]
- Guo, L.; Mishra, G.; Markham, J.E.; Li, M.; Tawfall, A.; Welti, R.; Wang, X. Connections between sphingosine kinase and phospholipase D in the abscisic acid signaling pathway in Arabidopsis. J. Biol. Chem. 2012, 287, 8286–8296. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Liang, H.; Yao, N.; Song, J.T.; Luo, S.; Lu, H.; Greenberg, J.T. Ceramides modulate programmed cell death in plants. Genes Dev. 2003, 17, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Ishikawa, T.; Fujiwara, M.; Fukao, Y.; Kawano, Y.; Kawai-Yamada, M.; Shimamoto, K. Plasma Membrane Microdomains Are Essential for Rac1-RbohB/H-Mediated Immunity in Rice. Plant Cell 2016, 26, 1966–1983. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Aki, T.; Yanagisawa, S.; Uchimiya, H.; Kawai-Yamada, M. Overexpression of BAX INHIBITOR-1 Links Plasma Membrane Microdomain Proteins to Stress. Plant Physiol. 2015, 169, 1333–1343. [Google Scholar] [CrossRef]
- Xie, L.J.; Chen, Q.F.; Chen, M.X.; Yu, L.J.; Huang, L.; Chen, L.; Wang, F.Z.; Xia, F.N.; Zhu, T.R.; Wu, J.X.; et al. Unsaturation of very-long-chain ceramides protects plant from hypoxia-induced damages by modulating ethylene signaling in Arabidopsis. PLoS Genet. 2015, 11, e1005143. [Google Scholar] [CrossRef]
- Ryan, P.R.; Liu, Q.; Sperling, P.; Dong, B.; Franke, S.; Delhaize, E. A higher plant Δ8 sphingolipid desaturase with a preference for (Z)-isomer formation confers aluminum tolerance to yeast and plants. Plant Physiol. 2007, 144, 1968–1977. [Google Scholar] [CrossRef][Green Version]
- Sperling, P.; Zähringer, U.; Heinz, E. A sphingolipid desaturase from higher plants. Identification of a new cytochrome b5 fusion protein. J. Biol. Chem. 1998, 273, 28590–28596. [Google Scholar] [CrossRef]
- Ternes, P.; Franke, S.; Zähringer, U.; Sperling, P.; Heinz, E. Identification and characterization of a sphingolipid Δ4-desaturase family. J. Biol. Chem. 2002, 277, 25512–25518. [Google Scholar] [CrossRef]
- Takakuwa, N.; Kinoshita, M.; Oda, Y.; Ohnishi, M. Isolation and characterization of the genes encoding Δ8-sphingolipid desaturase from Saccharomyces kluyveri and Kluyveromyces lactis. Curr. Microbiol. 2002, 45, 459–461. [Google Scholar] [CrossRef]
- Inagaki, M.; Oyama, A.; Arao, K.; Higuchi, R. Constituents of Crinoidea, 4. Isolation and structure of ceramides and glucocerebrosides from the feather star Comanthus japonica. Chem. Pharm. Bull. 2004, 52, 1307–1311. [Google Scholar] [CrossRef][Green Version]
- Tonon, T.; Sayanova, O.; Michaelson, L.V.; Qing, R.; Harvey, D.; Larson, T.R.; Li, Y.; Napier, J.A.; Graham, I.A. Fatty acid desaturases from the microalga Thalassiosira pseudonana. FEBS J. 2005, 272, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, G.Y.; Fang, W.; Xu, Q.; Tan, R.X. Δ10(E)-Sphingolipid desaturase involved in fusaruside mycosynthesis and stress adaptation in Fusarium graminearum. Sci. Rep. 2015, 5, 10486. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Zhang, G.J.; Zhang, X.J.; Yuan, J.H.; Deng, C.L.; Hu, Z.M.; Gao, W.J. Genes encoding Δ8-sphingolipid desaturase from various plants: Identification, biochemical functions, and evolution. J. Plant Res. 2016, 129, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Ohnishi, M.; Hotsubo, K.; Kojima, M.; Ito, S. Sphingoid base composition of cerebrosides from plant leaves. Biosci. Biotech. Biochem. 1997, 61, 351–353. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, T.; Ito, Y.; Kawai-Yamada, M. Molecular characterization and targeted quantitative profiling of the sphingolipidome in rice. Plant J. 2016, 88, 681–693. [Google Scholar] [CrossRef]
- Roach, C.; Feller, S.E.; Ward, J.A.; Shaikh, S.R.; Zerouga, M.; Stillwell, W. Comparison of cis and trans fatty acid containing phosphatidylcholines on membrane properties. Biochemistry 2004, 43, 6344–6351. [Google Scholar] [CrossRef]
- Akeson, M.A.; Munns, D.N.; Burau, R.G. Adsorption of Al3+ to phosphatidylcholine vesicles. Biochim. Biophys. Acta 1989, 986, 33–40. [Google Scholar] [CrossRef]
- Krtková, J.; Havelková, L.; Křepelová, A.; Fišer, R.; Vosolsobě, S.; Novotná, Z.; Martinec, J.; Schwarzerová, K. Loss of membrane fluidity and endocytosis inhibition are involved in rapid aluminum-induced root growth cessation in Arabidopsis thaliana. Plant Physiol. Biochem. 2012, 60, 88–97. [Google Scholar] [CrossRef]
- Maejima, E.; Watanabe, T.; Osaki, M.; Wagatsuma, T. Phosphorus deficiency enhances aluminum tolerance of rice (Oryza sativa) by changing the physicochemical characteristics of root plasma membranes and cell walls. J. Plant Physiol. 2014, 171, 9–15. [Google Scholar] [CrossRef]
- Huynh, V.B.; Repellin, A.; Zuily-Fodil, Y.; Pham-Thi, A.T. Aluminum stress response in rice: Effects on membrane lipid composition and expression of lipid biosynthesis genes. Physiol. Plant. 2012, 146, 272–284. [Google Scholar] [CrossRef]
- Pejchar, P.; Potocký, M.; Krčková, Z.; Brouzdová, J.; Daněk, M.; Martinec, J. Non-specific phospholipase C4 mediates response to aluminum toxicity in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.F.; Song, L.Y.; Yin, W.B.; Chen, Y.H.; Chen, L.; Li, J.L.; Wang, R.R.; Hu, Z.M. Isolation and functional characterisation of the genes encoding Δ(8)-sphingolipid desaturase from Brassica rapa. J. Genet. Genom. 2012, 39, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Markham, J.E.; Dietrich, C.R.; Jaworski, J.G.; Cahoon, E.B. Sphingolipid long-chain base hydroxylation is important for growth and regulation of sphingolipid content and composition in Arabidopsis. Plant Cell 2008, 20, 1862–1878. [Google Scholar] [CrossRef] [PubMed]
- Rennie, E.A.; Ebert, B.; Miles, G.P.; Cahoon, R.E.; Christiansen, K.M.; Stonebloom, S.; Khatab, H.; Twell, D.; Petzold, C.J.; Adams, P.D.; et al. Identification of a sphingolipid α-glucuronosyltransferase that is essential for pollen function in Arabidopsis. Plant Cell 2014, 26, 3314–3325. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.E.; Li, J.; Cahoon, E.B.; Jaworski, J.G. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 2006, 281, 22684–22694. [Google Scholar] [CrossRef]
- Ternes, P.; Wobbe, T.; Schwarz, M.; Albrecht, S.; Feussner, K.; Riezman, I.; Cregg, J.M.; Heinz, E.; Riezman, H.; Feussner, I.; et al. Two pathways of sphingolipid biosynthesis are separated in the yeast Pichia pastoris. J. Biol. Chem. 2011, 286, 11401–11414. [Google Scholar] [CrossRef]
- Owen, D.M.; Rentero, C.; Magenau, A.; Abu-Siniyeh, A.; Gaus, K. Quantitative imaging of membrane lipid order in cells and organisms. Nat. Protoc. 2011, 7, 24–35. [Google Scholar] [CrossRef]
- Watanabe, M.; Miyagi, A.; Nagano, M.; Kawai-Yamada, M.; Imai, H. Characterization of glucosylceramides in the Polygonaceae, Rumex obtusifolius L. injurious weed. Biosci. Biotechnol. Biochem. 2011, 75, 877–881. [Google Scholar] [CrossRef]
- Oura, T.; Kajiwara, S. Disruption of the sphingolipid Δ8-desaturase gene causes a delay in morphological changes in Candida albicans. Microbiology 2008, 154, 3795–3803. [Google Scholar] [CrossRef][Green Version]
- Michaelson, L.V.; Zäuner, S.; Markham, J.E.; Haslam, R.P.; Desikan, R.; Mugford, S.; Albrecht, S.; Warnecke, D.; Sperling, P.; Heinz, E.; et al. Functional characterization of a higher plant sphingolipid Δ4-desaturase: Defining the role of sphingosine and sphingosine-1-phosphate in Arabidopsis. Plant Physiol. 2009, 149, 487–498. [Google Scholar] [CrossRef]
- Luttgeharm, K.D.; Kimberlin, A.N.; Cahoon, R.E.; Cerny, R.L.; Napier, J.A.; Markham, J.E.; Cahoon, E.B. Sphingolipid metabolism is strikingly different between pollen and leaf in Arabidopsis as revealed by compositional and gene expression profiling. Phytochemistry 2015, 115, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Kashiwagi, M.; Tsuchihashi, K.; Akino, T.; Gasa, S. Substrate specificity and some other enzymatic properties of dihydroceramide desaturase (ceramide synthase) in fetal rat skin. J. Biochem. 1998, 123, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Imai, H.; Kawai-Yamada, M. Development of an LC–MS/MS method for the analysis of free sphingoid bases using 4-fluoro-7-nitrobenzofurazan (NBD-F). Lipids 2014, 49, 295–304. [Google Scholar] [CrossRef]
- Ishii, J.; Izawa, K.; Matsumura, S.; Wakamura, K.; Tanino, T.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. A simple and immediate method for simultaneously evaluating expression level and plasmid maintenance in yeast. J. Biolchem. 2009, 154, 701–708. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef]
- Mikami, M.; Toki, S.; Endo, M. Comparison of CRISPR/Cas9 expression constructs for efficient targeted mutagenesis in rice. Plant Mol. Biol. 2015, 88, 561–572. [Google Scholar] [CrossRef]
- Ishikawa, T.; Fang, L.; Rennie, E.A.; Sechet, J.; Yan, J.; Jing, B.; Moore, W.; Cahoon, E.B.; Scheller, H.V.; Kawai-Yamada, M.; et al. GLUCOSAMINE INOSITOLPHOSPHORYLCERAMIDE TRANSFERASE1 (GINT1) is a GlcNAc-Containing Glycosylinositol Phosphorylceramide Glycosyltransferase. Plant Physiol. 2018, 177, 938–952. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.M. Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, M.; Nagano, M.; Jin, S.; Miyagi, A.; Yamaguchi, M.; Kawai-Yamada, M.; Ishikawa, T. Plant-Unique cis/trans Isomerism of Long-Chain Base Unsaturation is Selectively Required for Aluminum Tolerance Resulting from Glucosylceramide-Dependent Plasma Membrane Fluidity. Plants 2020, 9, 19. https://doi.org/10.3390/plants9010019
Sato M, Nagano M, Jin S, Miyagi A, Yamaguchi M, Kawai-Yamada M, Ishikawa T. Plant-Unique cis/trans Isomerism of Long-Chain Base Unsaturation is Selectively Required for Aluminum Tolerance Resulting from Glucosylceramide-Dependent Plasma Membrane Fluidity. Plants. 2020; 9(1):19. https://doi.org/10.3390/plants9010019
Chicago/Turabian StyleSato, Masaya, Minoru Nagano, Song Jin, Atsuko Miyagi, Masatoshi Yamaguchi, Maki Kawai-Yamada, and Toshiki Ishikawa. 2020. "Plant-Unique cis/trans Isomerism of Long-Chain Base Unsaturation is Selectively Required for Aluminum Tolerance Resulting from Glucosylceramide-Dependent Plasma Membrane Fluidity" Plants 9, no. 1: 19. https://doi.org/10.3390/plants9010019
APA StyleSato, M., Nagano, M., Jin, S., Miyagi, A., Yamaguchi, M., Kawai-Yamada, M., & Ishikawa, T. (2020). Plant-Unique cis/trans Isomerism of Long-Chain Base Unsaturation is Selectively Required for Aluminum Tolerance Resulting from Glucosylceramide-Dependent Plasma Membrane Fluidity. Plants, 9(1), 19. https://doi.org/10.3390/plants9010019




