Abamectin Efficacy on the Potato Cyst Nematode Globodera pallida
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Test on Cysts
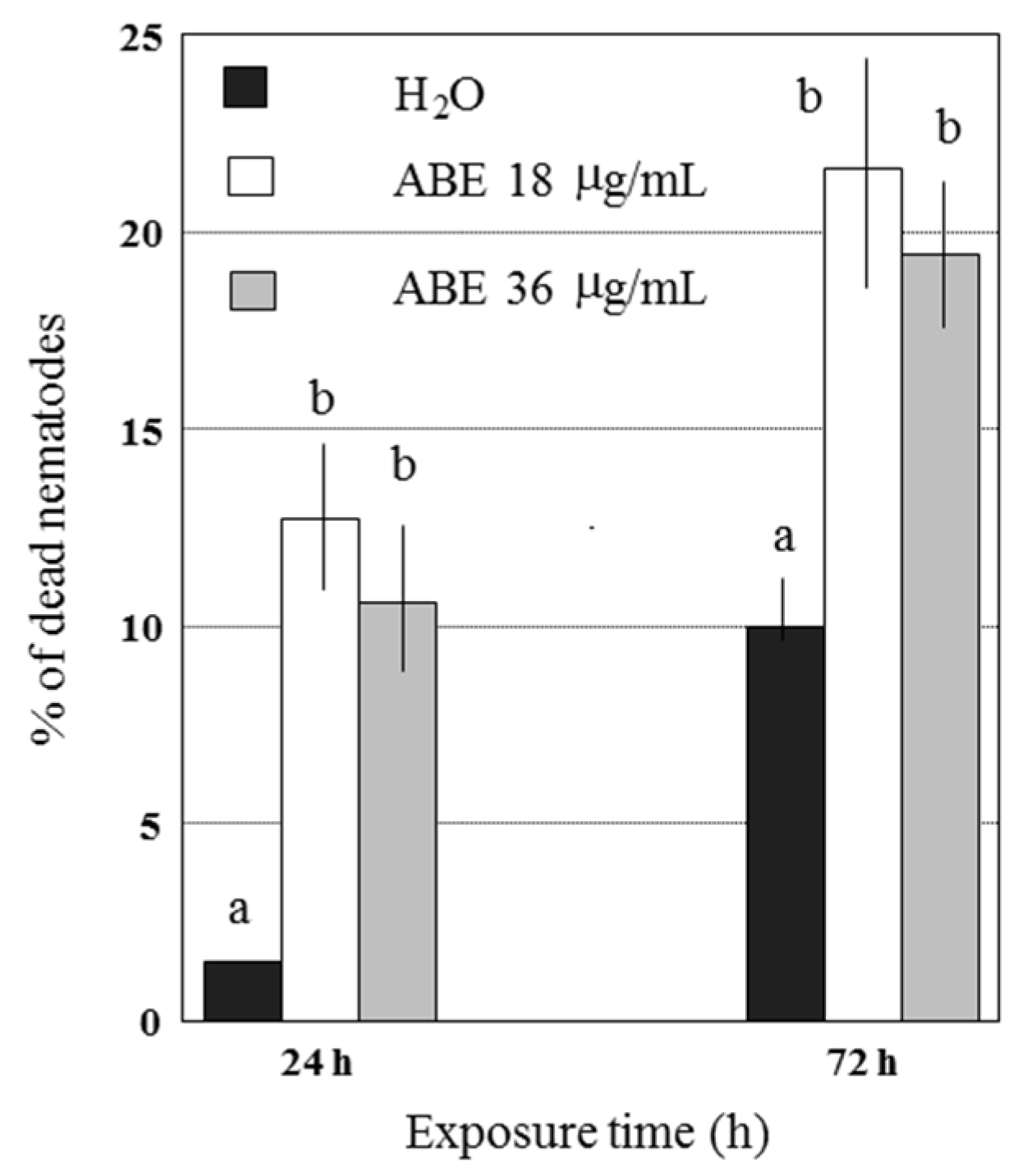
2.2. Abamectin Effect on Viability and Infectivity of G. pallida J2
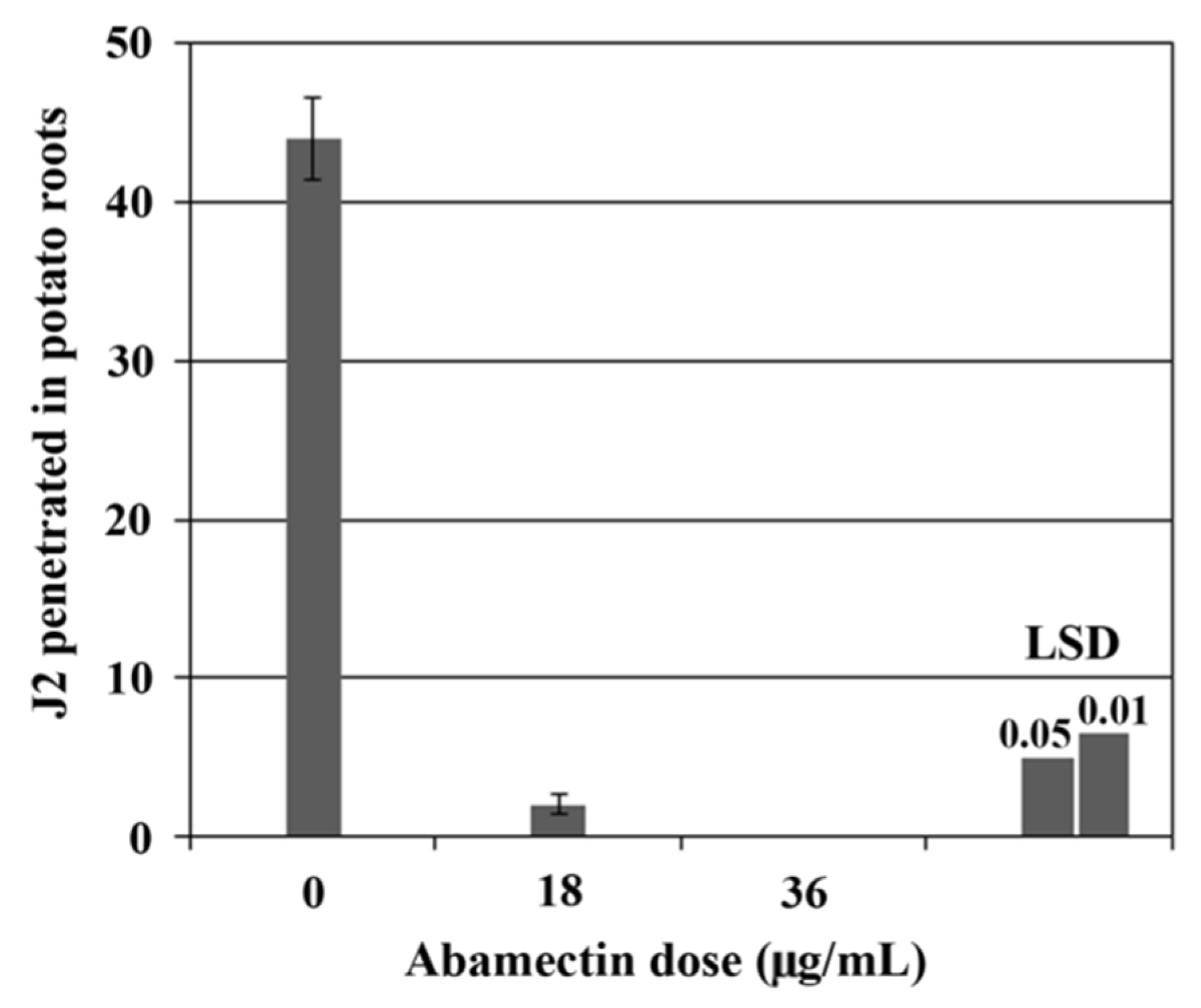
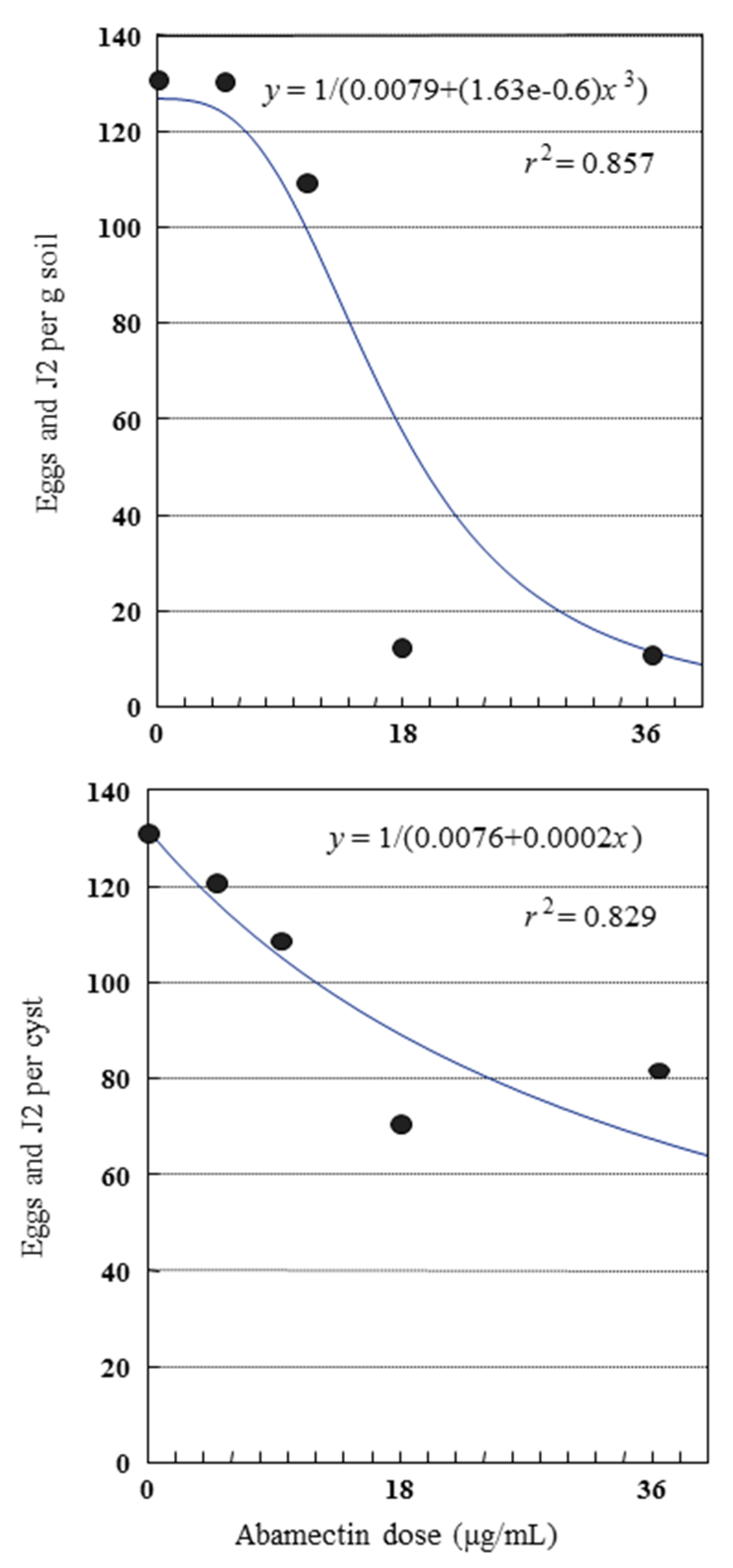
2.3. Glasshouse Experiment
3. Materials and Methods
3.1. Globodera Pallida Population
3.2. In Vitro Tests on Cysts
3.3. Abamectin Effect on Viability and Infectivity of G. pallida J2
3.4. Glasshouse Experiment
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- CABI Datasheet. Available online: http://www.cabi.org/isc/datasheet/27033#20127201272 (accessed on 25 November 2019).
- Greco, N.; Di Vito, M.; Brandonisio, A.; Giordano, I.; De Marinis, G. The effect of Globodera pallida and G. rostochiensis on potato yield. Nematologica 1982, 28, 379–386. [Google Scholar] [CrossRef]
- Jones, L.; Koehler, A.K.; Trnka, M.; Balek, J.; Challinor, A.J.; Atkinson, H.J.; Urwin, P.E. Climate change is predicted to alter the current pest status of Globodera pallida and G. rostochiensis in the United Kingdom. Glob. Chang. Biol. 2017, 23, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Fournet, S.; Kerlan, M.C.; Renault, L.; Dantec, J.P.; Rouaux, C.; Montarry, J. Selection of nematodes by resistant plants has implications for local adaptations and cross-virulence. Plant Pathol. 2013, 62, 184–193. [Google Scholar] [CrossRef]
- Been, T.H.; Schomaker, C.H. Fumigation of marine clay soils infested with Globodera pallida and G. rostochiensis using 1,3-dichloropropene and additional top soil treatments. Nematology 1999, 1, 3–14. [Google Scholar] [CrossRef]
- Sasanelli, N.; Dongiovanni, C.; Schepel, E.; Fumarola, G.; Van De Griend, P.; Santori, A.; Myrta, A. Dymethyl Disulfide (DMDS) in the control of the cyst nematode Globodera pallida on potato in Italy and in the Netherlands. In Proceedings of the International Public Procurement Conference, Berlin, Germany, 24–27 August 2015; pp. 245–246. [Google Scholar]
- Deliopoulos, T.; Devine, K.J.; Haydock, P.P.J.; Jones, P.W. Studies on the effect of mycorrhization of potato roots on the hatching activity of potato root leachate towards the potato cyst nematodes, Globodera pallida and G. rostochiensis. Nematology 2007, 9, 719–729. [Google Scholar]
- Aires, A.; Carvalho, R.; Da Conceicao, M.; Eduardo, R. Suppressing potato cyst nematode, Globodera rostochiensis, with extracts of Brassicacea plants. Am. J. Potato Res. 2009, 86, 327–333. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. App. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Timper, P. Utilization of biological control for managing plant-parasitic nematodes. In Biological Control of Plant-Parasitic Nematodes: Building Coherence between Microbial Ecology and Molecular Mechanisms; Davies, K., Spiegel, Y., Eds.; Springer: London, UK, 2011; pp. 259–289. [Google Scholar]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Renčo, M.; Sasanelli, N.; Papajova, I.; Maistrello, L. Nematicidal effect of chestnut tannin solutions on the potato cyst nematode Globodera rostochiensis (Woll.) Barhens. Helminthologia 2012, 49, 108–114. [Google Scholar] [CrossRef]
- Jansson, H.B.; Lopez-Llorca, L.V. Control of nematodes by Fungi. In Fungal Biotechnology in Agricultural, Food, and Enviromental Applications; Dekker, M., Ed.; CRC Press: Boca Raton FL, USA, 2004. [Google Scholar] [CrossRef]
- Dong, L.Q.; Zhang, K.Q. Microbial control of plant-parasitic nematodes: A five-party interaction. Plant Soil 2006, 288, 31–45. [Google Scholar] [CrossRef]
- Dybas, R.A. Abamectin use in crop protection. In Ivermectin and Abamectin; Campbell, W.C., Ed.; Springer: New York, NY, USA, 1989; pp. 287–310. [Google Scholar]
- Jones, C.R.; Samac, D.A. Biological control of fungi causing alfalfa seedling damping-off with a disease-suppressive strain of Streptomyces. Biol. Control 1996, 7, 196–204. [Google Scholar] [CrossRef]
- Sasanelli, N.; Toderas, I.; Ciccarese, F.; Erhan, D.; Rusu, S.; Bivol, A.; Iurcu-Straistaru, E. Biological limitators in the control of Meloidogyne incognita and Verticillium dahliae on eggplant. In Proceedings of the 9th International Conference of Zoologists, Chisinau, Republic of Moldova, 12–13 October 2016; pp. 160–161. [Google Scholar]
- Putter, I.; Maconnel, J.G.; Preiser, F.A.; Haidri, A.A.; Ristich, S.S.; Dybas, R.A. Avermectins: Novel insecticides, acaricides and nematicides from a soil microorganism. Experientia 1981, 37, 963–964. [Google Scholar] [CrossRef]
- Siddique, S.; Syed, Q.; Adnan, A.; Nadeem, M.; Irfan, M.; Qureshi, F.A. Production of Avermectin B1b From Streptomyces avermitilis 41445 by Batch Submerged Fermentation. Jundishapur J. Microbiol. 2013, 6, 7198. [Google Scholar] [CrossRef]
- Jansson, R.K.; Dybas, R.A. Avermectins: Biochemical mode of action, biological activity and agricultural importance. In Insecticides with Novel Modes of Action: Mechanism and Application; Ishaaya, I., Degheele, D., Eds.; Springer: New York, NY, USA, 1998; pp. 152–170. [Google Scholar]
- Khalil, M.H.; Allam, A.F.; Barakat, A.S. Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under greenhouse conditions. J. Plant Prot. Res. 2012, 2, 102–103. [Google Scholar]
- Cabrera, J.A.; Menjivar, R.D.; Dababat, A.; Sikora, R.A. Properties and nematicide performance of Avermectins. J. Phytopathol. 2013, 161, 65–69. [Google Scholar] [CrossRef]
- Leathwick, D.M.; Miller, C.M.; Sauermann, C.W.; Candy, P.M.; Ganesh, S.; Fraser, K.; Waghorn, T.S. The efficacy of plasma profile of abamectin plus levamisole combination anthelmintics administered as oral and pour-on formulations to cattle. Vet. Parasitol. 2016, 227, 85–92. [Google Scholar] [CrossRef]
- Khalil, M.S. Abamectin and Azadirachtin as Eco-friendly Promising Biorational Tools in Integrated Nematodes Management Programs. J. Plant Pathol. Microb. 2013, 4, 174. [Google Scholar] [CrossRef]
- Litskas, V.D.; Karamanlis, X.N.; Batzias, G.C.; Tsiouris, S.E. Are the parasiticidal avermectins resistant to dissipation in the environment? The case of eprinomectin. Environ. Int. 2013, 60, 48–55. [Google Scholar] [CrossRef]
- Camilotti Dionisio, A.; Rath, A. Abamectin in soils: Analytical methods, kinetics, sorption and dissipation. Chemosphere 2016, 151, 17–29. [Google Scholar] [CrossRef]
- Samac, D.; Kinkel, L.L. Suppression of the root-lesion nematode (Pratylenchus penetrans) in alfalfa (Medicago sativa) by Streptomyces spp. Plant Soil 2001, 235, 35–44. [Google Scholar] [CrossRef]
- Faske, T.R.; Starr, J.L. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to Abamectin. J. Nematol. 2006, 38, 240–244. [Google Scholar] [PubMed]
- Jayakumar, J. Bio-efficacy of Streptomyces avermitilis culture filtrates against root-knot nematode, Meloidogyne incognita and reniform nematodes, Rotylenchulus reniformis. J. Agric. Sci. 2009, 22, 567–571. [Google Scholar]
- Toderas, I.; Rusu, S.; Iurcu-Straistaru, E.; Erhan, D.; Poiras, N.; Bivol, A.; Sasanelli, N.; Rusu, V. Control of the root-knot nematode Meloidogyne incognita by Ivomec containing an exametabolite of Streptomyces avermitilis. In Proceedings of the 9th International Conference of Zoologists, Chisinau, Republic of Moldova, 12–13 October 2016; pp. 176–177. [Google Scholar]
- Cabrera, J.A.; Kiewnick, S.; Grimm, C.; Dababat, A.A.; Sikora, R.A. Efficacy of abamectin seed treatment on Pratylenchus zeae, Meloidogyne incognita and Heterodera schachtii. J. Plant Dis. Prot. 2009, 116, 124–128. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, H.; Ji, X.; Wang, K.; Wang, D.; Qiao, K. Effect of Abamectin on the Cereal Cyst Nematode (CCN, Heterodera avenae) and Wheat Yield. Plant Dis. 2017, 101, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Toderas, I.; Erhan, D.; Rusu, S.; Iurcu-Straistaru, E.; Bivol, A.; Sasanelli, N.; Toderas, L. In vitro effect of abamectin on the carrot cyst nematode Heterodera carotae. In Proceedings of the 9th International Conference of Zoologists, Chisinau, Republic of Moldova, 12–13 October 2016; pp. 174–175. [Google Scholar]
- Perry, R.N.; Moens, M. Survival of parasitic nematodes outside the host. In Molecular and Physiological Basis of Nematode Survival; Perry, R.N., Wharton, D.A., Eds.; CAB International: Wallingford, UK, 2011; pp. 1–27. [Google Scholar]
- Greco, N.; D’Addabbo, T.; Sasanelli, N.; Seinhorst, J.W.; Stea, V.; Brandonisio, A. Effects of temperature and length of exposure on the mortality of the carrot cyst nematode, Heterodera carotae. Int. J. Pest Manag. 1998, 44, 99–107. [Google Scholar] [CrossRef]
- Storey, R.J.M. The relationship between neutral lipid reserves and infectivity for hatched and dormant juveniles of Globodera spp. Ann. App. Biol. 1984, 104, 511–520. [Google Scholar] [CrossRef]
- Smus, J.P.; Ludlow, E.; Dallìere, N.; Luedtke, S.; Monfort, T.; Liley, C.; Urwin, P.; Walker, R.; O’Connor, V.; Jholden-Dye, L.; et al. Coherent anti-Stokes Raman scattering (CARS) spectroscopy in Caenorhabditis elegans and Globodera pallida: Evidence for an ivermectin-activated decrease in lipid stores. Pest Manag. Sci. 2017, 73, 2550–2558. [Google Scholar] [CrossRef]
- Krogh, K.A.; Søeborg, T.; Brodin, B.; Halling-Sørensen, B. Sorption and Mobility of Ivermectin in Different Soils. J. Environ. Qual. 2008, 37, 2202–2211. [Google Scholar] [CrossRef]
- Sasanelli, N.; Toderas, I.; Ircu-Straistaru, E.; Rusu, S.; Migunova, V.; Konrat, A. Yield losses caused by plant parasitic nematodes graphical estimation. In Proceedings of the International Symposium Functional Ecology of Animals, Chisinau, Republic of Moldova, 21 September 2018; pp. 319–329. [Google Scholar]
- Fenwick, D.W. Methods for the recovery and counting of cysts of Heterodera schachtii from soil. J. Helminthol. 1940, 18, 155–172. [Google Scholar] [CrossRef]
- EPPO. PM 7/40 (4) Globodera rostochiensis and Globodera pallida. EPPO Bull. 2017, 47, 174–197. [Google Scholar]
- Kort, J.; Ross, H.; Rumpenhorst, H.J.; Stoner, R. An International scheme for identifying and classifying pathotypes of potato cyst-nematodes Globodera rostochiensis and G. pallida. Nematologica 1977, 23, 333–339. [Google Scholar] [CrossRef]
- Clarke, A.J.; Perry, R.N. Hatching of cyst-nematodes. Nematologica 1977, 23, 350–368. [Google Scholar]
- Seinhorst, J.W.; Den Ouden, H. An improvement of Bijloo’s method for determining the egg content of Heterodera cysts. Nematologica 1966, 12, 170–171. [Google Scholar]
- Schneider-Orelli, O. Entomologisches Praktikum; HR Sauerlander: Aarau, Switzerland, 1947. [Google Scholar]
- Finney, D.J. Estimation of the median effective dose. In Probit Analysis; Cambridge University Press, Ed.; Bentley House: London, UK, 1971; pp. 20–49. [Google Scholar]
- Chen, S.Y.; Dickson, D.W. A technique for determining live second-stage juveniles of Heterodera glycines. J. Nematol. 2000, 32, 117–121. [Google Scholar] [PubMed]
- Lumaret, J.P.; Errouissi, F.; Floate, K.; Römbke, J.; Wardhaugh, K. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr. Pharm. Biotechnol. 2012, 13, 1004–1060. [Google Scholar] [CrossRef]
| Treatment | Concentration (g/mL) a.i. | Hatching Percentage | LSD | |||||
|---|---|---|---|---|---|---|---|---|
| Exposure Time (h) | ||||||||
| 24 | 48 | 96 | 192 | 384 | 0.05 | 0.01 | ||
| Untreated Control | 0 | 30.0 ± 1.4 a | 23.8 ± 0.5 | 20.8 ± 2.9 | 22.0 ± 0.6 | 16.4 ± 0.6 | 4.7 | 6.8 |
| Abamectin | 1.125 | 26.7 ± 1.3 | 21.5 + 0.6 | 18.0 ± 1.8 | 17.3 ± 1.6 | 14.8 ± 0.9 | 4.1 | 5.9 |
| Abamectin | 2.25 | 22.1 ± 1.7 | 18.0 ± 1.5 | 14.8 ± 2.5 | 15.4 ± 1.3 | 8.1 ± 0.8 | 5.2 | 7.4 |
| Abamectin | 4.5 | 21.8 ± 1.5 | 15.9 ± 0.8 | 13.5 ± 1.6 | 9.9 ± 0.4 | 4.1 ± 0.1 | 3.3 | 4.7 |
| Abamectin | 9.0 | 15.3 ± 0.4 | 14.4 ± 0.3 | 12.7 ± 0.4 | 5.4 ± 1.0 | 2.6 ± 0.8 | 2.1 | 3.0 |
| Abamectin | 18.0 | 15.1 ± 1.4 | 14.5 ± 1.1 | 10.0 ± 0.7 | 5.2 ± 0.9 | 1.8 ± 0.8 | 3.2 | 4.5 |
| Abamectin | 36.0 | 9.3 ± 0.9 | 8.0 ± 0.9 | 6.4 ± 0.1 | 5.0 ± 0.5 | 1.1 ± 0.8 | 2.3 | 3.3 |
| LSD | 0.05 | 3.9 | 2.7 | 5.2 | 3.1 | 2.2 | --- | --- |
| 0.01 | 5.5 | 3.7 | 7.3 | 4.2 | 3.1 | --- | --- | |
| ANOVA F values Factor A: Abamectin concentrations 137.2 ** | ||||||||
| Factor B: Exposure times 127.6 ** | ||||||||
| A × B 2.9 ** | ||||||||
| Abamectin Doses (g/mL) a.i. | Exposure Time (h) | LSD | ||||||
|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 96 | 192 | 384 | 0.05 | 0.01 | ||
| 1.125 | 11.0 ± 4.2 a | 9.7 ± 2.4 | 13.5 ± 6.6 | 21.4 ± 7.4 | 9.8 ± 5.3 | 18.0 | 25.7 | |
| 2.25 | 26.3 ± 5.6 | 24.4 ± 6.1 | 28.8 ± 12.1 | 30.0 ± 6.1 | 50.6 ± 4.7 | 23.4 | 33.3 | |
| 4.5 | 27.3 ± 5.0 | 33.2 ± 3.4 | 35.1 ± 7.6 | 55.0 ± 1.9 | 75.0 ± 0.4 | 13.9 | 19.8 | |
| 9.0 | 49.0 ± 1.4 | 39.5 ± 1.2 | 38.9 ± 1.9 | 75.5 ± 4.7 | 84.1 ± 5.0 | 10.3 | 14.7 | |
| 18.0 | 49.7 ± 4.6 | 39.1 ± 4.5 | 51.9 ± 3.4 | 76.4 ± 4.1 | 89.0 ± 4.9 | 13.7 | 19.4 | |
| 36.0 | 69.0 ± 3.0 | 66.4 ± 3.9 | 69.2 ± 0.4 | 77.3 ± 2.3 | 93.3 ± 5.2 | 10.6 | 15.1 | |
| LSD | 0.05 | 13.0 | 12.1 | 21.0 | 14.8 | 14.1 | --- | --- |
| 0.01 | 18.2 | 16.9 | 29.4 | 20.7 | 19.8 | --- | --- | |
| ANOVA F values Factor A: Abamectin concentrations 73.8 ** | ||||||||
| Factor B: Exposure times 30.1 ** | ||||||||
| A × B 2.1 ** | ||||||||
| Exposure Time (h) | Abamectin Concentrations (µg a.i./mL) Needed for Different % Mortalities | |||||
|---|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | 90 | 99.9 | |
| 24 | 13.2 (10.1–17.3) a | 23.1 (17.7–30.3) | 42.3 (32.3–55.4) | 86.4 (66.0–113.1) | 230.8 (176.2–302.3) | 2408.2 (1838–3154.2) |
| 48 | 18.1 (12.9–25.4) | 33.9 (24.1–47.6) | 66.7 (47.5–93.8) | 148.9 (105.9–209.3) | 449.0 (319.5–630.9) | 6250.0 (4447.3–8783.3) |
| 96 | 13.2 (9.8–17.8) | 24.6 (18.2–33.1) | 48.0 (35.6–64.6) | 106.1 (78.7–142.9) | 316.0 (234.5–425.7) | 4271.6 (3170.5–5755.1) |
| 192 | 4.5 (3.6–5.6) | 7.5 (6.0–9.3) | 12.8 (10.3–15.9) | 24.2 (19.5–30.2) | 58.3 (46.8–72.6) | 474.6 (381.1–591.1) |
| 384 | 2.9 (2.4–3.4) | 4.0 (3.4–4.7) | 5.7 (4.8–6.8) | 8.7 (7.4–10.3) | 15.5 (13.1–18.3) | 61.3 (51.8–72.6) |
| Treatment | Dose (µg/mL or g/m2) c | Application Time | Cysts/100 g soil | Eggs and J2/g Soil | Eggs and J2/cyst | Reproduction Rate Pf/Pi | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated control | 0 | --- | 98 ± 9.1 a | A b | 126 ± 10.8 | A | 130 ± 5.4 | A | 4.6 ± 0.4 | A |
| Abamectin | 4.5 | 2 wks after sowing | 107 ± 4.8 | A | 126 ± 8.0 | A | 119 ± 9.8 | A | 4.6 ± 0.3 | A |
| Abamectin | 9.0 | 2 wks after sowing | 101 ± 8.6 | A | 108 ± 7.4 | A | 111 ± 8.6 | AB | 3.9 ± 0.3 | A |
| Abamectin | 18.0 | 2 wks after sowing | 20 ± 2.6 | BC | 13 ± 2.1 | C | 68 ± 5.3 | C | 0.5 ± 0.1 | C |
| Abamectin | 36.0 | 2 wks after sowing | 18 ± 1.7 | C | 14 ± 1.6 | BC | 83 ± 9.7 | BC | 0.5 ± 0.1 | C |
| Fosthiazate | 0.3 | At sowing | 40 ± 3.6 | B | 40 ± 5.7 | B | 100 ± 8.9 | AB | 1.5 ± 0.2 | B |
| Treatment | Dose (µg/mL or g/m2) a.i. | Hatching (%) | |
|---|---|---|---|
| Untreated control | 0 | 51.1 ± 5.2 a | A b |
| Abamectin | 4.5 | 49.7 ± 2.3 | A |
| Abamectin | 9 | 34.2 ± 2.5 | B |
| Abamectin | 18 | 22.2 ± 1.2 | C |
| Abamectin | 36 | 4.6 ± 1.3 | D |
| Fosthiazate | 0.3 | 28.0 ± 3.0 | BC |
| Percentage Mortality | Abamectin Lethal Doses (µg/mL a.i.) |
|---|---|
| 50 | 14.4 (12.9–16.0) a |
| 60 | 17.2 (15.4–19.1) |
| 70 | 20.9 (18.8–23.2) |
| 80 | 26.3 (23.6–29.3) |
| 90 | 36.0 (32.3–40.1) |
| 99.9 | 131.3 (118.0–146.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasanelli, N.; Toderas, I.; Veronico, P.; Iurcu-Straistaru, E.; Rusu, S.; Melillo, M.T.; Caboni, P. Abamectin Efficacy on the Potato Cyst Nematode Globodera pallida. Plants 2020, 9, 12. https://doi.org/10.3390/plants9010012
Sasanelli N, Toderas I, Veronico P, Iurcu-Straistaru E, Rusu S, Melillo MT, Caboni P. Abamectin Efficacy on the Potato Cyst Nematode Globodera pallida. Plants. 2020; 9(1):12. https://doi.org/10.3390/plants9010012
Chicago/Turabian StyleSasanelli, Nicola, Ion Toderas, Pasqua Veronico, Elena Iurcu-Straistaru, Stefan Rusu, Maria Teresa Melillo, and Pierluigi Caboni. 2020. "Abamectin Efficacy on the Potato Cyst Nematode Globodera pallida" Plants 9, no. 1: 12. https://doi.org/10.3390/plants9010012
APA StyleSasanelli, N., Toderas, I., Veronico, P., Iurcu-Straistaru, E., Rusu, S., Melillo, M. T., & Caboni, P. (2020). Abamectin Efficacy on the Potato Cyst Nematode Globodera pallida. Plants, 9(1), 12. https://doi.org/10.3390/plants9010012






