Transcriptome Changes Induced by Different Potassium Levels in Banana Roots
Abstract
1. Introduction
2. Results
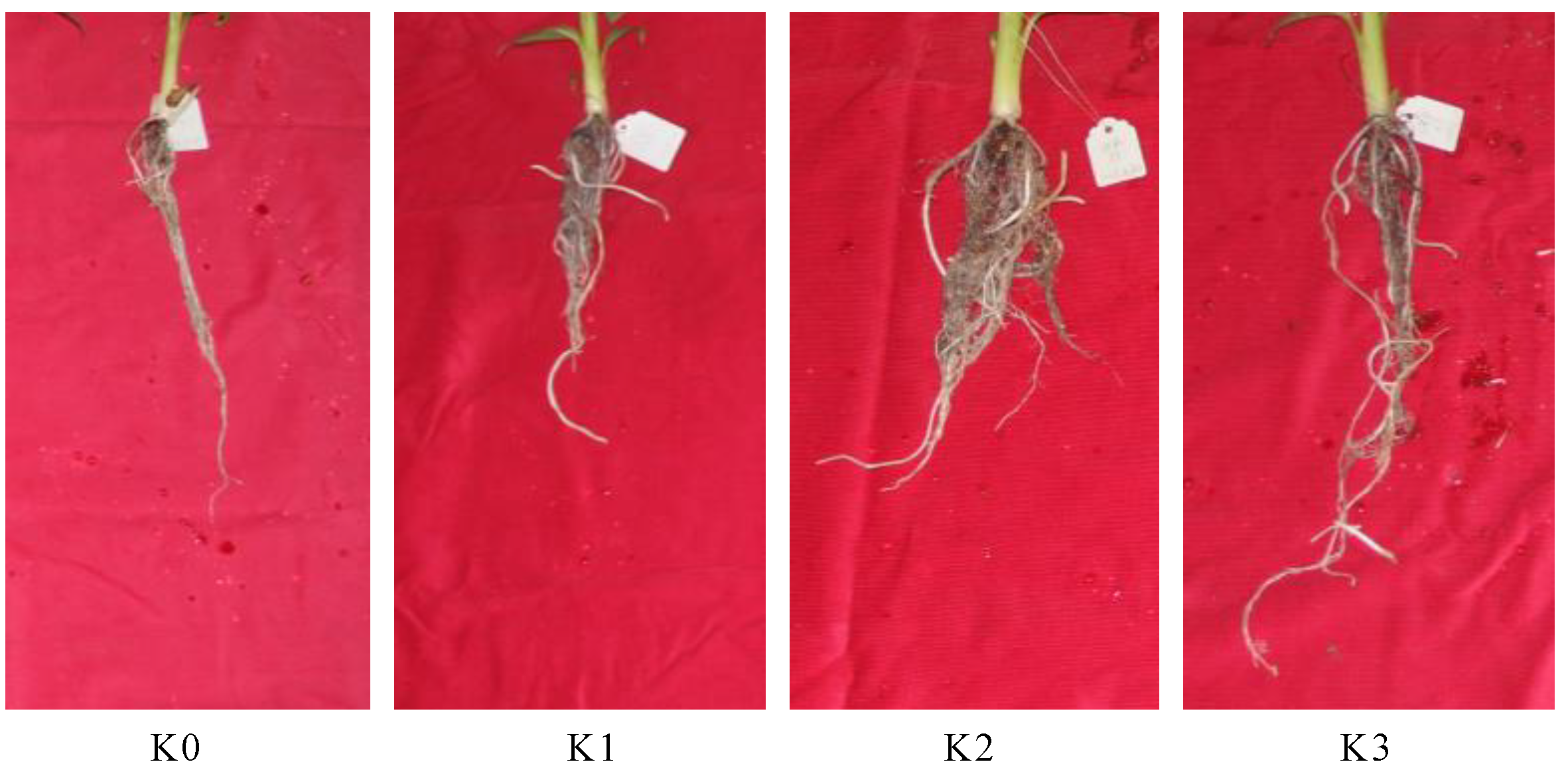
2.1. Morphology Changes of Banana Roots after Potassium Treatments
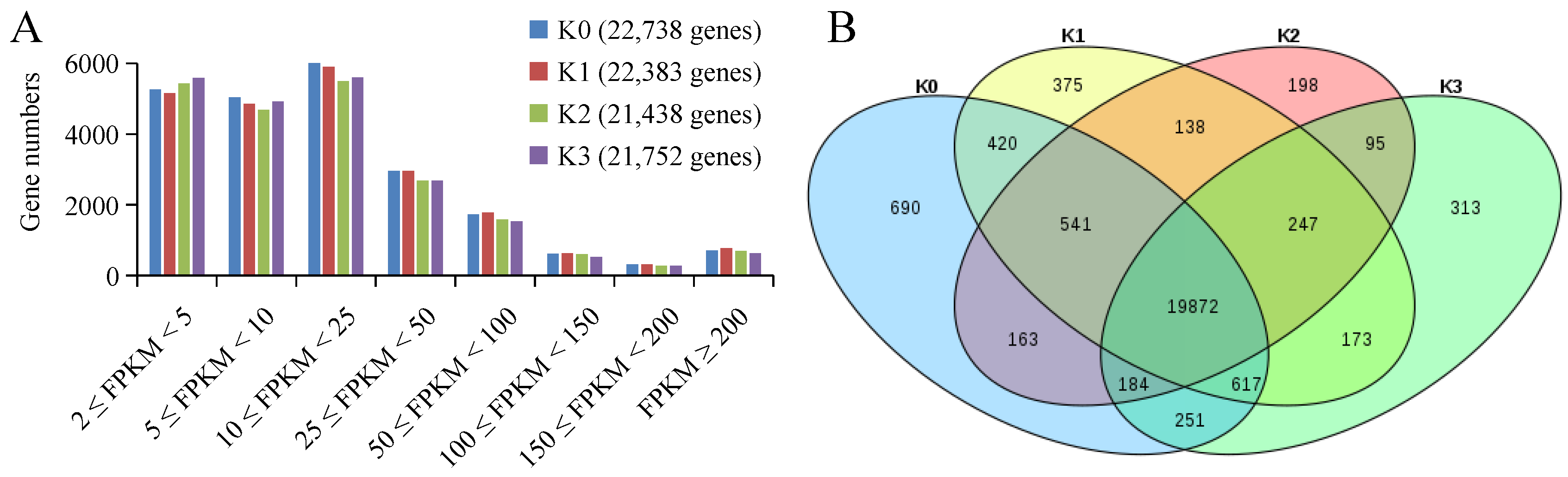
2.2. Overview of Transcriptome Sequencing Results
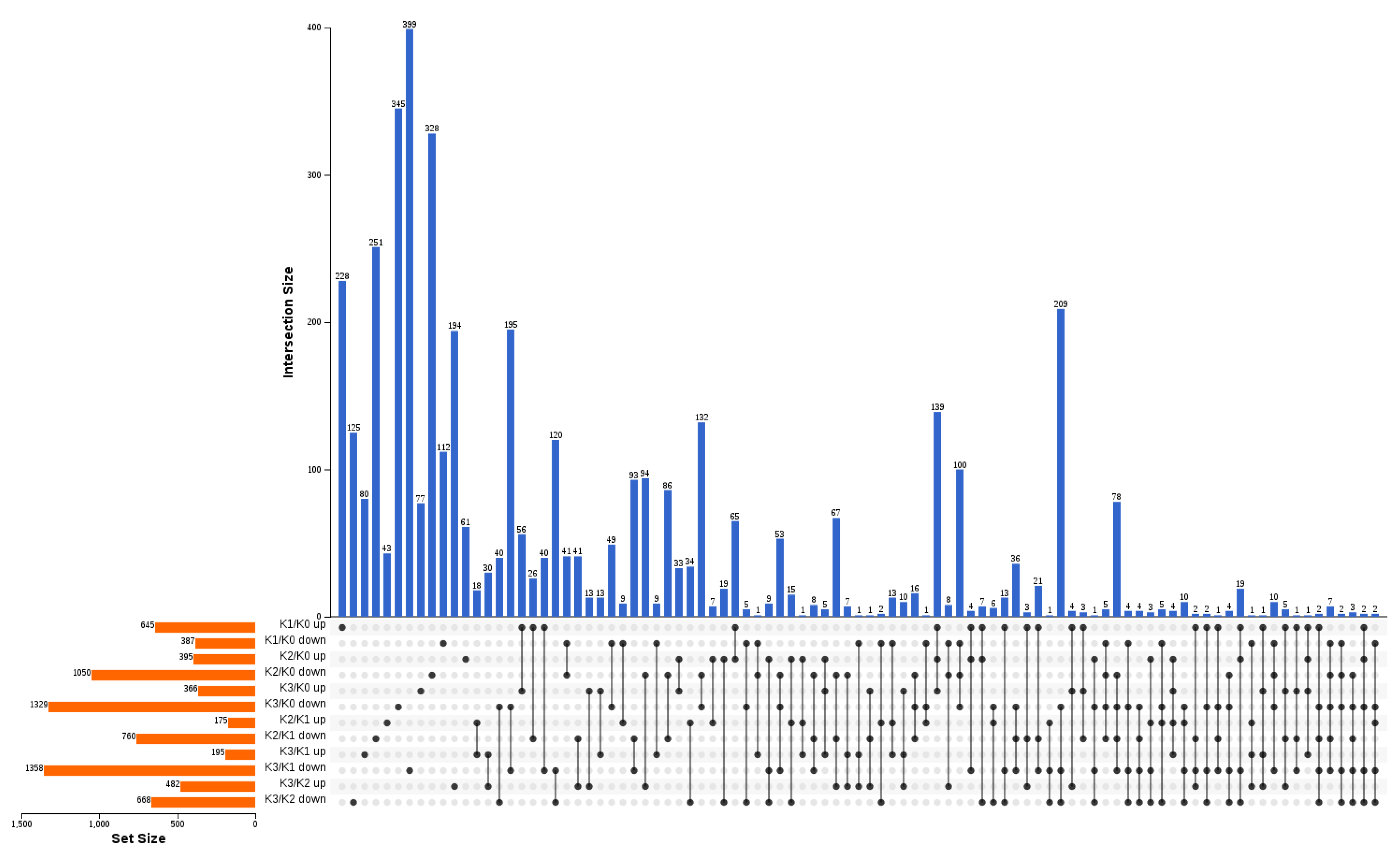
2.3. Different Concentration of Potassium Stresses Affect Genes Expression in Banana Roots
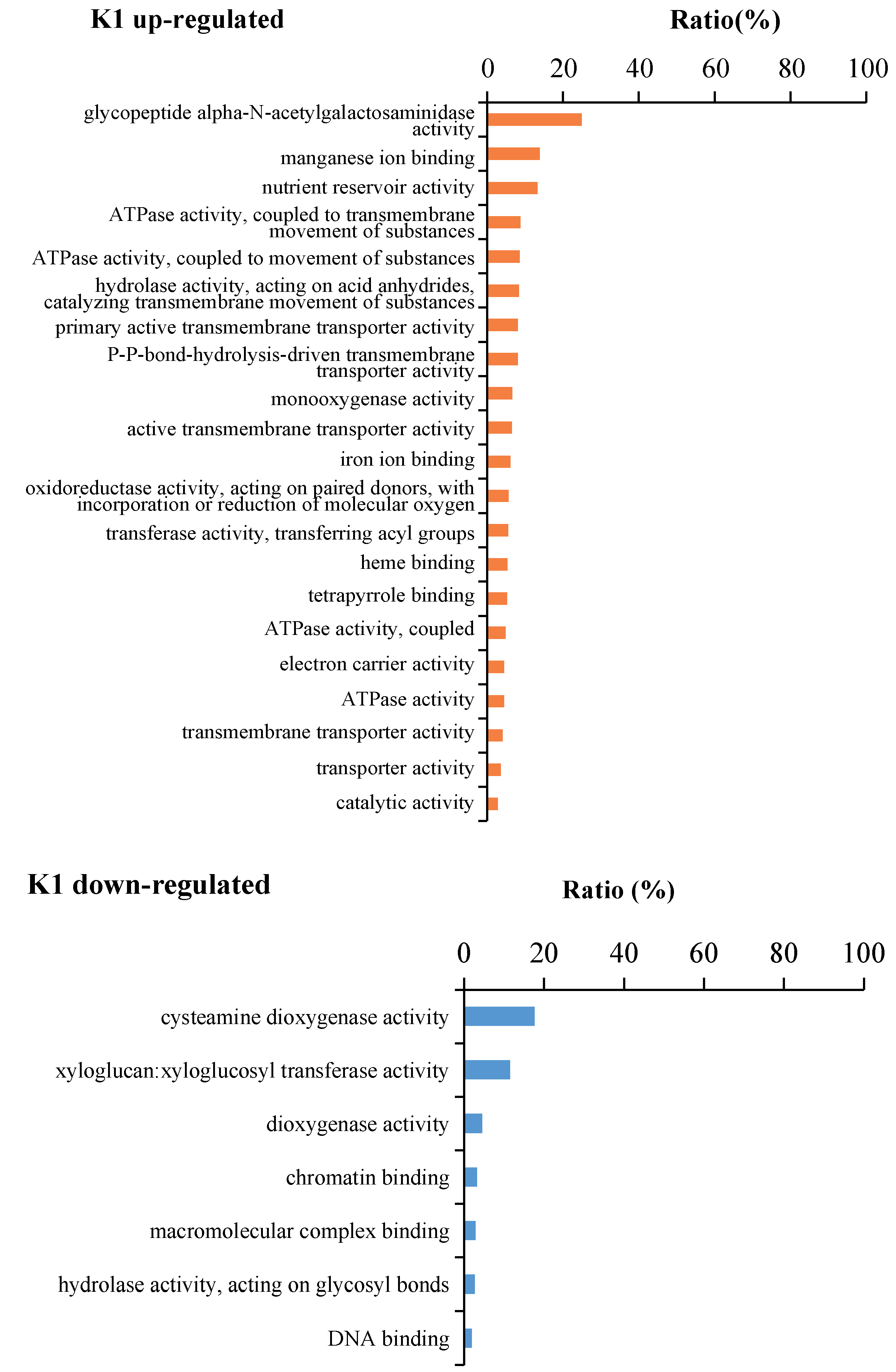
2.4. Gene function Enrichment Analysis of DEGs in Responses to K+ Treatments
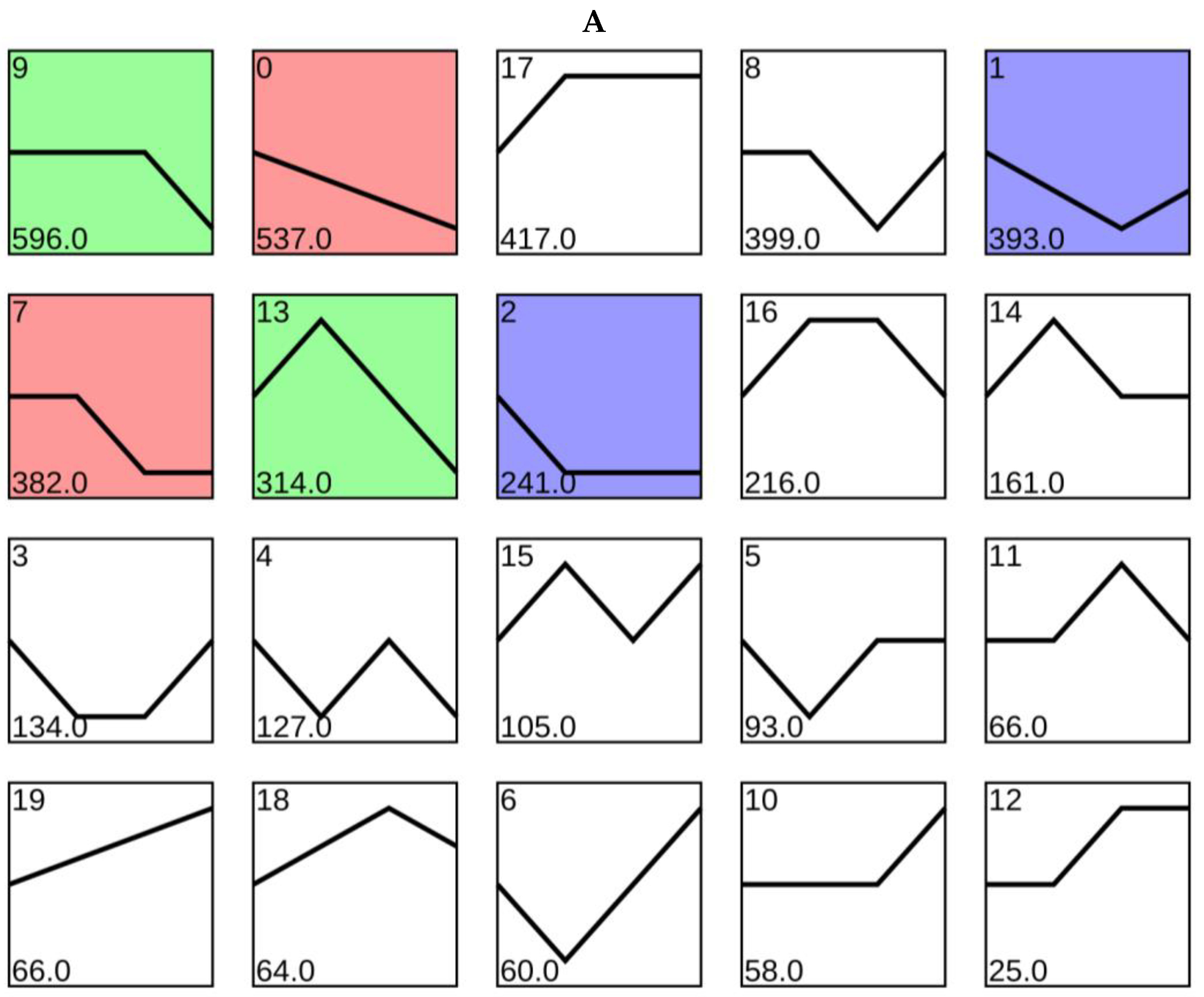
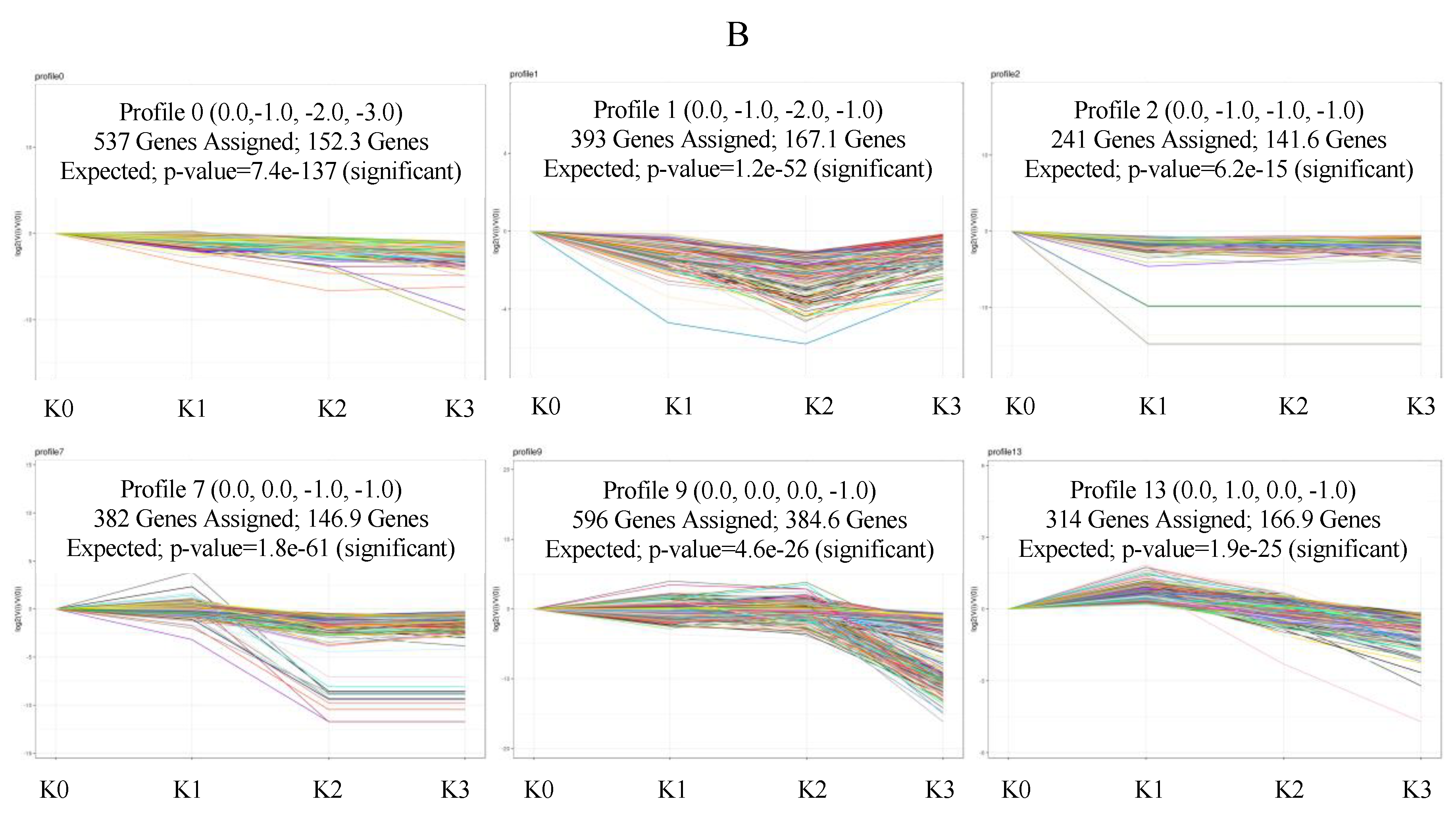
2.5. Expression Trend Analysis
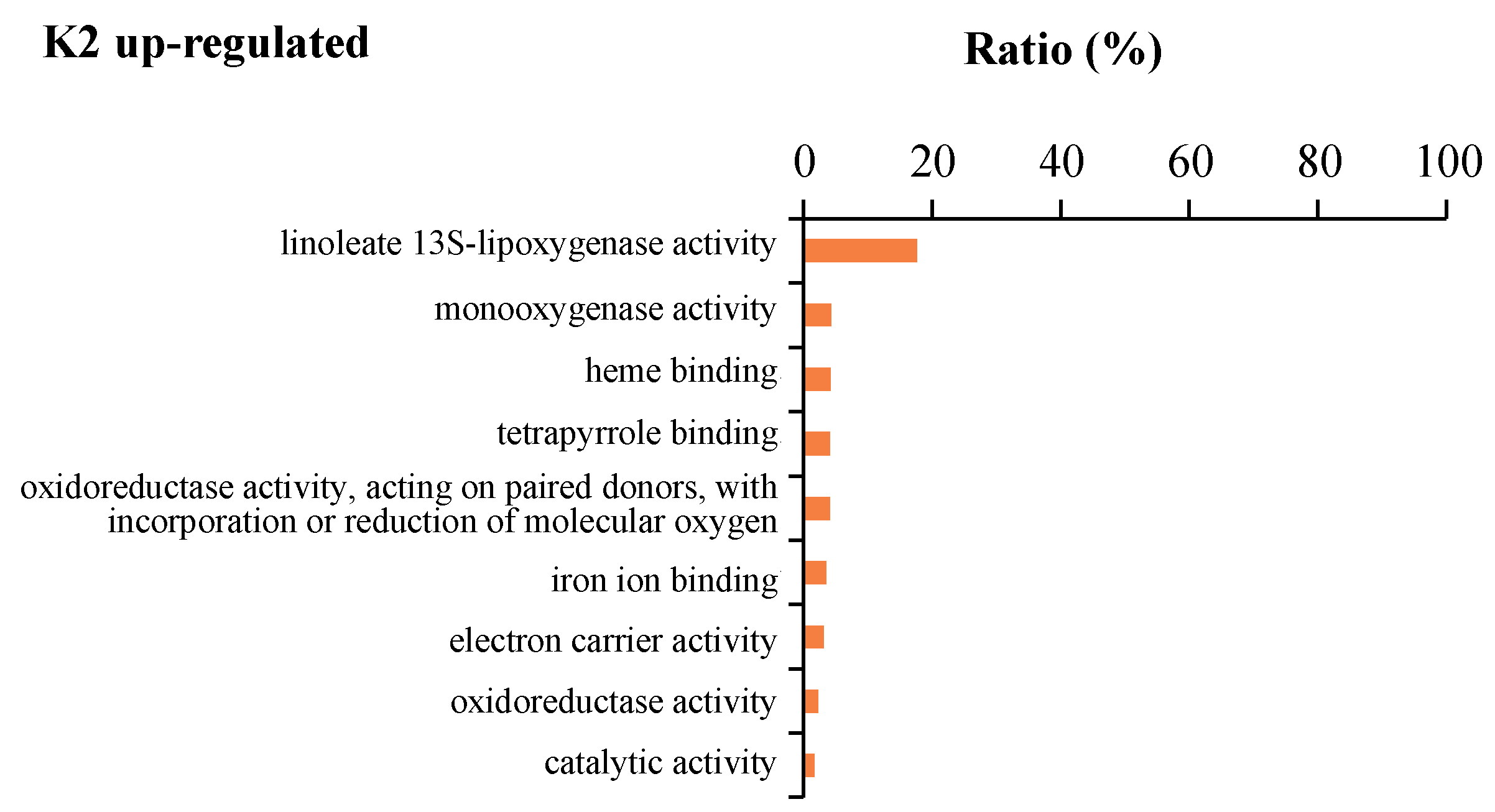
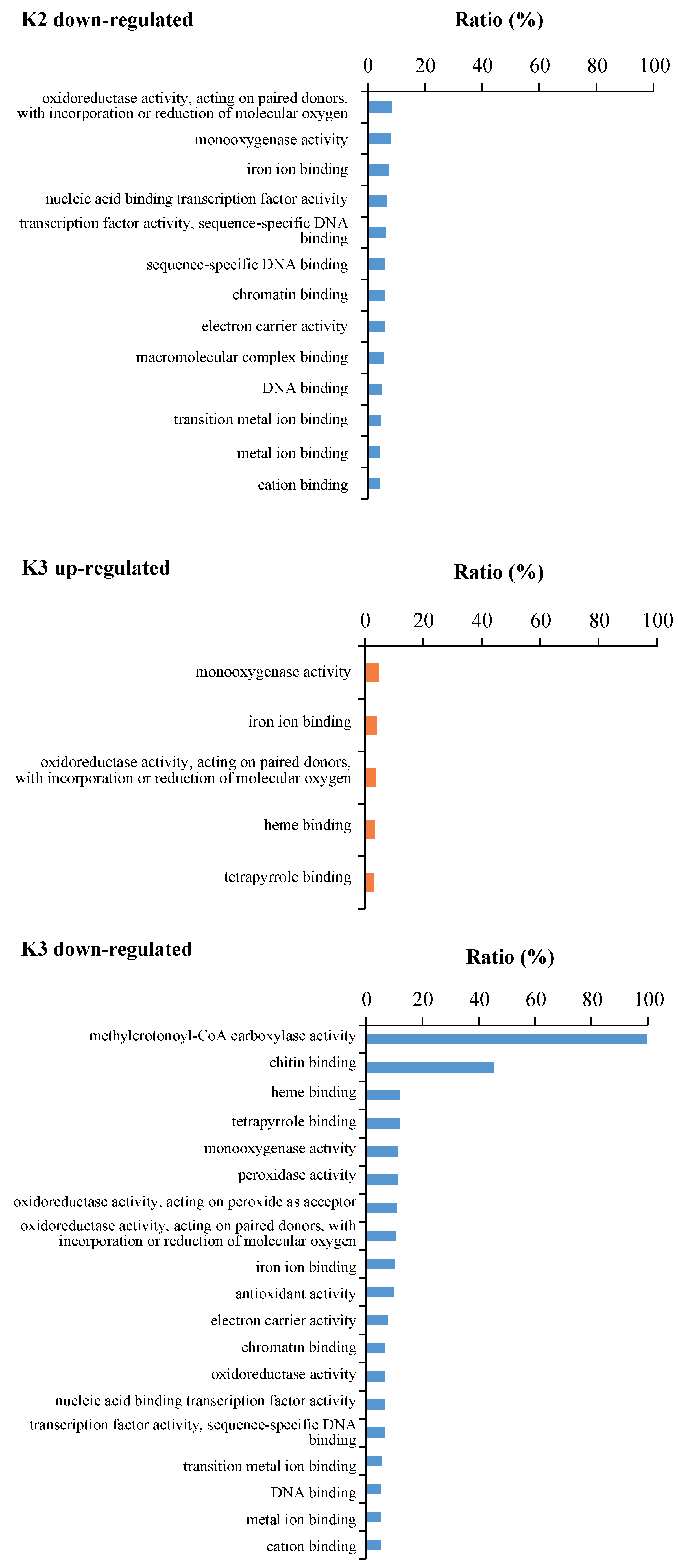
2.6. GO Enrichment Analysis of the Six Significant Gene Expression Patterns
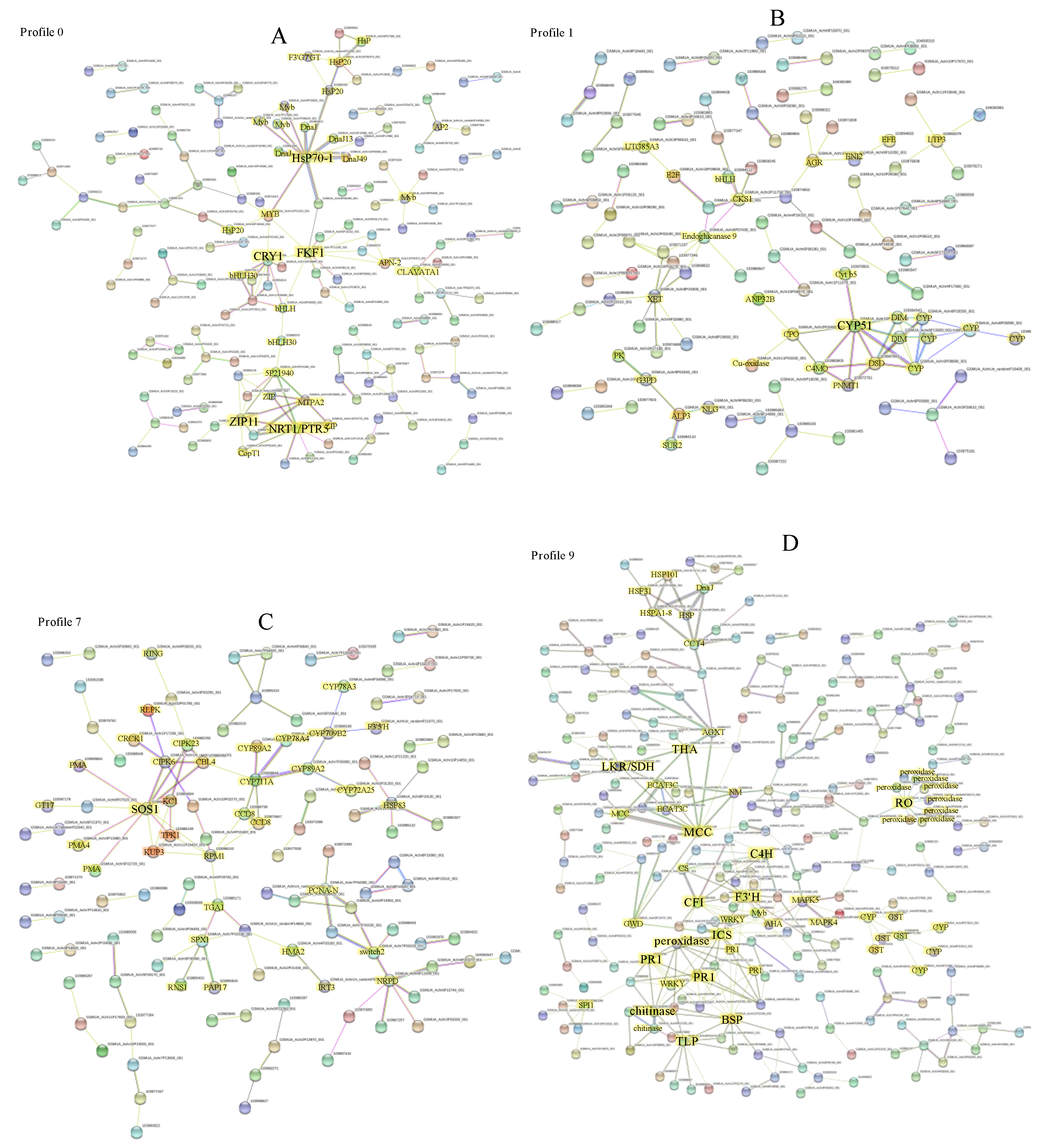
2.7. Interaction Network Construction of the DEGs in Significant Expression Patterns
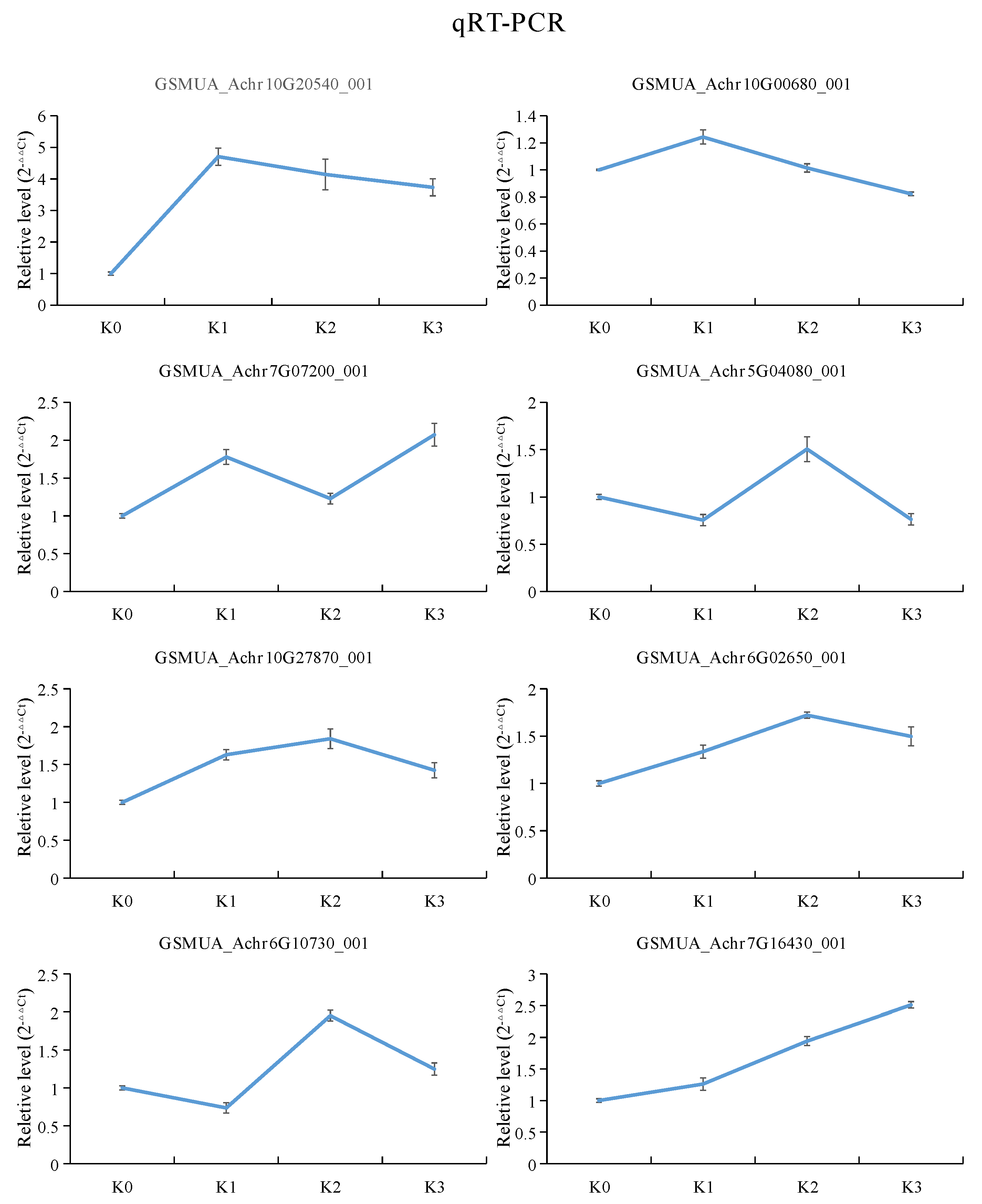
2.8. Validation of RNA Sequencing Data
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Different Potassium Treatments
4.2. Extraction of Total RNA from Banana Root under Different Potassium Stresses
4.3. RNA-Seq Library Preparation, Sequencing and Data Analysis
4.4. Data Statistics, GO Enrichment, Expression Trend Analysis and Interactive Network Construction of DEGs
4.5. RNA-Seq Reliability Verification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Annu. Rev. Plant Physiol. 2003, 31, 239–298. [Google Scholar] [CrossRef]
- Leigh, R.A.; Jones, R.G.W. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Ma, T.L.; Wu, W.H.; Wang, Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 2012, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Agarwal, R.M.; Tomar, N.S.; Shrivastava, M. Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L. cultivar Kent). J. Plant Interact. 2015, 10, 211–223. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tittal, M.; Mir, R.A.; Agarwal, R.M. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 2017, 254, 1953–1963. [Google Scholar] [CrossRef]
- Shabala, S. Salinity and programmed cell death: Unravelling mechanisms for ion specific signalling. J. Exp. Bot. 2009, 60, 709–712. [Google Scholar] [CrossRef]
- Shabala, S. Signalling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. Agron. Sustain. Dev. 2008, 2, 33–46. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Holzmueller, E.J.; Jose, S.; Jenkins, M.A. Influence of calcium, potassium, and magnesium on Cornus florida L. density and resistance to dogwood anthracnose. Plant Soil 2007, 290, 189–199. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Zhao, X.; Wu, Q. Potassium-induced plant resistance against soybean cyst nematode via root exudation of phenolic acids and plant pathogen-related genes. PLOS ONE 2018, 13, e0200903. [Google Scholar] [CrossRef] [PubMed]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. Int. J. Exp. Plant Biol. 2011, 180, 368. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, M. The Response of Arabidopsis to Low Potassium Availability. Ph.D. Thesis, Durham University, Durham, UK, 2018. [Google Scholar]
- Armengaud, P. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.D.; Giehl, R.F.H.; Swetlana, F.; Nicolaus, W. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-H.; Yu, H.-Q.; Wen, J.; Wang, X.-G.; Du, Q.; Wang, J.; Wang, Q. Response of root morphology, physiology and endogenous hormones in maize (Zea mays L.) to potassium deficiency. J. Integr. Agric. 2016, 15, 785–794. [Google Scholar] [CrossRef]
- Dun, X.; Shi, J.; Liu, H.; Wang, J.; Wang, X.; Wang, H. Genetic dissection of root morphological traits as related to potassium use efficiency in rapeseed under two contrasting potassium levels by hydroponics. Sci. China Life Sci. 2019, 62, 746–757. [Google Scholar] [CrossRef]
- Song, W.; Liu, S.; Meng, L.; Xue, R.; Wang, C.; Liu, G.; Dong, C.; Wang, S.; Dong, J.; Zhang, Y. Potassium deficiency inhibits lateral root development in tobacco seedlings by changing auxin distribution. Plant Soil 2015, 396, 163–173. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, P.; Zhang, Q.A.; Yan, C.; Yu, F.; Yi, J.; Fang, L. Effect of different levels of nitrogen, phosphorus, and potassium on root activity and chlorophyll content in leaves of Brassica oleracea seedlings grown in vegetable nursery substrate. Hortic. Environ. Biotechnol. 2017, 58, 5–11. [Google Scholar] [CrossRef]
- Shahzad, Z.; Amtmann, A. Food for thought: How nutrients regulate root system architecture. Curr. Opin. Plant Biol. 2017, 39, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, H.; Hao, Q.; Sha, A.; Shan, Z.; Chen, L.; Zhou, R.; Zhi, H.; Zhou, X. Transcript profile of the response of two soybean genotypes to potassium deficiency. PLOS ONE 2012, 7, e39856. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; He, X.; Wu, D.; Zhu, B.; Cai, S.; Nadira, U.A.; Jabeen, Z.; Zhang, G. Comparative transcriptome profiling of two Tibetan wild barley genotypes in responses to low potassium. PLOS ONE 2014, 9, e100567. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Ling, Q.; Fan, L.; Li, Y.; Hu, F.; Chen, J.; Huang, Z.; Deng, H.; Li, Q.; Qi, Y. Transcriptome profiling of sugarcane roots in response to low potassium stress. PLOS ONE 2015, 10, e0126306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, H.; Wang, H.; Cui, J.; Wang, J.; Hu, J.; Guo, L.; Qian, Q.; Xue, D. Transcriptome analysis of rice seedling roots in response to potassium deficiency. Sci. Rep. 2017, 7, 5523. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Liu, G.; Xiao, X.; Wang, P.; Gao, T.; Xu, M.; Han, Q.; Wang, Y.; Guo, T.; et al. Large-scale proteomics combined with transgenic experiments demonstrates an important role of jasmonic acid in potassium deficiency response in wheat and rice. Mol. Cell. Proteom. 2017, 16, 1889–1905. [Google Scholar] [CrossRef]
- Sansoulet, J.; Cabidoche, Y.M.; Cattan, P. Adsorption and transport of nitrate and potassium in an Andosol under banana (Guadeloupe, French West Indies). Eur. J. Soil Sci. 2007, 58, 478–489. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Shin, R. Nutrient sensing and signaling: NPKS. Annu. Rev. Plant Biol. 2007, 58, 47–69. [Google Scholar] [CrossRef]
- Fernández, F.G.; Brouder, S.M.; Volenec, J.J.; Beyrouty, C.A.; Hoyum, R. Soybean shoot and root response to localized water and potassium in a split-pot study. Plant Soil 2011, 344, 197–212. [Google Scholar] [CrossRef]
- Kellermeier, F.; Chardon, F.; Amtmann, A. Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiol. 2013, 161, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Xin, X.; Zhang, J.; Zhao, B.; Cheng, H.; Zhang, C.; Ma, D.; Chen, L. Potential root foraging strategy of wheat (Triticum aestivum L.) for potassium heterogeneity. Front. Plant Sci. 2018, 9, 1755. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; Shi, X.; Kang, Y.; Xie, C.; Peng, L.; Dong, C.; Shen, Q.; Xu, Y. Transcriptome analysis of differentially expressed genes induced by low and high potassium levels provides insight into fruit sugar metabolism of pear. Front. Plant Sci. 2017, 8, 938. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Zhang, Y.; Li, C.; Gong, S.; Yan, S.; Li, G.; Hu, G.; Ren, H.; Yang, J.; et al. Comparative transcriptome analysis of salt-sensitive and salt-tolerant maize reveals potential mechanisms to enhance salt resistance. Genes Genom. 2019, 41, 781–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, F.; Shen, Y.; Zhan, Q.; Chi, Y. Transcriptome analysis reveals candidate genes related to phosphorus starvation tolerance in sorghum. BMC Plant Biol. 2019, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Chen, Y.; Lu, Y.; Lu, L. De novo transcriptome analysis of tobacco seedlings and identification of the early response gene network under low-potassium stress. Genet. Mol. Res. 2016, 15, gmr.15038599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Liu, X.; Jiang, J. Comparative transcriptome profiling of two tomato genotypes in response to potassium-deficiency stress. Int. J. Mol. Sci. 2018, 19, 2402. [Google Scholar] [CrossRef]
- Calabrese, S.; Kohler, A.; Niehl, A.; Veneault-Fourrey, C.; Courty, P.E. Transcriptome analysis of the populus trichocarpa-rhizophagus irregularis mycorrhizal symbiosis: Regulation of plant and fungal transportomes under nitrogen starvation. Plant Cell Physiol. 2017, 58, 1003–1017. [Google Scholar] [CrossRef]
- Cai, H.; Lu, Y.; Xie, W.; Zhu, T.; Lian, X. Transcriptome response to nitrogen starvation in rice. J. Biosci. 2012, 37, 731–747. [Google Scholar] [CrossRef]
- Gelli, M.; Duo, Y.; Konda, A.; Zhang, C.; Holding, D.; Dweikat, I. Identification of differentially expressed genes between sorghum genotypes with contrasting nitrogen stress tolerance by genome-wide transcriptional profiling. BMC Genom. 2014, 15, 179. [Google Scholar] [CrossRef]
- Quan, X.; Zeng, J.; Ye, L.; Chen, G.; Han, Z.; Shah, J.M.; Zhang, G. Transcriptome profiling analysis for two tibetan wild barley genotypes in responses to low nitrogen. BMC Plant Biol. 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, M.; Undan, J.R.; Takagi, H.; Abe, A.; Yoshida, K.; Undan, J.Q.; Natsume, S.; Uemura, A.; Saitoh, H.; Matsumura, H.; et al. A cytochrome P450, OsDSS1, is involved in growth and drought stress responses in rice (Oryza sativa L.). Plant Mol. Biol. 2015, 88, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ding, L.; Zhu, J.K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 1996, 8, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K.; Xiong, L.L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell 1998, 10, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Cuin, T.A.; Shabala, N.S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 2005, 222, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.X.; Yuan, H.J.; Hu, J.; Wei, L.; Bao, A.K.; Zhang, J.L.; Wang, S.M. ZxSOS1 is essential for long-distance transport and spatial distribution of Na+, and K+, in the xerophyte Zygophyllum xanthoxylum. Plant Soil 2014, 374, 661–676. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Jeanguenin, L.; Alcon, C.; Duby, G.; Boeglin, M.; Cherel, I.; Gaillard, I.; Zimmermann, S.; Sentenac, H.; Véry, A.-A. AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. Cell Mol. Biol. 2011, 67, 570–582. [Google Scholar] [CrossRef]
- Wang, X.P.; Chen, L.M.; Liu, W.X.; Shen, L.K.; Wang, F.L.; Zhou, Y.; Zhang, Z.; Wu, W.H.; Wang, Y. AtKC1 and CIPK23 synergistically modulate AKT1-mediated low-potassium stress responses in Arabidopsis. Plant Physiol. 2016, 170, 2264–2277. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Pascaud, F.; Eckert, C.; Gajdanowicz, P.; Hashimoto, K.; Corratgé-Faillie, C.; Offenborn, J.N.; Lacombe, B.; Dreyer, I.; Thibaud, J.-B.; et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011, 21, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Halfter, U.; Ishitani, M.; Zhu, J.K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Nie, J.; Cao, C.; Jin, Y.; Yan, M.; Wang, F.; Liu, J.; Xiao, Y.; Liang, Y.; Zhang, W. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 2010, 188, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Latz, A.; Becker, D.; Hekman, M.; Müller, T.; Beyhl, D.; Marten, I.; Eing, C.; Fischer, A.; Dunkel, M.; Bertl, A.; et al. TPK1, a Ca2+-regulated Arabidopsis vacuole two-pore K+ channel is activated by 14-3-3 proteins. Plant J. 2007, 52, 449–459. [Google Scholar] [CrossRef]
- Isner, J.C.; Begum, A.; Nuehse, T.; Hetherington, A.M.; Maathuis, F.J.M. KIN7 kinase regulates the vacuolar TPK1 K+, channel during stomatal closure. Curr. Biol. 2018, 28, 466–472. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwak, J.M.; Schroeder, U.J.I. AtKUP1: An Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 1998, 10, 51–62. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, Z.; Zhang, R.; Xu, P.; Liu, H.; Yang, H.-Q.; Doblin, M.S.; Bacic, A.; Li, L. Blue light regulates secondary cell wall thickening via MYC2/MYC4 activation of the NST1-directed transcriptional network in Arabidopsis. Plant Cell 2018, 30, 2512–2528. [Google Scholar] [CrossRef]
- Yuan, N.; Balasubramanian, V.K.; Chopra, R.; Mendu, V. The photoperiodic flowering time regulator FKF1 negatively regulates cellulose biosynthesis. Plant Physiol. 2019, 180, 2240–2253. [Google Scholar] [CrossRef]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta 2006, 1763, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Zocchi, G.; Bashir, K.; Philippar, K.; Briat, J.F. Cellular iron homeostasis and metabolism in plant. Front. Plant Sci. 2013, 4, 490. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, S.; Sautron, E.; Finazzi, G.; Rivasseau, C.; Frelet-Barrand, A.; Pilon, M.; Rolland, N.; Seigneurin-Berny, D. HMA1 and PAA1, two chloroplast-envelope play distinct roles in chloroplast copper homeostasis. J. Exp. Bot. 2014, 65, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Sathiyamurthi, S.; Dhanasekaran, K. Effects of different levels and sources of zinc on dry matter production and nutrient uptake by cotton (Gossypium hirsutum L.) in salt affected soil. Asian J. Soil Sci. 2015, 9, 208–212. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Du, L.; Liu, X. Comparative transcriptome analysis of cadmium responses in Solanum nigrum and Solanum torvum. New Phytol. 2012, 196, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Garvin, D.F.; Kochian, L.V. Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots. Evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiol. 2002, 130, 1361–1370. [Google Scholar] [CrossRef]
- Drechsler, N.; Zheng, Y.; Bohner, A.; Nobmann, B.; von Wirén, N.; Kunze, R.; Rausch, C. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the Stelar K+ Outward Rectifier SKOR in Arabidopsis. Plant Physiol. 2015, 169, 2832–2847. [Google Scholar]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Banavath, J.N.; Chakradhar, T.; Pandit, V.; Konduru, S.; Guduru, K.K.; Akila, C.S.; Podha, S.; Puli, C.O.R. Stress inducible overexpression of AtHDG11 leads to improved drought and salt stress tolerance in peanut (Arachis hypogaea L.). Front. Chem. 2018, 6, 3–4. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhuang, L.; Gao, Y.; Huang, B. Abscisic acid mediation of drought priming-enhanced heat tolerance in tall fescue (Festuca arundinacea) and Arabidopsis. Physiol. Plant. 2019, 167, 488–501. [Google Scholar] [CrossRef]
- Qu, J.; Tao, X.-Y.; Teng, P.; Zhang, Y.; Guo, C.-L.; Hu, L.; Qian, Y.-N.; Jiang, C.-Y.; Liu, W.-T. Blocking ATP-sensitive potassium channel alleviates morphine tolerance by inhibiting HSP70-TLR4-NLRP3-mediated neuroinflammation. J. Neuroinflamm. 2017, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Ficker, E.; Dennis, A.T.; Wang, L.; Brown, A.M. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 2003, 92, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Waditee, R.; Bhuiyan, N.H.; Rai, V.; Aoki, K.; Tanaka, Y.; Hibino, T.; Suzuki, S.; Takano, J.; Jagendorf, A.T.; Takabe, T.; et al. Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tang, G.; Granier, F.; Bouchez, D.; Galili, G. A T-DNA insertion knockout of the bifunctional lysine-ketoglutarate reductase/saccharopine dehydrogenase gene elevates lysine levels in Arabidopsis seeds. Plant Physiol. 2001, 126, 1539–1545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moulin, M.; Deleu, C.; Larher, F. L-lysine catabolism is osmo-regulated at the level of lysine-ketoglutarate reductase and saccharopine dehydrogenase in rapeseed leaf discs. Plant Physiol. Biochem. 2000, 38, 577–585. [Google Scholar] [CrossRef]
- Stepansky, A.; Galili, G. Synthesis of the Arabidopsis bifunctional lysine-ketoglutarate reductase/saccharopine dehydrogenase enzyme of lysine catabolism is concertedly regulated by metabolic and stress-associated signals. Plant Physiol. 2003, 133, 1407. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tang, G.; Galili, G.; Stepansky, A. Regulation of lysine catabolism in Arabidopsis through concertedly regulated synthesis of the two distinct gene products of the composite AtLKR/SDH locus. J. Exp. Bot. 2005, 56, 525. [Google Scholar]
- Aubert, S.; Alban, C.; Bligny, R.; Douce, R. Induction of β-methylcrotonyl-coenzyme A carboxylase in higher plant cells during carbohydrate starvation: Evidence for a role of MCCase in leucine catabolism. FEBS Lett. 1996, 383, 175–180. [Google Scholar] [CrossRef]
- Anderson, M.D.; Che, P.; Song, J.; Nikolau, B.J.; Wurtele, E.S. 3-methylcrotonyl-coenzyme a carboxylase is a component of the mitochondrial leucine catabolic pathway in plants1. Plant Physiol. 1998, 118, 1127–1138. [Google Scholar] [CrossRef]
- Binder, S. Branched-chain amino acid metabolism in Arabidopsis thaliana. Arabidopsis Book 2010, 8, e0137. [Google Scholar] [CrossRef]
- Ding, G.; Che, P.; Ilarslan, H.; Wurtele, E.S.; Nikolau, B.J. Genetic dissection of methylcrotonyl CoA carboxylase indicates a complex role for mitochondrial leucine catabolism during seed development and germination. Plant J. 2012, 70, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Hu, H. Methylcrotonyl-CoA carboxylase regulates triacylglycerol accumulation in the model diatom Phaeodactylum tricornutum. Plant Cell 2014, 26, 1681–1697. [Google Scholar] [CrossRef]
- Wang, X.; Wurtele, E.S.; Nikolau, B.J. Regulation of β-methylcrotonyl-coenzyme A carboxylase activity by biotinylation of the apoenzyme. Plant Physiol. 1995, 108, 1133–1139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bell-Lelong, D.A.; Cusumano, J.C.; Meyer, K.; Chapple, C. Cinnamate-4-hydroxylase expression in Arabidopsis (Regulation in response to development and the environment). Plant Physiol. 1997, 113, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Cominelli, E.; Bailey, P.; Parr, A.; Mehrtens, F.; Jones, J.; Tonelli, C.; Weisshaar, B.; Martin, C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000, 19, 6150–6161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L.; Wang, H.; Gao, R.; Zhang, J.; Hu, B.; Chang, Y. Cloning and expression analysis of phenylalanine ammonia-lyase (PAL) gene family and cinnamate 4-hydroxylase (C4H) from Dryopteris fragrans. Biologia 2015, 70, 606–614. [Google Scholar] [CrossRef]
- Huang, B.; Duan, Y.; Yi, B.; Sun, L.; Lu, B.; Yu, X.; Sun, H.; Zhang, H.; Chen, W. Characterization and expression profiling of cinnamate 4-hydroxylase gene from Salvia miltiorrhiza in rosmarinic acid biosynthesis pathway. Russ. J. Plant Physiol. 2008, 55, 390–399. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, P.Y.; Lee, Y.K.; Lim, Y.P.; Lee, M.C. cDNA cloning and sequence analysis of the rice cinnamate-4-hydroxylase gene, a cytochrome P450-dependent monooxygenase involved in the general phenylpropanoid pathway. J. Plant Biol. 2005, 48, 311–318. [Google Scholar]
- Betz, C.; Mccollum, T.G.; Mayer, R.T. Differential expression of two cinnamate 4-hydroxylase genes in ’Valencia’ orange (Citrus sinensis Osbeck). Plant Mol. Biol. 2001, 46, 741–748. [Google Scholar] [CrossRef]
- Sadeghi, M.; Dehghan, S.; Fischer, R.; Wenzel, U.; Vilcinskas, A.; Kavousi, H.R.; Rahnamaeian, M. Isolation and characterization of isochorismate synthase and cinnamate 4-hydroxylase during salinity stress, wounding, and salicylic acid treatment in Carthamus tinctorius. Plant Signal. Behav. 2013, 8, 2420–2423. [Google Scholar] [CrossRef]
- Jonggeun, K.; Bosung, C.; Natarajan, S.; Hanhong, B. Expression analysis of kenaf cinnamate 4-hydroxylase (C4H) ortholog during developmental and stress responses. Plant Omics 2013, 6, 65–72. [Google Scholar]
- Phimchan, P.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Enzymatic changes in phenylalanine ammonia-lyase, cinnamic-4-hydroxylase, capsaicin synthase, and peroxidase activities in Capsicum under drought stress. J. Agric. Food Chem. 2014, 62, 7057. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Aoki, T.; Sato, S.; Nakamura, Y.; Tabata, S.; Ayabe, S.-I. A cluster of genes encodes the two types of chalcone isomerase involved in the biosynthesis of general flavonoids and legume-specific 5-deoxy(iso)flavonoids in Lotus japonicus. Plant Physiol. 2003, 131, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Mehdy, M.C.; Lamb, C.J. Chalcone isomerase cDNA cloning and mRNA induction by fungal elicitor, wounding and infection. EMBO J. 1987, 6, 1527–1533. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Zhang, X.; Zhu, L.; Wang, X.; Guo, N.; Xing, H. Overexpression of chalcone isomerase (CHI) increases resistance against Phytophthora sojae in soybean. J. Plant Biol. 2018, 61, 309–319. [Google Scholar] [CrossRef]
- Walia, H.; Wilson, C.; Zeng, L.; Ismail, A.M.; Condamine, P.; Close, T.J. Genome-wide transcriptional analysis of salinity stressed japonica and indica rice genotypes during panicle initiation stage. Plant Mol. Biol. 2007, 63, 609–623. [Google Scholar] [CrossRef]
- Zamora, P.; Pardo, A.; Fierro, A.; Prieto, H.; Zuniga, G.E. Molecular characterization of the chalcone isomerase gene family in Deschampsia antarctica. Polar Biol. 2013, 36, 1269–1280. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Khlestkina, E.K.; Bergès, H.; Salina, E.A. The homoeologous genes encoding chalcone–flavanone isomerase in Triticum aestivum L.: Structural characterization and expression in different parts of wheat plant. Gene 2014, 538, 334–341. [Google Scholar] [CrossRef]
- Seitz, C.; Ameres, S.; Forkmann, G. Identification of the molecular basis for the functional difference between flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase. FEBS Lett. 2007, 581, 3429–3434. [Google Scholar] [CrossRef]
- Siegel, S.M. Studies on the biosynthesis of lignins. Physiol. Plantarum 1954, 7, 41–50. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Assembly and enlargement of the primary cell wall in plants. Annu. Rev. Cell Dev. Biol. 1997, 13, 171–201. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant Soil 2001, 230, 135–143. [Google Scholar] [CrossRef]
- Balzergue, C.; Dartevelle, T.; Godon, C.; Laugier, E.; Meisrimler, C.; Teulon, J.-M.; Creff, A.; Bissler, M.; Brouchoud, C.; Hagège, A.; et al. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat. Commun. 2017, 8, 15300. [Google Scholar] [CrossRef]
- Loon, L.C.V.; Strien, E.A.V. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arabidopsis Book 2011, 9, e0156. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Garcion, C.; Lohmann, A.; Lamodiere, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.P. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef]
- Kovacs, I.; Durner, J.; Lindermayr, C. Crosstalk between nitric oxide and glutathione is required for nonexpressor of pathogenesis-related genes 1 (NPR1)-dependent defense signaling in Arabidopsis thaliana. New Phytol. 2015, 208, 860–872. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Li, K.; Kang, Z.; Cantu, D.; Dubcovsky, J. A conserved Puccinia striiformis protein interacts with wheat NPR1 and reduces induction of pathogenesis-related genes in response to pathogens. Mol. Plant Microbe Int. 2016, 29, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, S.; Mou, Z. Nuclear localization of NPR1 is required for regulation of salicylate tolerance, isochorismate synthase 1 expression and salicylate accumulation in Arabidopsis. J. Plant Physiol. 2010, 167, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite roles of salicylic acid receptors NPR1 and NPR3/ NPR4 in transcriptional regulation of plant immunity. Cell 2018, 173, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Balić, I.; Vizoso, P.; Nilo-Poyanco, R.; Sanhueza, D.; Olmedo, P.; Sepúlveda, P.; Arriagada, C.; Defilippi, B.G.; Meneses, C.; Campos-Vargas, R. Transcriptome analysis during ripening of table grape berry cv. Thompson Seedless. PLOS ONE 2018, 13, e0190087. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, H.; Zheng, J.; Ye, Y.; Pan, L. Identification of strong promoters based on the transcriptome of Bacillus licheniformis. Biotechnol. Lett. 2017, 39, 873–881. [Google Scholar] [CrossRef] [PubMed]
| Raw Data | Clean Data | Q20 (%) | GC (%) | Unique Mapped | Multiple Mapped | Mapping Ratio (%) | |
|---|---|---|---|---|---|---|---|
| K0 | 28,043,505 | 27,574,822 | 94.01 | 53.96 | 21,674,956 | 92,502 | 79.23 |
| K1 | 30,165,251 | 29,675,721 | 94.13 | 53.58 | 22,955,324 | 74,451 | 77.27 |
| K2 | 35,061,437 | 34,465,589 | 94.02 | 53.95 | 27,718,870 | 94,409 | 79.81 |
| K3 | 24,438,772 | 24,024,081 | 94.15 | 54.26 | 20,030,295 | 50,940 | 83.37 |
| GO Terms | Description | Out | All | p.Adjust | Ratio (%) | |
|---|---|---|---|---|---|---|
| Profile 0 | GO:0003682 | chromatin binding | 23 | 585 | 0.007589488 | 3.93 |
| GO:0003700 | transcription factor activity, sequence-specific DNA binding | 35 | 1046 | 0.006265828 | 3.35 | |
| GO:0001071 | nucleic acid binding transcription factor activity | 35 | 1057 | 0.006265828 | 3.31 | |
| GO:0003677 | DNA binding | 77 | 2659 | 0.00010964 | 2.90 | |
| Profile 1 | GO:0016705 | oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen | 17 | 460 | 0.007235669 | 3.70 |
| Profile 7 | GO:0008131 | primary amine oxidase activity | 3 | 10 | 0.01050813 | 30.00 |
| GO:0016641 | oxidoreductase activity, acting on the CH-NH2 group of donors, oxygen as acceptor | 3 | 14 | 0.013873365 | 21.43 | |
| GO:0048038 | quinone binding | 4 | 25 | 0.01050813 | 16.00 | |
| GO:0016638 | oxidoreductase activity, acting on the CH-NH2 group of donors | 3 | 24 | 0.04293938 | 12.50 | |
| GO:0004713 | protein tyrosine kinase activity | 4 | 50 | 0.044647033 | 8.00 | |
| GO:0004497 | monooxygenase activity | 11 | 255 | 0.012179367 | 4.31 | |
| GO:0016705 | oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen | 16 | 460 | 0.01050813 | 3.48 | |
| GO:0008324 | cation transmembrane transporter activity | 15 | 434 | 0.012179367 | 3.46 | |
| GO:0009055 | electron carrier activity | 17 | 506 | 0.01050813 | 3.36 | |
| GO:0005506 | iron ion binding | 13 | 420 | 0.040286999 | 3.10 | |
| GO:0003700 | transcription factor activity, sequence-specific DNA binding | 32 | 1046 | 0.000516422 | 3.06 | |
| GO:0001071 | nucleic acid binding transcription factor activity | 32 | 1057 | 0.000516422 | 3.03 | |
| GO:0043565 | sequence-specific DNA binding | 19 | 644 | 0.013547559 | 2.95 | |
| GO:0004674 | protein serine/threonine kinase activity | 26 | 1080 | 0.023435321 | 2.41 | |
| GO:0004672 | protein kinase activity | 33 | 1498 | 0.023435321 | 2.20 | |
| GO:0003677 | DNA binding | 53 | 2659 | 0.012179367 | 1.99 | |
| GO:0046914 | transition metal ion binding | 39 | 1957 | 0.040286999 | 1.99 | |
| GO:1901363 | heterocyclic compound binding | 123 | 7912 | 0.035080217 | 1.55 | |
| GO:0097159 | organic cyclic compound binding | 123 | 7923 | 0.035080217 | 1.55 | |
| Profile 9 | GO:0004485 | methylcrotonoyl-CoA carboxylase activity | 2 | 2 | 0.010998085 | 100.00 |
| GO:0008061 | chitin binding | 6 | 11 | 1.37 × 10−6 | 54.55 | |
| GO:0004084 | branched-chain-amino-acid transaminase activity | 2 | 4 | 0.037470895 | 50.00 | |
| GO:0052654 | L-leucine transaminase activity | 2 | 4 | 0.037470895 | 50.00 | |
| GO:0052655 | L-valine transaminase activity | 2 | 4 | 0.037470895 | 50.00 | |
| GO:0052656 | L-isoleucine transaminase activity | 2 | 4 | 0.037470895 | 50.00 | |
| GO:0004568 | chitinase activity | 6 | 22 | 8.26 × 10−5 | 27.27 | |
| GO:0008762 | UDP-N-acetylmuramate dehydrogenase activity | 5 | 38 | 0.018018015 | 13.16 | |
| GO:0004601 | peroxidase activity | 15 | 160 | 1.86 × 10−5 | 9.38 | |
| GO:0016684 | oxidoreductase activity, acting on peroxide as acceptor | 15 | 166 | 2.48 × 10−5 | 9.04 | |
| GO:0016209 | antioxidant activity | 16 | 191 | 2.48 × 10−5 | 8.38 | |
| GO:0020037 | heme binding | 30 | 446 | 1.74 × 10−7 | 6.73 | |
| GO:0046906 | tetrapyrrole binding | 30 | 455 | 1.86 × 10−7 | 6.59 | |
| GO:0050660 | flavin adenine dinucleotide binding | 9 | 157 | 0.043604233 | 5.73 | |
| GO:0004497 | monooxygenase activity | 12 | 255 | 0.045573268 | 4.71 | |
| GO:0016705 | oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen | 20 | 460 | 0.010998085 | 4.35 | |
| GO:0004553 | hydrolase activity, hydrolyzing O-glycosyl compounds | 20 | 488 | 0.019555184 | 4.10 | |
| GO:0005506 | iron ion binding | 17 | 420 | 0.037470895 | 4.05 | |
| GO:0016491 | oxidoreductase activity | 69 | 1744 | 1.74 × 10−7 | 3.96 | |
| GO:0016798 | hydrolase activity, acting on glycosyl bonds | 20 | 516 | 0.035644596 | 3.88 | |
| GO:0003824 | catalytic activity | 224 | 9724 | 3.52 × 10−5 | 2.30 | |
| Profile 13 | GO:0030145 | manganese ion binding | 5 | 43 | 0.01549229 | 11.63 |
| GO:0045735 | nutrient reservoir activity | 5 | 45 | 0.01549229 | 11.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Li, R.; Lin, F.; Xiong, Y.; Wang, L.; Wang, B.; Guo, J.; Hu, C. Transcriptome Changes Induced by Different Potassium Levels in Banana Roots. Plants 2020, 9, 11. https://doi.org/10.3390/plants9010011
He Y, Li R, Lin F, Xiong Y, Wang L, Wang B, Guo J, Hu C. Transcriptome Changes Induced by Different Potassium Levels in Banana Roots. Plants. 2020; 9(1):11. https://doi.org/10.3390/plants9010011
Chicago/Turabian StyleHe, Yingdui, Ruimei Li, Fei Lin, Ying Xiong, Lixia Wang, Bizun Wang, Jianchun Guo, and Chengxiao Hu. 2020. "Transcriptome Changes Induced by Different Potassium Levels in Banana Roots" Plants 9, no. 1: 11. https://doi.org/10.3390/plants9010011
APA StyleHe, Y., Li, R., Lin, F., Xiong, Y., Wang, L., Wang, B., Guo, J., & Hu, C. (2020). Transcriptome Changes Induced by Different Potassium Levels in Banana Roots. Plants, 9(1), 11. https://doi.org/10.3390/plants9010011





