Potential of Grasses in Phytolith Production in Soils Contaminated with Cadmium
Abstract
1. Introduction
2. Results and Discussion
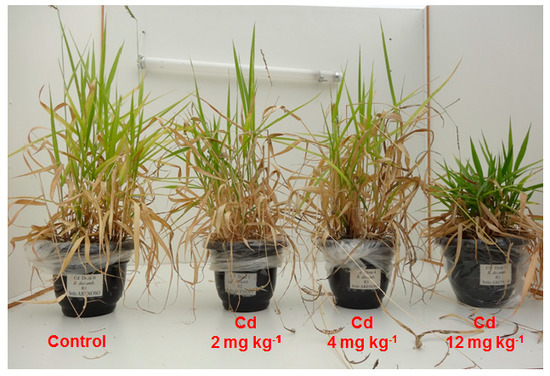
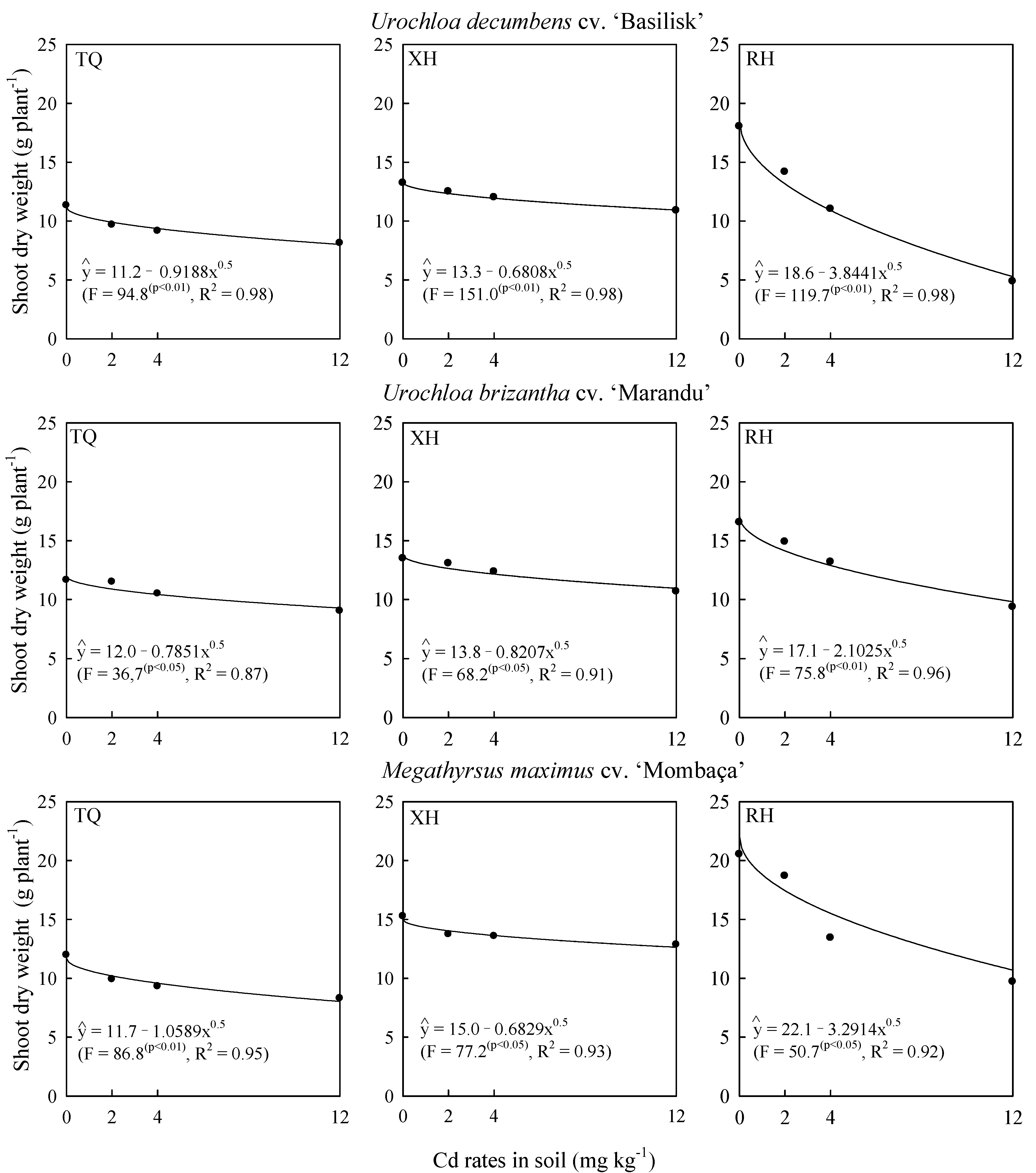
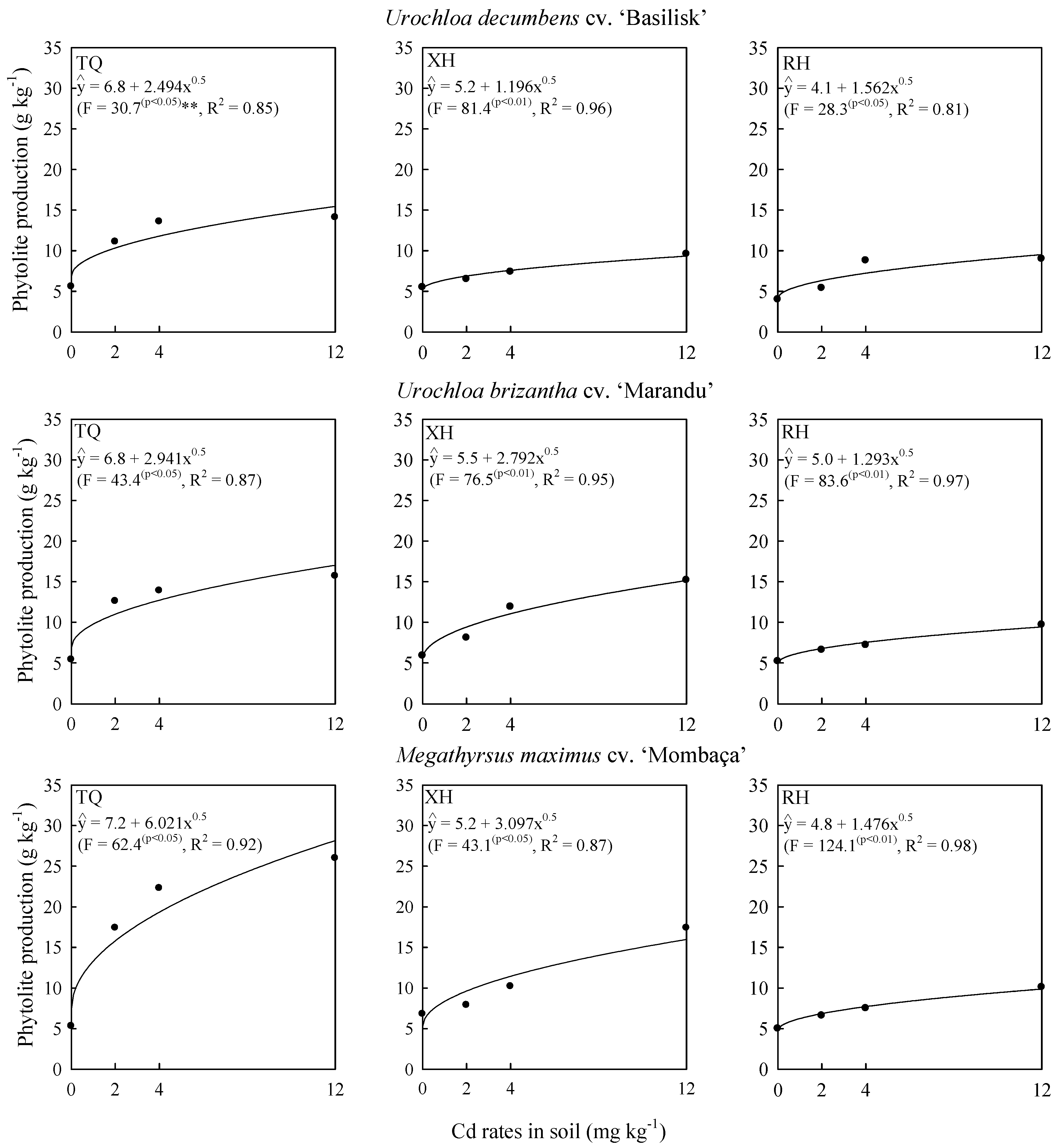
2.1. Cadmium Effects on Grass Biomass and Phytolith Production
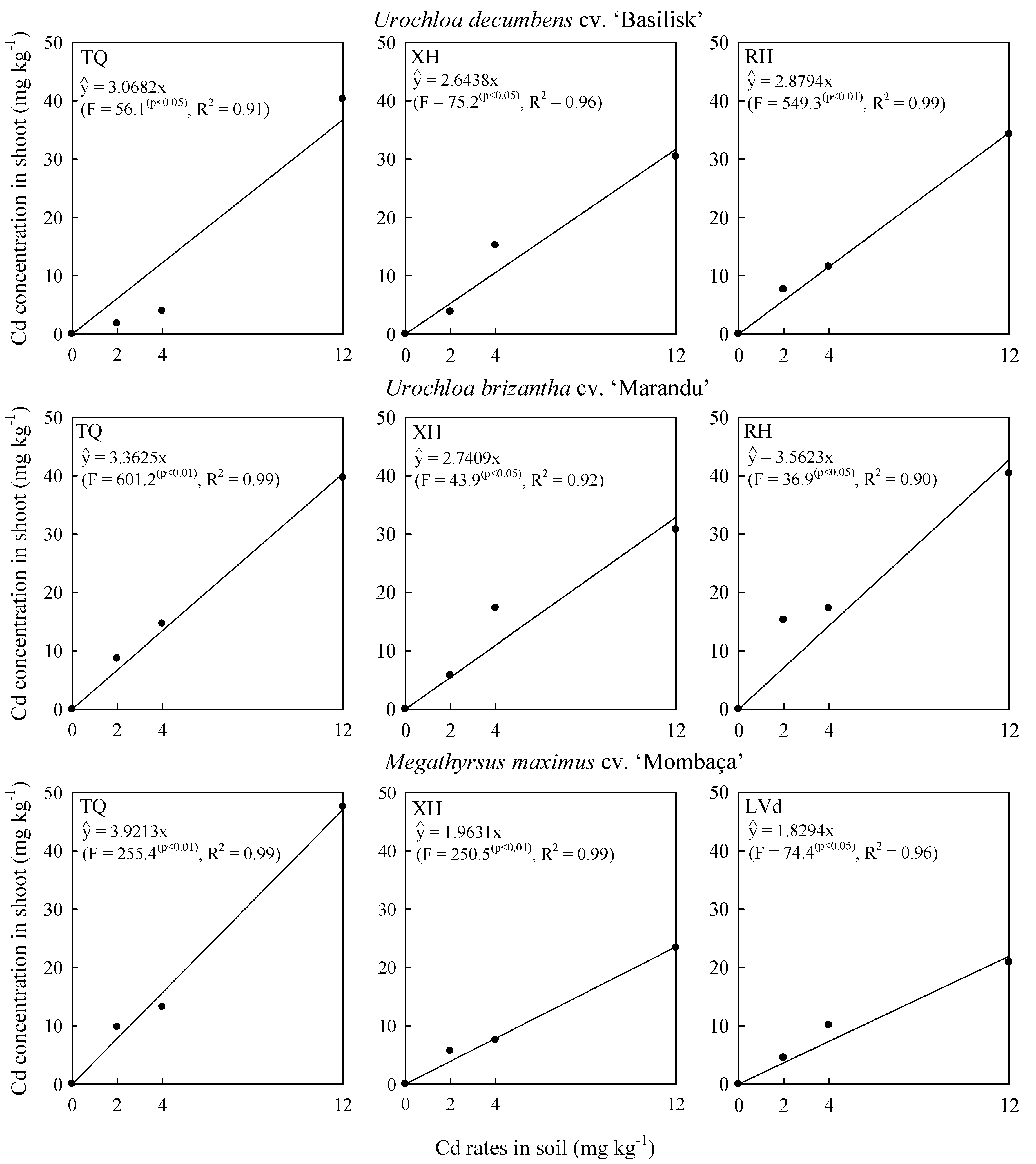
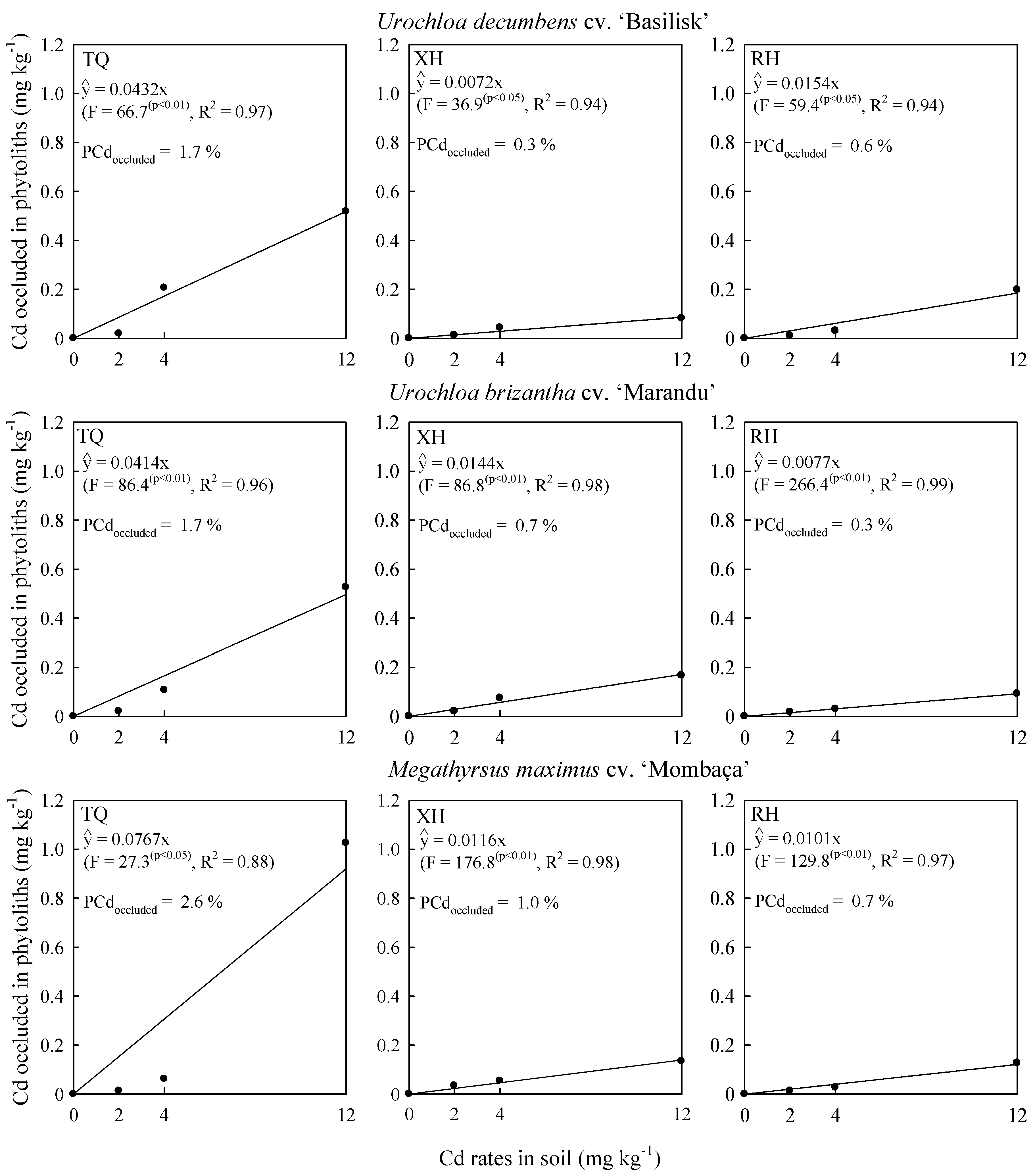
2.2. Cadmium Concentration in Shoot and Phytolith
3. Materials and Methods
3.1. Experimental Conditions
3.2. Measurements
3.3. Statistics and Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Kashiflrshad, M. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2010, 62, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Buján, E. Elemental composition of phytoliths in modern plants (Ericaceae). Quat. Int. 2013, 287, 114–120. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Cornelis, J.T. Impact of rice cultivar and organ on elemental composition of phytoliths and the release of bio-available silicon. Front. Plant Sci. 2014, 5, 529. [Google Scholar] [CrossRef] [PubMed]
- Zare, A.A.; Khoshgoftarmanesh, A.H.; Malakouti, M.J.; Bahrami, H.A.; Chaney, R.L. Root uptake and shoot accumulation of cadmium by lettuce at various Cd:Zn ratios in nutrient solution. Ecotoxicol. Environ. Saf. 2018, 148, 441–446. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; He, C.; Li, X.; Zhang, W.; Xu, F.; Lin, Y.; Wang, L. Inhibition of cadmium ion uptake in rice (Oryza sativa) cells by a wall-bound form of silicon. New Phytol. 2013, 200, 691–699. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Zhang, Y.; Chai, T. Silicon attenuates cadmium toxicity in Solanum nigrum L. by reducing cadmium uptake and oxidative stress. Plant Physiol. Biochem. 2013, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, K.; Xu, J.; Liang, J.; Lu, X.; Yang, J.; Zhu, Q. Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes. Field Crops Res. 2003, 83, 271–281. [Google Scholar] [CrossRef]
- Zhang, G.; Fukami, M.; Sekimoto, H. Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. Field Crops Res. 2002, 77, 93–98. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Nie, Q.; Zhang, W.; Zhang, F. Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ. Exp. Bot. 2008, 62, 300–307. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, H.; Li, Z.; Zhuang, P.; Gao, B. Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour. Technol. 2010, 101, 2063–2066. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, G. Genotypic variation in kernel heavy metal concentrations in barley and as affected by soil factors. J. Plant Nutr. 2002, 25, 1163–1173. [Google Scholar] [CrossRef]
- Oliva, S.R.; Mingorance, M.D.; Leidi, E.O. Effects of silicon on copper toxicity in Erica andevalensis Cabezudo and Rivera: A potential species to remediate contaminated soils. J. Environ. Monit. 2011, 13, 591–596. [Google Scholar] [CrossRef]
- Baker, A.J. Accumulators and excluders strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Antonkiewicz, J.; Gruba, P.; Pająk, M. Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill. Open Chem. 2018, 16, 1143–1152. [Google Scholar] [CrossRef]
- Roma, C.F.D.C.; Cecato, U.; Soares Filho, C.V.; Santos, G.T.D.; Ribeiro, O.L.; Iwamoto, B.S. Morphogenetic and tillering dynamics in Tanzania grass fertilized and non-fertilized with nitrogen according to season. Revista Brasileira de Zootecnia 2012, 41, 565–573. [Google Scholar] [CrossRef]
- Silva, E.B.; Fonseca, F.G.; Alleoni, L.R.F.; Nascimento, S.S.; Grazziotti, P.H.; Nardis, B.O. Availability and toxicity of cadmium to forage grasses grown in contaminated soil. Int. J. Phytoremediat. 2016, 18, 847–852. [Google Scholar] [CrossRef]
- Pan, W.; Song, Z.; Liu, H.; Müeller, K.; Yang, X.; Zhang, X.; Li, Z.; Liu, X.; Qiu, S.; Hao, Q.; et al. Impact of grassland degradation on soil phytolith carbon sequestration in Inner Mongolian steppe of China. Geoderma 2017, 308, 86–92. [Google Scholar] [CrossRef]
- Ru, N.; Yang, X.; Song, Z.; Liu, H.; Hao, Q.; Liu, X.; Wu, X. Phytoliths and phytolith carbon occlusion in aboveground vegetation of sandy grasslands in eastern Inner Mongolia, China. Sci. Total Environ. 2018, 625, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, Z.; Liu, H.; Bolan, N.S.; Wang, H.; Li, Z. Plant silicon content in forests of north China and its implications for phytolith carbon sequestration. Ecol. Res. 2015, 30, 347–355. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Liu, H.; Van Zwieten, L.; Song, A.; Li, Z.; Hao, Q.; Zhang, X.; Wang, H. Phytolith accumulation in broadleaf and conifer forests of northern China: Implications for phytolith carbon sequestration. Geoderma 2018, 312, 36–44. [Google Scholar] [CrossRef]
- Parr, J.F. Effect of fire on phytolith coloration. Geoarchaeology 2006, 21, 171–185. [Google Scholar] [CrossRef]
- Song, Z.; McGrouther, K.; Wang, H. Occurrence, turnover and carbon sequestration potential of phytoliths in terrestrial ecosystems. Earth-Sci. Rev. 2016, 158, 19–30. [Google Scholar] [CrossRef]
- Zuo, X.; Lu, H. Carbon sequestration within millet phytoliths from dry-farming of crops in China. Chin. Sci. Bull. 2011, 56, 3451–3456. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Cetesb. São Paulo State Environmental Agency. Report on Standard Values for Soils and Groundwater in the São Paulo State; Cetesb: São Paulo, Brazil, 2005. Available online: http://www.cetesb.sp.gov.br/Solo/relatorios/tabela_valores_2005.pdf (accessed on 10 August 2015).
- Soil Survey Staff. Soil Taxonomy: Keys to Soil Taxonomy, 11th ed.; Department of Agriculture, Natural Resources and Conservation Services: Washington, DC, USA, 2010; pp. 123–257. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual for Methods of Soil Analysis, 3rd ed.; Embrapa: Brasília, Brazil, 2017; pp. 198–247. (In Portuguese) [Google Scholar]
- USEPA. United States Environmental Protection Agency. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils and Oils—Method 3052—SW—846, 1994; EPA: Washington, DC, USA, 2007. Available online: http:www.epa.gov/epaosver/hazwaste/test/3052.pdf (accessed on 8 October 2018).
- Sparks, D.L. Kinetics of metal sorption reactions. In Metal Speciation and Contamination of Soil; Allen, H.E., Huang, C.P., Bailey, G.W., Bowers, A.R., Eds.; Lewis Publishers: Chelsea, MI, USA, 1995; pp. 35–58. [Google Scholar]
- Parr, J.F.; Lentfer, C.J.; Boyd, W.E. A comparative analysis of wet and dry ashing techniques for the extraction of phytoliths from plant material. J. Archaeol. Sci. 2001, 28, 875–886. [Google Scholar] [CrossRef]
| Attribute | Unit | Soil | ||
|---|---|---|---|---|
| TQ | XH | RH | ||
| pH (a) water | - | 5.1 | 5.4 | 5.5 |
| P (b) | mg kg−1 | 0.2 | 0.1 | 0.2 |
| K (b) | mmolc kg−1 | 0.4 | 0.1 | 0.2 |
| Ca (c) | mmolc kg−1 | 6.7 | 4.50 | 8.1 |
| Mg (c) | mmolc kg−1 | 3.5 | 1.8 | 3.9 |
| Al (c) | mmolc kg−1 | 7.8 | 4.2 | 1.6 |
| CEC (d) | mmolc kg−1 | 40.6 | 71.4 | 49.2 |
| Organic carbon | g kg−1 | 3.5 | 5.8 | 5.2 |
| Cd (e) | mg kg−1 | 0.0 | 0.0 | 0.0 |
| P max (f) | mg kg−1 | 100 | 200 | 250 |
| Si (g) | mg kg−1 | 558 | 330 | 119 |
| Sand (h) | g kg−1 | 830 | 580 | 310 |
| Loam (h) | g kg−1 | 110 | 70 | 180 |
| Clay (h) | g kg−1 | 60 | 350 | 510 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Melo Farnezi, M.M.; de Barros Silva, E.; Lopes dos Santos, L.; Christofaro Silva, A.; Grazziotti, P.H.; Taline Prochnow, J.; Marinho Pereira, I.; Costa Ilhéu Fontan, I.d. Potential of Grasses in Phytolith Production in Soils Contaminated with Cadmium. Plants 2020, 9, 109. https://doi.org/10.3390/plants9010109
de Melo Farnezi MM, de Barros Silva E, Lopes dos Santos L, Christofaro Silva A, Grazziotti PH, Taline Prochnow J, Marinho Pereira I, Costa Ilhéu Fontan Id. Potential of Grasses in Phytolith Production in Soils Contaminated with Cadmium. Plants. 2020; 9(1):109. https://doi.org/10.3390/plants9010109
Chicago/Turabian Stylede Melo Farnezi, Múcio Mágno, Enilson de Barros Silva, Lauana Lopes dos Santos, Alexandre Christofaro Silva, Paulo Henrique Grazziotti, Jeissica Taline Prochnow, Israel Marinho Pereira, and Ivan da Costa Ilhéu Fontan. 2020. "Potential of Grasses in Phytolith Production in Soils Contaminated with Cadmium" Plants 9, no. 1: 109. https://doi.org/10.3390/plants9010109
APA Stylede Melo Farnezi, M. M., de Barros Silva, E., Lopes dos Santos, L., Christofaro Silva, A., Grazziotti, P. H., Taline Prochnow, J., Marinho Pereira, I., & Costa Ilhéu Fontan, I. d. (2020). Potential of Grasses in Phytolith Production in Soils Contaminated with Cadmium. Plants, 9(1), 109. https://doi.org/10.3390/plants9010109